Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Cell Cultures
2.2. Single Cell Cytosolic Calcium Video Imaging
2.3. SP Enzyme Immunoassay (EIA)
2.4. Immunocytochemical Quantification of PAR2 Internalization
2.5. Statistical Analysis
3. Results
3.1. PbTx-1-Induces an Increase of [Ca2+]i in DRG Sensory Neurons
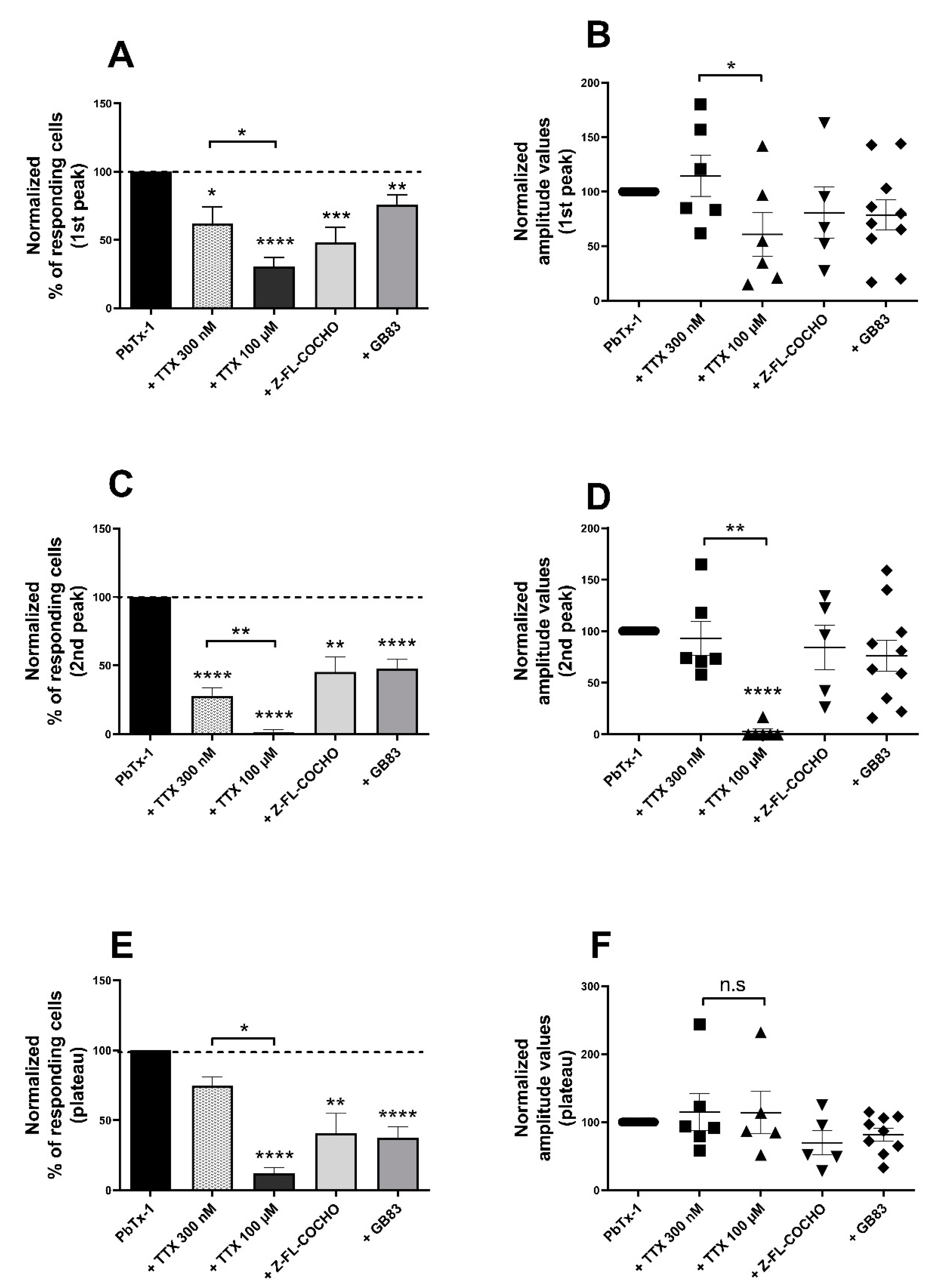
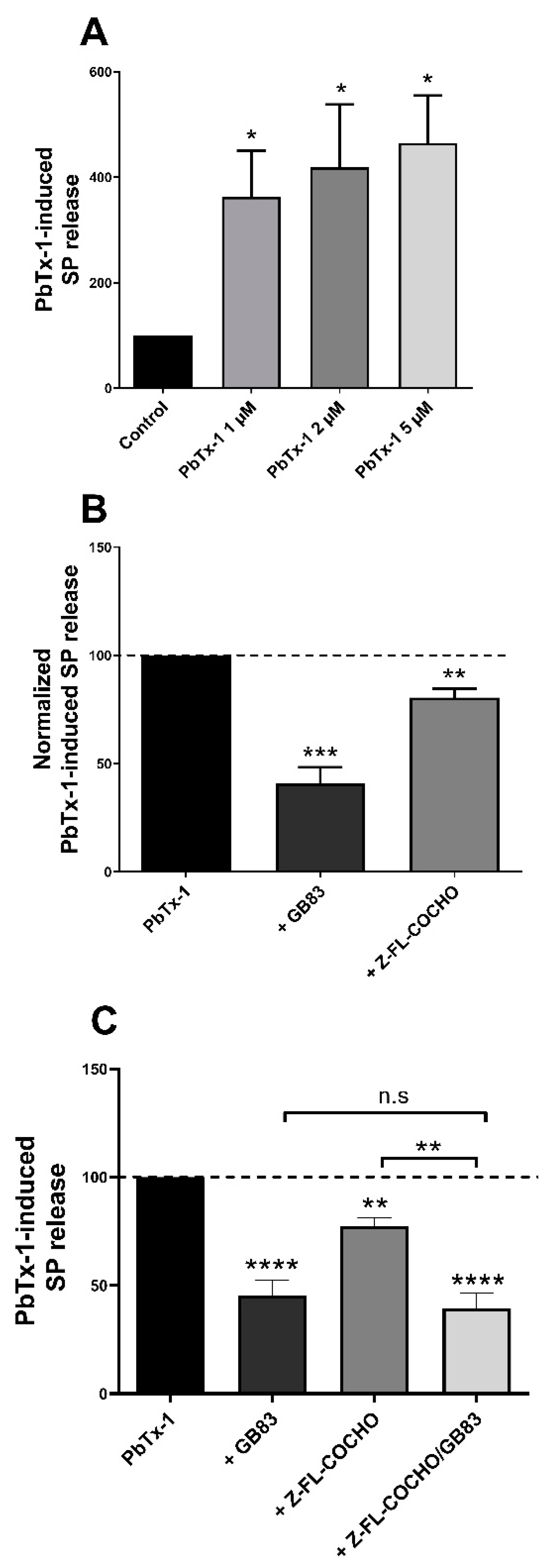
3.2. PbTx-1-Induced [Ca2+]i Increase and SP Release in DRG Neurons Are Mediated by Nav Channels, Cat-S and PAR2
3.3. PbTx-1 Induced Cat-S- and PAR2-Dependent SP Release from Co-Cultured Sensory Neurons and Keratinocytes
3.4. P-CTX-2 Induced PAR2 Internalization Independtly from Cat-S in Sensory Neurons
3.5. PKC, PKA, IP3R and TRPV4 Are Involved in the [Ca2+]i Increase Induced by PbTx-1 in Sensory Neurons
3.6. PKC, PKA, IP3R, and TRPV4 Are Involved in the SP Release Induced by PbTx-1 in the Co-Culture
4. Discussion
4.1. PbTx-1 Induce [Ca2+]i Increase and SP Release from Sensory Neurons and Co-Culture Model
4.2. Mechanisms Involved in the [Ca2+]i Increase and SP Release Induced by PbTx-1
4.3. Internalization of PAR2 Induced by P-CTX-2 Is Not Mediated by Cat-S in Sensory Neurons
4.4. PKC, PKA, IP3R and TRPV4 Involvement in PbTx-1-Induced [Ca2+]i Increase and SP Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cat-S | Cathepsin S |
| CTXs | Ciguatoxins |
| Nav | Voltage-dependent sodium channel |
| NSP | Neurotoxic shellfish poisoning |
| PAR2 | Protease-activated receptor 2 |
| PbTxs | Brevetoxins |
| P-CTX-2 | Pacific-ciguatoxin-2 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| SP | Substance P |
| TRPV4 | The transient receptor potential vanilloid 4 |
| TTX | Tetrodotoxin |
| TTX-r Nav | Tetrodotoxin-resistant Nav channel |
| TTX-s Nav | Tetrodotoxin-sensitive Nav channel |
References
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef] [Green Version]
- Morris, P.D.; Campbell, D.S.; Taylor, T.J.; Freeman, J.I. Clinical and epidemiological features of neurotoxic shellfish poisoning in North Carolina. Am. J. Public Health 1991, 81, 471–474. [Google Scholar] [CrossRef] [Green Version]
- FAO. Assessement and Management of Biotoxin Risks in Bivalve Molluscs; FAO: Rome, Italy, 2011; p. 358. [Google Scholar]
- Kirkpatrick, B.; Fleming, L.E.; Backer, L.C.; Bean, J.A.; Tamer, R.; Kirkpatrick, G.; Kane, T.; Wanner, A.; Dalpra, D.; Reich, A.; et al. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae 2006, 5, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Backer, L.C.; Fleming, L.E.; Rowan, A.; Cheng, Y.-S.; Benson, J.; Pierce, R.H.; Zaias, J.; Bean, J.; Bossart, G.D.; Johnson, D.; et al. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2003, 2, 19–28. [Google Scholar] [CrossRef]
- Backer, L.C.; Kirkpatrick, B.; Fleming, L.E.; Cheng, Y.S.; Pierce, R.; Bean, J.A.; Clark, R.; Johnson, D.; Wanner, A.; Tamer, R.; et al. Occupational Exposure to Aerosolized Brevetoxins during Florida Red Tide Events: Effects on a Healthy Worker Population. Environ. Health Perspect. 2005, 113, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, B.; Fleming, L.E.; Squicciarini, D.; Backer, L.C.; Clark, R.; Abraham, W.; Benson, J.; Cheng, Y.S.; Johnson, D.; Pierce, R.; et al. Literature Review of Florida Red Tide: Implications for Human Health Effects. Harmful Algae 2004, 3, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Dalpra, D.; Tamer, R.; Zaias, J.; Cheng, Y.S.; Pierce, R.; et al. Initial Evaluation of the Effects of Aerosolized Florida Red Tide Toxins (Brevetoxins) in Persons with Asthma. Environ. Health Perspect. 