Regulation of the Extracellular Matrix by Ciliary Machinery
Abstract
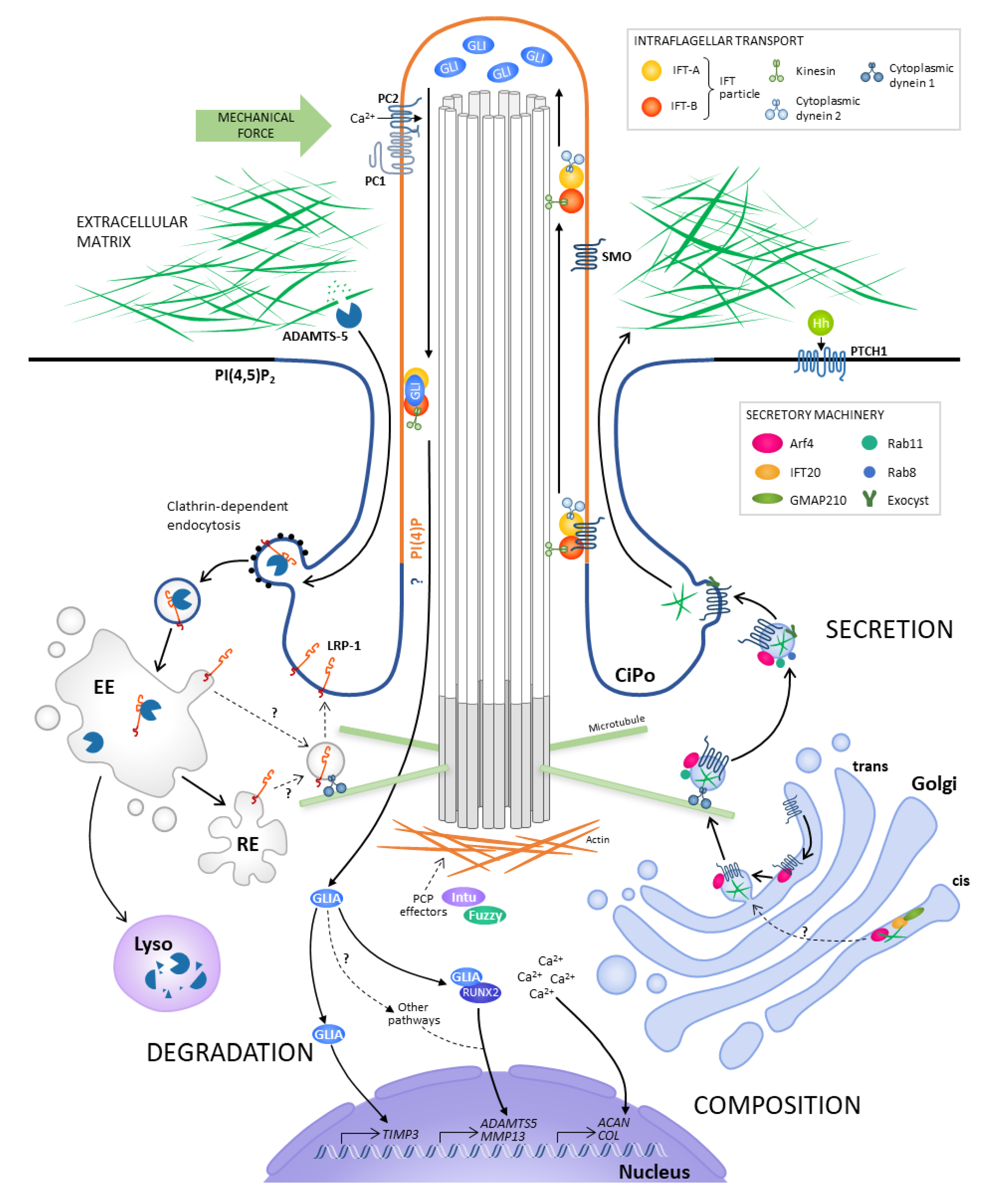
:1. Introduction
2. Regulation of Matrix Composition
2.1. Ciliary Signalling Regulates Matrix Phenotype
2.2. Mechanical Forces Regulate Matrix Composition through Ciliary Signalling
3. Regulation of Matrix Secretion
3.1. Primary Cilia Are Proposed to Act in a Matrix-Cilium-Golgi Continuum
3.2. IFT20 Links the Golgi to the Cilium
3.3. Polarisation of Secretion Occurs at the Immune Synapse
4. Regulation of Matrix Degradation
4.1. Ciliary Signalling has been Linked to Transcriptional Control of Protease Expression
4.2. Primary Cilia have been Associated with Endocytic Control of Protease Activity
5. Regulation of Matrix Organisation
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wheatley, D.N. Primary cilia in normal and pathological tissues. Pathobiology 1995, 63, 222–238. [Google Scholar] [CrossRef]
- Kobayashi, T.; Dynlacht, B.D. Regulating the transition from centriole to basal body. J. Cell Biol. 2011, 193, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Mick, D.U.; Rodrigues, R.B.; Leib, R.D.; Adams, C.M.; Chien, A.S.; Gygi, S.P.; Nachury, M.V. Proteomics of Primary Cilia by Proximity Labeling. Dev. Cell 2015, 35, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, J.L.; Beales, P.L. The nonmotile ciliopathies. Genet. Med. 2009, 11, 386–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvet, J.P. Polycystic kidney disease: Primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int. 1993, 43, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Seeger-Nukpezah, T.; Golemis, E.A. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012, 24, 652–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huangfu, D.; Liu, A.; Rakeman, A.S.; Murcia, N.S.; Niswander, L.; Anderson, K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003, 426, 83–87. [Google Scholar] [CrossRef]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.R.; Reiter, J.F. Vertebrate Smoothened functions at the primary cilium. Nature 2005, 437, 1018–1021. [Google Scholar] [CrossRef]
- Rohatgi, R.; Milenkovic, L.; Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007, 317, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Bangs, F.; Anderson, K.V. Primary cilia and Mammalian Hedgehog signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028175. [Google Scholar] [CrossRef] [PubMed]
- Riddle, R.D.; Johnson, R.L.; Laufer, E.; Tabin, C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 1993, 75, 1401–1416. [Google Scholar] [CrossRef]
- Vortkamp, A.; Lee, K.; Lanske, B.; Segre, G.V.; Kronenberg, H.M.; Tabin, C.J. Regulation of rate of cartilage differentiation by Indian Hedgehog and PTH-related protein. Science 1996, 273, 613–622. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Soegiarto, D.W.; Yang, Y.; Lanske, B.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Investig. 2005, 115, 1734–1742. [Google Scholar] [CrossRef]
- Rosenbaum, J.L.; Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002, 3, 813–825. [Google Scholar] [CrossRef]
- Song, B.; Haycraft, C.J.; Seo, H.S.; Yoder, B.K.; Serra, R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 2007, 305, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Yang, S. Deletion of IFT80 impairs epiphyseal and articular cartilage formation due to disruption of chondrocyte differentiation. PLoS ONE 2015, 10, e0130618. [Google Scholar] [CrossRef] [Green Version]