2005, 113, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.E.; Friedman, M.A.; Jin, D.; Beet, A.; Kirkpatrick, B.; Reich, A.; Kirkpatrick, G.; Ullmann, S.G.; Fleming, L.E.; Hoagland, P. Neurological illnesses associated with Florida red tide (Karenia brevis) blooms. Harmful Algae 2019, 82, 73–81. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Higgins, C.; Davidson, K.; Veszelovszki, A.; Payne, D.; Hungerford, J.; Higman, W. Potential Threats Posed by New or Emerging Marine Biotoxins in UK Waters and Examination of Detection Methodology Used in Their Control: Brevetoxins. Mar. Drugs 2015, 13, 1224–1254. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.J.; Leggett, S.R.; Carter, B.J.; Colle, C. Effects of brevetoxin exposure on the immune system of loggerhead sea turtles. Aquat. Toxicol. 2010, 97, 293–303. [Google Scholar] [CrossRef]
- Bossart, G.D.; Baden, D.G.; Ewing, R.Y.; Roberts, B.; Wright, S.D. Brevetoxicosis in manatees (Trichechus manatus latirostris) from the 1996 epizootic: Gross, histologic, and immunohistochemical features. Toxicol. Pathol. 1998, 26, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Fire, S.E.; Fauquier, D.; Flewelling, L.J.; Henry, M.; Naar, J.; Pierce, R.; Wells, R.S. Brevetoxin exposure in bottlenose dolphins (Tursiops truncatus) associated with Karenia brevis blooms in Sarasota Bay, Florida. Mar. Biol. 2007, 152, 827–834. [Google Scholar] [CrossRef]
- Fire, S.E.; Flewelling, L.J.; Stolen, M.; Durden, W.N.; de Wit, M.; Spellman, A.C.; Wang, Z. Brevetoxin-associated mass mortality event of bottlenose dolphins and manatees along the east coast of Florida, USA. Mar. Ecol. Prog. Ser. 2015, 526, 241–251. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zhou, Y.; Irvin, C.M.; Pierce, R.H.; Naar, J.; Backer, L.C.; Fleming, L.E.; Kirkpatrick, B.; Baden, D.G. Characterization of Marine Aerosol for Assessment of Human Exposure to Brevetoxins. Environ. Health Perspect. 2005, 113, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Pierce, R.H.; Henry, M.S.; Blum, P.C.; Hamel, S.L.; Kirkpatrick, B.; Cheng, Y.S.; Zhou, Y.; Irvin, C.M.; Naar, J.; Weidner, A.; et al. Brevetoxin composition in water and marine aerosol along a Florida beach: Assessing potential human exposure to marine biotoxins. Harmful Algae 2005, 4, 965–972. [Google Scholar] [CrossRef]
- Lombet, A.; Bidard, J.N.; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987, 219, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Gawley, R.E.; Rein, K.S.; Kinoshita, M.; Baden, D.G. Binding of brevetoxins and ciguatoxin to the voltage-sensitive sodium channel and conformational analysis of brevetoxin B. Toxicon 1992, 30, 780–785. [Google Scholar] [CrossRef]
- Whitney, P.L.; Baden, D.G. Complex association and dissociation kinetics of brevetoxin binding to voltage-sensitive rat brain sodium channels. Nat. Toxins 1996, 4, 261–270. [Google Scholar] [CrossRef]
- Dechraoui, M.-Y.; Naar, J.; Pauillac, S.; Legrand, A.-M. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon 1999, 37, 125–143. [Google Scholar] [CrossRef]
- Wood, J.N.; Akopian, A.N.; Baker, M.; Ding, Y.; Geoghegan, F.; Nassar, M.; Malik-Hall, M.; Okuse, K.; Poon, L.; Ravenall, S.; et al. Sodium Channels in Primary Sensory Neurons: Relationship to Pain States. In Novartis Foundation Symposia; Bock, G., Goode, J.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 159–172. ISBN 978-0-471-48530-8. [Google Scholar]
- Baden, D.G. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB J. 1989, 3, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- McFarren, E.F.; Tanabe, H.; Silva, F.J.; Wilson, W.B.; Campbell, J.E.; Lewis, K.H. The occurrence of a ciguatera-like poison in oysters, clams, and Gymnodinium breve cultures. Toxicon 1965, 3, 111–123. [Google Scholar] [CrossRef]
- Le Garrec, R.; L’Herondelle, K.; Le Gall-Ianotto, C.; Lebonvallet, N.; Leschiera, R.; Buhe, V.; Talagas, M.; Vetter, I.; Lewis, R.J.; Misery, L. Release of neuropeptides from a neuro-cutaneous co-culture model: A novel in vitro model for studying sensory effects of ciguatoxins. Toxicon 2016, 116, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhoff, M.; Vergnolle, N.; Young, S.H.; Tognetto, M.; Amadesi, S.; Ennes, H.S.; Trevisani, M.; Hollenberg, M.D.; Wallace, J.L.; Caughey, G.H.; et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000, 6, 151–158. [Google Scholar] [CrossRef]
- Henry, J.L. Substance P and pain: An updating. Trends Neurosci. 1980, 3, 95–97. [Google Scholar] [CrossRef]
- Vergnolle, N.; Ferazzini, M.; D’Andrea, M.R.; Buddenkotte, J.; Steinhoff, M. Proteinase-activated receptors: Novel signals for peripheral nerves. Trends Neurosci. 2003, 26, 496–500. [Google Scholar] [CrossRef]
- L’Herondelle, K.; Pierre, O.; Fouyet, S.; Leschiera, R.; Le Gall-Ianotto, C.; Philippe, R.; Buscaglia, P.; Mignen, O.; Talagas, M.; Lewis, R.J.; et al. PAR2, keratinocytes, and cathesin S mediate the sensory effects of ciguatoxins reponsible for ciguatera poisoning. J. Investig. Dermatol. 2020, in press. [Google Scholar]
- Steinhoff, M.; Corvera, C.U.; Thoma, M.S.; Kong, W.; McAlpine, B.E.; Caughey, G.H.; Ansel, J.C.; Bunnett, N.W. Proteinase-activated receptor-2 in human skin: Tissue distribution and activation of keratinocytes by mast cell tryptase. Exp. Dermatol. 1999, 8, 282–294. [Google Scholar] [CrossRef]
- Vergnolle, N.; Wallace, J.L.; Bunnett, N.W.; Hollenberg, M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001, 22, 146–152. [Google Scholar] [CrossRef]
- Bao, Y.; Hou, W.; Hua, B. Protease-activated receptor 2 signalling pathways: A role in pain processing. Expert Opin. Ther. Targets 2014, 18, 15–27. [Google Scholar] [CrossRef]
- Clark, A.K.; Wodarski, R.; Guida, F.; Sasso, O.; Malcangio, M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 2010, 58, 1710–1726. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.B.; Reddy, V.B.; Lerner, E.A. Cathepsin S Signals via PAR2 and Generates a Novel Tethered Ligand Receptor Agonist. PLoS ONE 2014, 9, e99702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Lieu, T.; Barlow, N.; Sostegni, S.; Haerteis, S.; Korbmacher, C.; Liedtke, W.; Jimenez-Vargas, N.N.; Vanner, S.J.; Bunnett, N.W. Neutrophil Elastase Activates Protease-activated Receptor-2 (PAR2) and Transient Receptor Potential Vanilloid 4 (TRPV4) to Cause Inflammation and Pain. J. Biol. Chem. 2015, 290, 13875–13887. [Google Scholar] [CrossRef] [Green Version]