- McGlashan, S.R.; Haycraft, C.J.; Jensen, C.G.; Yoder, B.K.; Poole, C.A. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpkmice lacking the primary cilia protein polaris. Matrix Biol. 2007, 26, 234–246. [Google Scholar] [CrossRef]
- Ali, S.A.; Niu, B.; Cheah, K.S.E.; Alman, B. Unique and overlapping GLI1 and GLI2 transcriptional targets in neoplastic chondrocytes. PLoS ONE 2019, 14, e0211333. [Google Scholar] [CrossRef] [Green Version]
- Haycraft, C.J.; Zhang, Q.; Song, B.; Jackson, W.S.; Detloff, P.J.; Serra, R.; Yoder, B.K. Intraflagellar transport is essential for endochondral bone formation. Development 2007, 134, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.F.; Serra, R. Ift88 regulates Hedgehog signaling, Sfrp5 expression, and β-catenin activity in post-natal growth plate. J. Orthop. Res. 2013, 31, 350–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, C.L.; Chapple, J.P.; Knight, M.M. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: A feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthr. Cartil. 2014, 22, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Analysis of the morphology and function of primary cilia in connective tissues:A cellular cybernetic probe? Cell Motil. 1985, 5, 175–193. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Spring, K.R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001, 184, 71–79. [Google Scholar] [CrossRef]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.H.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Liu, W.; Murcia, N.S.; Duan, Y.; Weinbaum, S.; Yoder, B.K.; Schwiebert, E.; Satlin, L.M. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am. J. Physiol. Ren. Physiol. 2005, 289, 978–988. [Google Scholar] [CrossRef] [Green Version]
- Lehman, J.M.; Michaud, E.J.; Schoeb, T.R.; Aydin-Son, Y.; Miller, M.; Yoder, B.K. The Oak Ridge Polycystic Kidney mouse: Modeling ciliopathies of mice and men. Dev. Dyn. 2008, 237, 1960–1971. [Google Scholar] [CrossRef] [Green Version]
- The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 1994, 77, 881–894. [Google Scholar] [CrossRef] [Green Version]
- Mangos, S.; Lam, P.; Zhao, A.; Liu, Y.; Mudumana, S.; Vasilyev, A.; Liu, A.; Drummond, I.A. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis. Model. Mech. 2010, 123, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.Y.; Parker, E.; Ibrahim, S.; Shortland, J.R.; El Nahas, M.; Haylor, J.L.; Ong, A.C.M. Haploinsufficiency of Pkd2 is associated with increased tubular cell proliferation and interstitial fibrosis in two murine Pkd2 models. Nephrol. Dial. Transplant. 2006, 21, 2078–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Happé, H.; Leonhard, W.N.; van der Wal, A.; van de Water, B.; Lantinga-van Leeuwen, I.S.; Breuning, M.H.; de Heer, E.; Peters, D.J.M. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum. Mol. Genet. 2009, 18, 2532–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, J. Fibrosis and progression of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1327–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wann, A.K.T.; Zuo, N.; Haycraft, C.J.; Jensen, C.G.; Poole, C.A.; McGlashan, S.R.; Knight, M.M. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012, 26, 1663–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovater, M.B.; Olteanu, D.; Hanson, E.L.; Cheng, N.L.; Siroky, B.; Fintha, A.; Komlosi, P.; Liu, W.; Satlin, L.M.; Bell, P.D.; et al. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal. 2008, 4, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, T.T.; Knight, M.M. Purinergic pathway suppresses the release of NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J. Cell. Physiol. 2006, 209, 845–853. [Google Scholar] [CrossRef]