- Böhm, S.K.; Kong, W.; Bromme, D.; Smeekens, S.P.; Anderson, D.C.; Connolly, A.; Kahn, M.; Nelken, N.A.; Coughlin, S.R.; Payan, D.G.; et al. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 1996, 314, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Vargas, N.N.; Pattison, L.A.; Zhao, P.; Lieu, T.; Latorre, R.; Jensen, D.D.; Castro, J.; Aurelio, L.; Le, G.T.; Flynn, B.; et al. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, E7438–E7447. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Mihara, K.; Chung, H.; Renaux, B.; Lau, C.S.; Muruve, D.A.; DeFea, K.A.; Bouvier, M.; Hollenberg, M.D. Neutrophil Elastase Acts as a Biased Agonist for Proteinase-activated Receptor-2 (PAR-2). J. Biol. Chem. 2011, 286, 24638–24648. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Metcalf, M.; Bunnett, N.W. Biased Signaling of Protease-Activated Receptors. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nystedt, S.; Emilsson, K.; Wahlestedt, C.; Sundelin, J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9208–9212. [Google Scholar] [CrossRef] [Green Version]
- Böhm, S.K.; Khitin, L.M.; Grady, E.F.; Aponte, G.; Payan, D.G.; Bunnett, N.W. Mechanisms of Desensitization and Resensitization of Proteinase-activated Receptor-2. J. Biol. Chem. 1996, 271, 22003–22016. [Google Scholar] [CrossRef] [Green Version]
- Amadesi, S.; Cottrell, G.S.; Divino, L.; Chapman, K.; Grady, E.F.; Bautista, F.; Karanjia, R.; Barajas-Lopez, C.; Vanner, S.; Vergnolle, N.; et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cɛ- and A-dependent mechanisms in rats and mice. J. Physiol. 2006, 575, 555–571. [Google Scholar] [CrossRef]
- Amadesi, S.; Grant, A.D.; Cottrell, G.S.; Vaksman, N.; Poole, D.P.; Rozengurt, E.; Bunnett, N.W. Protein Kinase D Isoforms Are Expressed in Rat and Mouse Primary Sensory Neurons and Are activated by Agonists Of Protease-Activated Receptor 2. J. Comp. Neurol. 2009, 516, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouin, O.; L’Herondelle, K.; Buscaglia, P.; Le Gall-Ianotto, C.; Philippe, R.; Legoux, N.; Mignen, O.; Buhé, V.; Leschiera, R.; Sakka, M.; et al. Major Role for TRPV1 and InsP3R in PAR2-Elicited Inflammatory Mediator Production in Differentiated Human Keratinocytes. J. Investig. Dermatol. 2018, 138, 1564–1572. [Google Scholar] [CrossRef] [Green Version]
- Amadesi, S. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef]
- Mrozkova, P.; Spicarova, D.; Palecek, J. Hypersensitivity Induced by Activation of Spinal Cord PAR2 Receptors Is Partially Mediated by TRPV1 Receptors. PLoS ONE 2016, 11, e0163991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Wang, S.; Tominaga, M.; Yamamoto, S.; Fukuoka, T.; Higashi, T.; Kobayashi, K.; Obata, K.; Yamanaka, H.; Noguchi, K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Investig. 2007, 117, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef]
- Déry, O.; Thoma, M.S.; Wong, H.; Grady, E.F.; Bunnett, N.W. Trafficking of proteinase-activated receptor-2 and Beta-arrestion-1 tagged with green fluorescent protein. J. Biol. Chem. 1999, 274, 18524–18535. [Google Scholar] [CrossRef] [Green Version]
- DeFea, K.A.; Zalevsky, J.; Thoma, M.S.; Déry, O.; Mullins, R.D.; Bunnett, N.W. β-Arrestin-Dependent Endocytosis of Proteinase-Activated Receptor 2 Is Required for Intracellular Targeting of Activated ERK1/2. J. Cell Biol. 2000, 148, 1267–1281. [Google Scholar] [CrossRef]
- Stalheim, L.; Ding, Y.; Gullapalli, A.; Paing, M.M.; Wolfe, B.L.; Morris, D.R.; Trejo, J. Multiple Independent Functions of Arrestins in the Regulation of Protease-Activated Receptor-2 Signaling and Trafficking. Mol. Pharmacol. 2005, 67, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Lau, C.S.; Mathur, M.; Wang, P.; DeFea, K.A. Differential effects of beta-arrestins on the internalization, desensitization and ERK1/2 activation downstream of protease activated receptor-2. Am. J. Physiol. Cell Physiol. 2007, 293, C346–C357. [Google Scholar] [CrossRef]