- Hooper, K.M.; Boletta, A.; Germino, G.G.; Hu, Q.; Ziegelstein, R.C.; Sutters, M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am. J. Physiol. Ren. Physiol. 2005, 289, F521–F530. [Google Scholar] [CrossRef] [Green Version]
- Rais, Y.; Reich, A.; Simsa-Maziel, S.; Moshe, M.; Idelevich, A.; Kfir, T.; Miosge, N.; Monsonego-Ornan, E. The growth plate’s response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell. Mol. Life Sci. 2015, 72, 597–615. [Google Scholar] [CrossRef]
- Toomer, K.A.; Fulmer, D.; Guo, L.; Drohan, A.; Peterson, N.; Swanson, P.; Brooks, B.; Mukherjee, R.; Body, S.; Lipschutz, J.H.; et al. A role for primary cilia in aortic valve development and disease. Dev. Dyn. 2017, 246, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Villalobos, E.; Criollo, A.; Schiattarella, G.G.; Altamirano, F.; French, K.M.; May, H.I.; Jiang, N.; Nguyen, N.U.N.; Romero, D.; Roa, J.C.; et al. Fibroblast Primary Cilia Are Required for Cardiac Fibrosis. Circulation 2019, 139, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Palumbo, K.; Cordazzo, C.; Dees, C.; Akhmetshina, A.; Tomcik, M.; Zerr, P.; Avouac, J.; Gusinde, J.; Zwerina, J.; et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012, 64, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.E.; Kaminski, N.; Thienpont, B.; Hogg, J.C.; Vanaudenaerde, B.M.; Wuyts, W.A. Gene correlation network analysis to identify regulatory factors in idiopathic pulmonary fibrosis. Thorax 2019, 74, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Klena, N.T.; Gabriel, G.C.; Liu, X.; Kim, A.J.; Lemke, K.; Chen, Y.; Chatterjee, B.; Devine, W.; Damerla, R.R.; et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 2015, 521, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, A.D.; van der Heiden, K.; Poelmann, R.E.; Hierck, B.P. Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation 2012, 83, S56–S61. [Google Scholar] [CrossRef]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Van der Heiden, K.; Hierck, B.P.; Krams, R.; de Crom, R.; Cheng, C.; Baiker, M.; Pourquie, M.J.B.M.; Alkemade, F.E.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 2008, 196, 542–550. [Google Scholar] [CrossRef]
- Dinsmore, C.; Reiter, J.F. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 2016, 17, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.A.; Jensen, C.G.; Snyder, J.A.; Gray, C.G.; Hermanutz, V.L.; Wheatley, D.N. Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol. Int. 1997, 21, 483–494. [Google Scholar] [CrossRef]
- Jensen, C.G.; Poole, C.A.; McGlashan, S.R.; Marko, M.; Issa, Z.I.; Vujcich, K.V.; Bowser, S.S. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol. Int. 2004, 28, 101–110. [Google Scholar] [CrossRef]