- Zhao, P.; Lieu, T.; Barlow, N.; Metcalf, M.; Veldhuis, N.A.; Jensen, D.D.; Kocan, M.; Sostegni, S.; Haerteis, S.; Baraznenok, V.; et al. Cathepsin S Causes Inflammatory Pain via Biased Agonism of PAR2 and TRPV4. J. Biol. Chem. 2014, 289, 27215–27234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driessen, C.; Bryant, R.A.R.; Lennon-Duménil, A.-M.; Villadangos, J.A.; Bryant, P.W.; Shi, G.-P.; Chapman, H.A.; Ploegh, H.L. Cathepsin S Controls the Trafficking and Maturation of Mhc Class II Molecules in Dendritic Cells. J. Cell Biol. 1999, 147, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-S.; deRoos, P.; Honey, K.; Beers, C.; Rudensky, A.Y. A Role for Cathepsin L and Cathepsin S in Peptide Generation for MHC Class II Presentation. J. Immunol. 2002, 168, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.B.; Shimada, S.G.; Sikand, P.; LaMotte, R.H.; Lerner, E.A. Cathepsin S elicits itch and signals via protease-activated receptors. J. Investig. Dermatol. 2010, 130, 1468–1470. [Google Scholar] [CrossRef] [Green Version]
- Sostegni, S.; Diakov, A.; McIntyre, P.; Bunnett, N.; Korbmacher, C.; Haerteis, S. Sensitisation of TRPV4 by PAR2 is independent of intracellular calcium signalling and can be mediated by the biased agonist neutrophil elastase. Pflugers Arch. 2015, 467, 687–701. [Google Scholar] [CrossRef]
- Zhao, P.; Pattison, L.A.; Jensen, D.D.; Jimenez-Vargas, N.N.; Latorre, R.; Lieu, T.; Jaramillo, J.O.; Lopez-Lopez, C.; Poole, D.P.; Vanner, S.J.; et al. Protein kinase D and Gβγ mediate sustained nociceptive signaling by biased agonists of protease-activated receptor-2. J. Biol. Chem. 2019, 294, 10649–10662. [Google Scholar] [CrossRef]
- Talagas, M.; Lebonvallet, N.; Leschiera, R.; Sinquin, G.; Elies, P.; Haftek, M.; Pennec, J.-P.; Ressnikoff, D.; Padula, V.L.; Garrec, R.L.; et al. Keratinocytes Communicate with Sensory Neurons via Synaptic-like Contacts. Ann. Neurol. 2020, 88, 1205–1219. [Google Scholar] [CrossRef]
- Le Gall-Ianotto, C.; Andres, E.; Hurtado, S.P.; Pereira, U.; Misery, L. Characterization of the first coculture between human primary keratinocytes and the dorsal root ganglion-derived neuronal cell line F-11. Neuroscience 2012, 210, 47–57. [Google Scholar] [CrossRef]
- Dib-Hajj, S.; Black, J.A.; Cummins, T.R.; Waxman, S.G. NaN/Nav1.9: A sodium channel with unique properties. Trends Neurosci. 2002, 25, 253–259. [Google Scholar] [CrossRef]
- Barry, G.D.; Suen, J.Y.; Le, G.T.; Cotterell, A.; Reid, R.C.; Fairlie, D.P. Novel Agonists and Antagonists for Human Protease Activated Receptor 2. J. Med. Chem. 2010, 53, 7428–7440. [Google Scholar] [CrossRef]
- Poole, D.P.; Amadesi, S.; Veldhuis, N.A.; Abogadie, F.C.; Lieu, T.; Darby, W.; Liedtke, W.; Lew, M.J.; McIntyre, P.; Bunnett, N.W. Protease-activated Receptor 2 (PAR-2) Protein and Transient Receptor Potential Vanilloid 4 (TRPV4) Protein Coupling Is Required for Sustained Inflammatory Signaling. J. Biol. Chem. 2013, 288, 5790–5802. [Google Scholar] [CrossRef] [Green Version]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Touska, F.; Sattler, S.; Malsch, P.; Lewis, R.J.; Reeh, P.W.; Zimmermann, K. Ciguatoxins Evoke Potent CGRP Release by Activation of Voltage-Gated Sodium Channel Subtypes NaV1.9, NaV1.7 and NaV1.1. Mar. Drugs 2017, 15, 269. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.; Deuis, J.R.; Inserra, M.C.; Collins, L.S.; Namer, B.; Cabot, P.J.; Reeh, P.W.; Lewis, R.J.; Vetter, I. Analgesic treatment of ciguatoxin-induced cold allodynia. Pain 2013, 154, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Molgó, J.; Meunier, F.A.; Colasante, C.; Poulain, B. Marine Toxins Affecting Quantal Acetylcholine Release and Transmission at the Vertebrate Neuromuscular Junction. In Advances in Organ Biology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 2, pp. 249–284. ISBN 978-0-7623-0222-2. [Google Scholar]