- McGlashan, S.R.; Jensen, C.G.; Poole, C.A. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 2006, 54, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The Intraflagellar Transport Protein IFT20 Is Associated with the Golgi Complex and Is Required for Cilia Assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonassen, J.A.; Agustin, J.S.; Follit, J.A.; Pazour, G.J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 2008, 183, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Follit, J.A.; San Agustin, J.T.; Xu, F.; Jonassen, J.A.; Samtani, R.; Lo, C.W.; Pazour, G.J. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. Plos Genet. 2008, 4, e1000315. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.R.; Reiter, J.F. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J. Cell Biol. 2012, 197, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knodler, A.; Feng, S.; Zhang, J.; Zhang, X.; Das, A.; Peranen, J.; Guo, W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. 2010, 107, 6346–6351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernabé-Rubio, M.; Alonso, M.A. Routes and machinery of primary cilium biogenesis. Cell. Mol. Life Sci. 2017, 74, 4077–4095. [Google Scholar] [CrossRef]
- Zuo, X.; Guo, W.; Lipschutz, J.H. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell 2009, 20, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Polgar, N.; Lee, A.J.; Lui, V.H.; Napoli, J.A.; Fogelgren, B. The exocyst gene sec10 regulates renal epithelial monolayer homeostasis and apoptotic sensitivity. Am. J. Physiol. Cell Physiol. 2015, 309, C190–C201. [Google Scholar] [CrossRef] [Green Version]
- Fogelgren, B.; Lin, S.Y.; Zuo, X.; Jaffe, K.M.; Park, K.M.; Reichert, R.J.; Bell, P.D.; Burdine, R.D.; Lipschutz, J.H. The exocyst protein sec10 interacts with polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet. 2011, 7, e1001361. [Google Scholar] [CrossRef] [Green Version]
- Keady, B.T.; Samtani, R.; Tobita, K.; Tsuchya, M.; San Agustin, J.T.; Follit, J.A.; Jonassen, J.A.; Subramanian, R.; Lo, C.W.; Pazour, G.J. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev. Cell 2012, 22, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Noda, K.; Kitami, M.; Kitami, K.; Kaku, M.; Komatsu, Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc. Natl. Acad. Sci. USA 2016, 113, E2589–E2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, P.; Bolton, A.D.; Funari, V.; Hong, M.; Boyden, E.D.; Lu, L.; Manning, D.K.; Dwyer, N.D.; Moran, J.L.; Prysak, M.; et al. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N. Engl. J. Med. 2010, 362, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitami, M.; Yamaguchi, H.; Ebina, M.; Kaku, M.; Chen, D.; Komatsu, Y. IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage. Biochem. Biophys. Res. Commun. 2019, 509, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, A.; Witkos, T.M.; Unger, S.; Schneider, J.; Follit, J.A.; Hermann, J.; Welting, T.; Fano, V.; Hietala, M.; Vatanavicharn, N.; et al. Hypomorphic mutations of TRIP11 cause odontochondrodysplasia. Jci Insight 2019, 4, e124701. [Google Scholar] [CrossRef]
- Stinchcombe, J.C.; Randzavola, L.O.; Angus, K.L.; Mantell, J.M.; Verkade, P.; Griffiths, G.M. Mother Centriole Distal Appendages Mediate Centrosome Docking at the Immunological Synapse and Reveal Mechanistic Parallels with Ciliogenesis. Curr. Biol. 2015, 25, 3239–3244. [Google Scholar] [CrossRef] [Green Version]
- Tanos, B.E.; Yang, H.J.; Soni, R.; Wang, W.J.; Macaluso, F.P.; Asara, J.M.; Tsou, M.F.B. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013, 27, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Gawden-Bone, C.M.; Frazer, G.L.; Richard, A.C.; Ma, C.Y.; Strege, K.; Griffiths, G.M. PIP5 Kinases Regulate Membrane Phosphoinositide and Actin Composition for Targeted Granule Secretion by Cytotoxic Lymphocytes. Immunity 2018, 49, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Chavez, M.; Ena, S.; Van Sande, J.; de Kerchove d’Exaerde, A.; Schurmans, S.; Schiffman, S.N. Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Dev. Cell 2015, 34, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gonzalo, F.R.; Phua, S.C.; Roberson, E.C.; Garcia, G.; Abedin, M.; Schurmans, S.; Inoue, T.; Reiter, J.F. Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev. Cell 2015, 34, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Das, V.; Nal, B.; Dujeancourt, A.; Thoulouze, M.I.; Galli, T.; Roux, P.; Dautry-Varsat, A.; Alcover, A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse: Involvement of SNARE complexes. Immunity 2004, 20, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Finetti, F.; Paccani, S.R.; Riparbelli, M.G.; Giacomello, E.; Perinetti, G.; Pazour, G.J.; Rosenbaum, J.L.; Baldari, C.T. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009, 11, 1332–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finetti, F.; Patrussi, L.; Masi, G.; Onnis, A.; Galgano, D.; Lucherini, O.M.; Pazour, G.J.; Baldari, C.T. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. J. Cell Sci. 2014, 127, 1924–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onnis, A.; Finetti, F.; Patrussi, L.; Gottardo, M.; Cassioli, C.; Spanò, S.; Baldari, C.T. The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ. 2015, 22, 1687–1699. [Google Scholar] [CrossRef] [Green Version]
- Stoetzel, C.; Bär, S.; De Craene, J.O.; Scheidecker, S.; Etard, C.; Chicher, J.; Reck, J.R.; Perrault, I.; Geoffroy, V.; Chennen, K.; et al. A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat. Commun. 2016, 7, 13586. [Google Scholar] [CrossRef] [PubMed]
- Kösling, S.K.; Fansa, E.K.; Maffini, S.; Wittinghofer, A. Mechanism and dynamics of INPP5E transport into and inside the ciliary compartment. Biol. Chem. 2018, 399, 277–292. [Google Scholar] [CrossRef]
- Van der Kraan, P.M.; Van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [Green Version]
- McGlashan, S.R.; Cluett, E.C.; Jensen, C.G.; Poole, C.A. Primary Cilia in osteoarthritic chondrocytes: From chondrons to clusters. Dev. Dyn. 2008, 237, 2013–2020. [Google Scholar] [CrossRef]
- Lin, A.C.; Seeto, B.L.; Bartoszko, J.M.; Khoury, M.A.; Whetstone, H.; Ho, L.; Hsu, C.; Ali, A.S.; Alman, B.A. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat. Med. 2009, 15, 1421–1425. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Q.; Lanske, B.; Fleming, B.C.; Terek, R.; Wei, X.; Zhang, G.; Wang, S.; Li, K.; Wei, L. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res. Ther. 2014, 16, R11. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Thirunavukkarasu, K.; Pei, Y.; Wei, T. Characterization of the human ADAMTS-5 (aggrecanase-2) gene promoter. Mol. Biol. Rep. 2007, 34, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhou, J.; Wei, X.; Zhang, J.; Fleming, B.C.; Terek, R.; Pei, M.; Chen, Q.; Liu, T.; Wei, L. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthr. Cartil. 2012, 20, 755–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, A.P.; Martin, J.A.; Zhang, Q.; Sheffield, V.C.; Morcuende, J.A. Cartilage abnormalities associated with defects of chondrocytic primary cilia in Bardet-Biedl syndrome mutant mice. J. Orthop. Res. 2009, 27, 1093–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffield, I.D.; McGee, M.A.; Glenn, S.J.; Baek, D.Y.; Coleman, J.M.; Dorius, B.K.; Williams, C.; Rose, B.J.; Sanchez, A.E.; Goodman, M.A.; et al. Osteoarthritis-like changes in Bardet-Biedl syndrome mutant ciliopathy mice (Bbs1M390R/M390R): Evidence for a role of Primary Cilia in cartilage homeostasis and regulation of inflammation. Front. Physiol. 2018, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Ramaswamy, G.; Serra, R. Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthr. Cartil. 2012, 20, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, R.T.; Chavally-Mis, B.; Herbage, D.; Eastwood, M.; Brown, R.A. Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen. 2000, 8, 226–237. [Google Scholar] [CrossRef]