- McCarthy, P.W.; Lawson, S.N. Cell type and conduction velocity of rat primary sensory neurons with substance p-like immunoreactivity. Neuroscience 1989, 28, 745–753. [Google Scholar] [CrossRef]
- Steinhoff, M.; Bienenstock, J.; Schmelz, M.; Maurer, M.; Wei, E.; Bíró, T. Neurophysiological, Neuroimmunological, and Neuroendocrine Basis of Pruritus. J. Investig. Dermatol. 2006, 126, 1705–1718. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.; Leffler, A.; Babes, A.; Cendan, C.M.; Carr, R.W.; Kobayashi, J.; Nau, C.; Wood, J.N.; Reeh, P.W. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 2007, 447, 856–859. [Google Scholar] [CrossRef]
- Ramsdell, J. The Molecular and Integrative Basis to Brevetoxin Toxicity. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, Second Edition; CRC Press Inc.: Boca Raton, FL, USA, 2008; pp. 519–550. [Google Scholar]
- Inserra, M.C.; Israel, M.R.; Caldwell, A.; Castro, J.; Deuis, J.R.; Harrington, A.M.; Keramidas, A.; Garcia-Caraballo, S.; Maddern, J.; Erickson, A.; et al. Multiple sodium channel isoforms mediate the pathological effects of Pacific ciguatoxin-1. Sci. Rep. 2017, 7, 42810. [Google Scholar] [CrossRef] [Green Version]
- Benn, S.C.; Costigan, M.; Tate, S.; Fitzgerald, M.; Woolf, C.J. Developmental Expression of the TTX-Resistant Voltage-Gated Sodium Channels Nav1.8 (SNS) and Nav1.9 (SNS2) in Primary Sensory Neurons. J. Neurosci. 2001, 21, 6077–6085. [Google Scholar] [CrossRef] [Green Version]
- Morfey, M. A review into the role of voltage gated sodium channels 1.8 and 1.9 in pain states: And medical uses of this knowledge. Plymouth Stud. Sci. 2012, 5, 294–303. [Google Scholar]
- Yamaoka, K.; Inoue, M.; Miyazaki, K.; Hirama, M.; Kondo, C.; Kinoshita, E.; Miyoshi, H.; Seyama, I. Synthetic Ciguatoxins Selectively Activate Nav1.8-derived Chimeric Sodium Channels Expressed in HEK293 Cells. J. Biol. Chem. 2009, 284, 7597–7605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.L.; Narahashi, T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J. Neurosci. 1992, 12, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.R.; Derian, C.K.; Leturcq, D.; Baker, S.M.; Brunmark, A.; Ling, P.; Darrow, A.L.; Santulli, R.J.; Brass, L.F.; Andrade-Gordon, P. Characterization of Protease-activated Receptor-2 Immunoreactivity in Normal Human Tissues. J. Histochem. Cytochem. 1998, 46, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, Z.-F.; Liao, M.-F.; Yao, W.-L.; Wang, J.; Wang, X.-R. Blocking PAR2 attenuates oxaliplatin-induced neuropathic pain via TRPV1 and releases of substance P and CGRP in superficial dorsal horn of spinal cord. J. Neurol. Sci. 2015, 352, 62–67. [Google Scholar] [CrossRef]
- Vergnolle, N.; Bunnett, N.W.; Sharkey, K.A.; Brussee, V.; Compton, S.J.; Grady, E.F.; Cirino, G.; Gerard, N.; Basbaum, A.I.; Andrade-Gordon, P.; et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat. Med. 2001, 7, 821–826. [Google Scholar] [CrossRef]