- Wu, Q.Q.; Zhang, Y.; Chen, Q. Indian hedgehog Is an Essential Component of Mechanotransduction Complex to Stimulate Chondrocyte Proliferation. J. Biol. Chem. 2001, 276, 35290–35296. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, A.; Wada, M.; Ikeda, F.; Hata, K.; Matsubara, T.; Nifuji, A.; Noda, M.; Amano, K.; Yamaguchi, A.; Nishimura, R.; et al. Ihh/Gli2 signaling promotes osteoblast differentiation by regulating Runx2 expression and function. Mol. Biol. Cell 2007, 18, 2411–2418. [Google Scholar] [CrossRef] [Green Version]
- Tetsunaga, T.; Nishida, K.; Furumatsu, T.; Naruse, K.; Hirohata, S.; Yoshida, A.; Saito, T.; Ozaki, T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthr. Cartil. 2011, 19, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Kamekura, S.; Kawasaki, Y.; Hoshi, K.; Shimoaka, T.; Chikuda, H.; Maruyama, Z.; Komori, T.; Sato, S.; Takeda, S.; Karsenty, G.; et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006, 54, 2462–2470. [Google Scholar] [CrossRef]
- Thompson, C.L.; Patel, R.; Kelly, T.A.N.; Wann, A.K.T.; Hung, C.T.; Chapple, J.P.; Knight, M.M. Hedgehog signalling does not stimulate cartilage catabolism and is inhibited by Interleukin-1β. Arthritis Res. Ther. 2015, 17, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlashan, S.R.; Knight, M.M.; Chowdhury, T.T.; Joshi, P.; Jensen, C.G.; Kennedy, S.; Poole, C.A. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol. Int. 2010, 34, 441–446. [Google Scholar] [CrossRef]
- Denoble, A.E.; Huffman, K.M.; Stabler, T.V.; Kelly, S.J.; Hershfield, M.S.; McDaniel, G.E.; Coleman, R.E.; Kraus, V.B. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. USA 2011, 108, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.M.; Yamamoto, K.; Vincent, T.L.; Nagase, H.; Troeberg, L.; Saklatvala, J. Interleukin-1 acts via the JNK-2 signaling pathway to induce aggrecan degradation by human chondrocytes. Arthritis Rheumatol. 2015, 67, 1826–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockel, J.S.; Yu, C.; Whetstone, H.; Craft, A.M.; Reilly, K.; Ma, H.; Tsushima, H.; Puviindran, V.; Al-Jazrawe, M.; Keller, G.M.; et al. Hedgehog inhibits β-catenin activity in synovial joint development and osteoarthritis. J. Clin. Investig. 2016, 126, 1649–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.A.; Al-Jazrawe, M.; Ma, H.; Whetstone, H.; Poon, R.; Farr, S.; Naples, M.; Adeli, K.; Alman, B.A. Regulation of Cholesterol Homeostasis by Hedgehog Signaling in Osteoarthritic Cartilage. Arthritis Rheumatol. 2016, 68, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, T.H.; Hotary, K.B.; Sabeh, F.; Saltiel, A.R.; Allen, E.D.; Weiss, S.J. A Pericellular Collagenase Directs the 3-Dimensional Development of White Adipose Tissue. Cell 2006, 125, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopinke, D.; Roberson, E.C.; Reiter, J.F. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell 2017, 170, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Gendron, C.; Kashiwagi, M.; Hughes, C.; Caterson, B.; Nagase, H. TIMP-3 inhibits aggrecanase-mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. Febs Lett. 2003, 555, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Sahebjam, S.; Khokha, R.; Mort, J.S. Increased collagen and aggrecan degradation with age in the joints of Timp3-/- mice. Arthritis Rheum. 2007, 56, 905–909. [Google Scholar] [CrossRef]
- Morris, K.J.; Cs-Szabo, G.; Cole, A.A. Characterization of TIMP-3 in human articular talar cartilage. Connect. Tissue Res. 2010, 51, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Gendron, C.; Kashiwagi, M.; Ngee, H.L.; Enghild, J.J.; Thøgersen, I.B.; Hughes, C.; Caterson, B.; Nagase, H. Proteolytic activities of human ADAMTS-5: Comparative studies with ADAMTS-4. J. Biol. Chem. 2007, 282, 18294–18306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kevorkian, L.; Young, D.A.; Darrah, C.; Donell, S.T.; Shepstone, L.; Porter, S.; Brockbank, S.M.V.; Edwards, D.R.; Parker, A.E.; Clark, I.M. Expression Profiling of Metalloproteinases and Their Inhibitors in Cartilage. Arthritis Rheum. 2004, 50, 131–141. [Google Scholar] [CrossRef]
- Naito, S.; Shiomi, T.; Okada, A.; Kimura, T.; Chijiiwa, M.; Fujita, Y.; Yatabe, T.; Komiya, K.; Enomoto, H.; Fujikawa, K.; et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol. Int. 2007, 57, 703–711. [Google Scholar] [CrossRef]
- Yamamoto, K.; Troeberg, L.; Scilabra, S.D.; Pelosi, M.; Murphy, C.L.; Strickland, D.K.; Nagase, H. LRP-1-mediated endocytosis regulates extracellular activity of ADAMTS-5 in articular cartilage. FASEB J. 2013, 27, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Santamaria, S.; Botkjaer, K.A.; Dudhia, J.; Troeberg, L.; Itoh, Y.; Murphy, G.; Nagase, H. Inhibition of Shedding of Low-Density Lipoprotein Receptor–Related Protein 1 Reverses Cartilage Matrix Degradation in Osteoarthritis. Arthritis Rheumatol. 2017, 69, 1246–1256. [Google Scholar] [CrossRef] [Green Version]
- Coveney, C.R.; Collins, I.; Mc Fie, M.; Chanalaris, A.; Yamamoto, K.; Wann, A.K.T. Cilia protein IFT88 regulates extracellular protease activity by optimizing LRP-1–mediated endocytosis. FASEB J. 2018, fj.201800334. [Google Scholar]
- Nandadasa, S.; Kraft, C.M.; Wang, L.W.; O’Donnell, A.; Patel, R.; Gee, H.Y.; Grobe, K.; Cox, T.C.; Hildebrandt, F.; Apte, S.S. Secreted metalloproteases ADAMTS9 and ADAMTS20 have a non-canonical role in ciliary vesicle growth during ciliogenesis. Nat. Commun. 2019, 10, 953. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Insinna, C.; Ott, C.; Stauffer, J.; Pintado, P.A.; Rahajeng, J.; Baxa, U.; Walia, V.; Cuenca, A.; Hwang, Y.S.; et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat. Cell Biol. 2015, 17, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Rainey, M.A.; Arya, P.; Dutta, S.; George, M.; Storck, M.D.; McComb, R.D.; Muirhead, D.; Todd, G.L.; Gould, K.; et al. Endocytic recycling protein EHD1 regulates primary cilia morphogenesis and SHH signaling during neural tube development. Sci. Rep. 2016, 6, 20727. [Google Scholar] [CrossRef]
- Field, M.C.; Carrington, M. The trypanosome flagellar pocket. Nat. Rev. Microbiol. 2009, 7, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Molla-Herman, A.; Ghossoub, R.; Blisnick, T.; Meunier, A.; Serres, C.; Silbermann, F.; Emmerson, C.; Romeo, K.; Bourdoncle, P.; Schmitt, A.; et al. The ciliary pocket: An endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 2010, 123, 1785–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schou, K.B.; Mogensen, J.B.; Morthorst, S.K.; Nielsen, B.S.; Aleliunaite, A.; Serra-Marques, A.; Fürstenberg, N.; Saunier, S.; Bizet, A.A.; Veland, I.R.; et al. KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nat. Commun. 2017, 8, 14177. [Google Scholar] [CrossRef] [Green Version]