- Gillet, L.; Roger, S.; Besson, P.; Lecaille, F.; Gore, J.; Bougnoux, P.; Lalmanach, G.; Le Guennec, J.-Y. Voltage-gated Sodium Channel Activity Promotes Cysteine Cathepsin-dependent Invasiveness and Colony Growth of Human Cancer Cells. J. Biol. Chem. 2009, 284, 8680–8691. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour. Neurobiol. Pain 2019, 6, 100032. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.K.; Malcangio, M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Petanceska, S.; Canoll, P.; Devi, L.A. Expression of Rat Cathepsin S in Phagocytic Cells. J. Biol. Chem. 1996, 271, 4403–4409. [Google Scholar] [CrossRef] [Green Version]
- Barclay, J.; Clark, A.K.; Ganju, P.; Gentry, C.; Patel, S.; Wotherspoon, G.; Buxton, F.; Song, C.; Ullah, J.; Winter, J.; et al. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain 2007, 130, 225–234. [Google Scholar] [CrossRef]
- Schwarz, G.; Weber, E.; Schmid, H.; Schwarz, G.; Braun, M.; Schröter, C.J.; Burster, T.; Flad, T.; Dressel, D.; Kalbacher, H. Cathepsin S Activity is Detectable in Human Keratinocytes and is Selectively Upregulated upon Stimulation with Interferon-γ. J. Investig. Dermatol. 2002, 119, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Sudarsanam, S.; Virca, G.D.; March, C.J.; Srinivasan, S. An approach to computer-aided inhibitor design: Application to cathepsin L. J. Comput. Aided Mol. Des. 1992, 6, 223–233. [Google Scholar] [CrossRef]
- Komatsu, N.; Takata, M.; Otsuki, N.; Toyama, T.; Ohka, R.; Takehara, K.; Saijoh, K. Expression and Localization of Tissue Kallikrein mRNAs in Human Epidermis and Appendages. J. Investig. Dermatol. 2003, 121, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, N.; Saijoh, K.; Sidiropoulos, M.; Tsai, B.; Levesque, M.A.; Elliott, M.B.; Takehara, K.; Diamandis, E.P. Quantification of Human Tissue Kallikreins in the Stratum Corneum: Dependence on Age and Gender. J. Investig. Dermatol. 2005, 125, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Stefansson, K.; Brattsand, M.; Roosterman, D.; Kempkes, C.; Bocheva, G.; Steinhoff, M.; Egelrud, T. Activation of Proteinase-Activated Receptor-2 by Human Kallikrein-Related Peptidases. J. Investig. Dermatol. 2008, 128, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, S.R.; Sloss, C.M.; Cameron, P.; Kanke, T.; McKenzie, R.C.; Plevin, R. The role of intracellular Ca2+ in the regulation of proteinase-activated receptor-2 mediated nuclear factor kappa B signalling in keratinocytes. Br. J. Pharmacol. 2005, 145, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Cormia, F.E.; Dougherty, J.W. Proteolytic Activity in Development of Pain and Itching. J. Investig. Dermatol. 1960, 35, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Nauroy, P.; Nyström, A. Kallikreins: Essential epidermal messengers for regulation of the skin microenvironment during homeostasis, repair and disease. Matrix Biol. Plus 2020, 6–7, 100019. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Hansen, K.K.; Saifeddine, M.; Vergnolle, N.; Tea, I.; Blaber, M.; Blaber, S.I.; Scarisbrick, I.; Diamandis, E.P.; Hollenberg, M.D. Kallikrein-mediated cell signalling: Targeting proteinase-activated receptors (PARs). Biol. Chem. 2006, 387. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Eissa, A.; Mihara, K.; Oikonomopoulou, K.; Saifeddine, M.; Renaux, B.; Diamandis, E.; Hollenberg, M.D. Proteinase-activated receptors (PARs): Differential signalling by kallikrein-related peptidases KLK8 and KLK14. Biol. Chem. 2012, 393, 421–427. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Li, H.-C.; Cowan, A.A.; Liu, S.; Zhang, Y.-K.; Song, X.-J. Chronic compression or acute dissociation of dorsal root ganglion induces cAMP-dependent neuronal hyperexcitability through activation of PAR2. Pain 2012, 153, 1426–1437. [Google Scholar] [CrossRef]
- Hidalgo, J.; Liberona, J.L.; Molgó, J.; Jaimovich, E. Pacific ciguatoxin-1b effect over Na+and K+currents, inositol 1,4,5-triphosphate content and intracellular Ca 2+signals in cultured rat myotubes. Br. J. Pharmacol. 2002, 137, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Liberona, J.L.; Cárdenas, J.C.; Reyes, R.; Hidalgo, J.; Molgó, J.; Jaimovich, E. Sodium-dependent action potentials induced by brevetoxin-3 trigger both IP3 increase and intracellular Ca2+ release in rat skeletal myotubes. Cell Calcium 2008, 44, 289–297. [Google Scholar] [CrossRef]
- Heller, S.; O’Neil, R.G. Molecular Mechanisms of TRPV4 Gating. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Frontiers in Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 113–125. ISBN 978-0-8493-4048-2. [Google Scholar]
- Dunn, K.M.; Hill-Eubanks, D.C.; Liedtke, W.B.; Nelson, M.T. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc. Natl. Acad. Sci. USA 2013, 110, 6157–6162. [Google Scholar] [CrossRef] [Green Version]
- Dahan, D.; Ducret, T.; Quignard, J.-F.; Marthan, R.; Savineau, J.-P.; Estève, E. Implication of the ryanodine receptor in TRPV4-induced calcium response in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L824–L833. [Google Scholar] [CrossRef] [Green Version]
- Boehmerle, W.; Huehnchen, P.; Lee, S.L.L.; Harms, C.; Endres, M. TRPV4 inhibition prevents paclitaxel-induced neurotoxicity in preclinical models. Exp. Neurol. 2018, 306, 64–75. [Google Scholar] [CrossRef]
- Lundberg, J.M.; Martling, C.-R.; Saria, A. Substance P and capsaicin-induced contraction of human bronchi. Acta Physiol. Scand. 1983, 119, 49–53. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baraniuk, J.N.; Belvisi, M.G. Neuropeptides in the Respiratory Tract: Part, I. Am. Rev. Respir. Dis. 1991, 144, 1187–1198. [Google Scholar] [CrossRef]
- Su, X.; Camerer, E.; Hamilton, J.R.; Coughlin, S.R.; Matthay, M.A. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J. Immunol. 2005, 175, 2598–2605. [Google Scholar] [CrossRef] [Green Version]
- Ollerenshaw, S.L.; Jarvist, D.; Sullivan, C.E.; Woolcock, A.J. Substance P immunoreactive nerves in airways from asthmatics and nonasthmatics. Eur. Respir. J. 1991, 4, 673–682. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierre, O.; Fouchard, M.; Buscaglia, P.; Le Goux, N.; Leschiera, R.; Mignen, O.; Fluhr, J.W.; Misery, L.; Le Garrec, R. Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling. Cells 2020, 9, 2704. https://doi.org/10.3390/cells9122704
Pierre O, Fouchard M, Buscaglia P, Le Goux N, Leschiera R, Mignen O, Fluhr JW, Misery L, Le Garrec R. Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling. Cells. 2020; 9(12):2704. https://doi.org/10.3390/cells9122704
Chicago/Turabian StylePierre, Ophélie, Maxime Fouchard, Paul Buscaglia, Nelig Le Goux, Raphaël Leschiera, Olivier Mignen, Joachim W. Fluhr, Laurent Misery, and Raphaële Le Garrec. 2020. "Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling" Cells 9, no. 12: 2704. https://doi.org/10.3390/cells9122704
APA StylePierre, O., Fouchard, M., Buscaglia, P., Le Goux, N., Leschiera, R., Mignen, O., Fluhr, J. W., Misery, L., & Le Garrec, R. (2020). Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling. Cells, 9(12), 2704. https://doi.org/10.3390/cells9122704