- Clement, C.A.; Ajbro, K.D.; Koefoed, K.; Vestergaard, M.L.; Veland, I.R.; Henriques de Jesus, M.P.R.; Pedersen, L.B.; Benmerah, A.; Andersen, C.Y.; Larsen, L.A.; et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013, 3, 1806–1814. [Google Scholar] [CrossRef] [Green Version]
- Kanai, Y.; Wang, D.; Hirokawa, N. KIF13B enhances the endocytosis of LRP1 by recruiting LRP1 to caveolae. J. Cell Biol. 2014, 204, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Demmel, L.; Schmidt, K.; Lucast, L.; Havlicek, K.; Zankel, A.; Koestler, T.; Reithofer, V.; de Camilli, P.; Warren, G. The endocytic activity of the flagellar pocket in Trypanosoma brucei is regulated by an adjacent phosphatidylinositol phosphate kinase. J. Cell Sci. 2014, 127, 2351–2364. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, V.; Rbaibi, Y.; Pastor-Soler, N.M.; Carattino, M.D.; Weisz, O.A. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc. Natl. Acad. Sci. USA 2014, 111, 8506–8511. [Google Scholar] [CrossRef] [Green Version]
- Galletta, B.J.; Cooper, J.A. Actin and endocytosis: Mechanisms and phylogeny. Curr. Opin. Cell Biol. 2009, 21, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.M. Variation of collagen fiber alignment in a joint surface: A scanning electron microscope study of the tibial plateau in dog, rabbit, and man. J. Orthop. Res. 1991, 9, 246–257. [Google Scholar] [CrossRef]
- Benninghoff, A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion - Zweiter Teil: Der Aufbau des Gelenkknorpels in seinen Beziehungen zur Funktion. Z. Für Zellforsch. Und Mikrosk. Anat. 1925, 2, 783–862. [Google Scholar]
- Korhonen, R.K.; Wong, M.; Arokoski, J.; Lindgren, R.; Helminen, H.J.; Hunziker, E.B.; Jurvelin, J.S. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med. Eng. Phys. 2002, 24, 99–108. [Google Scholar] [CrossRef]
- Shirazi, R.; Shirazi-Adl, A.; Hurtig, M. Role of cartilage collagen fibrils networks in knee joint biomechanics under compression. J. Biomech. 2008, 41, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Notzli, H.; Clark, J. Deformation of loaded articular cartilage prepared for scanning electron microscopy with rapid freezing and freeze-substitution fixation. J. Orthop. Res. 1997, 15, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Rieppo, J.; Hyttinen, M.M.; Halmesmaki, E.; Ruotsalainen, H.; Vasara, A.; Kiviranta, I.; Jurvelin, J.S.; Helminen, H.J. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthr. Cartil. 2009, 17, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julkunen, P.; Iivarinen, J.; Brama, P.A.; Arokoski, J.; Jurvelin, J.S.; Helminen, H.J. Maturation of collagen fibril network structure in tibial and femoral cartilage of rabbits. Osteoarthr. Cartil. 2010, 18, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Van Turnhout, M.C.; Schipper, H.; Engel, B.; Buist, W.; Kranenbarg, S.; Van Leeuwen, J.L. Postnatal development of collagen structure in ovine articular cartilage. BMC Dev. Biol. 2010, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnum, C.E.; Wilsman, N.J. Orientation of Primary Cilia of Articular Chondrocytes in Three-Dimensional Space. Anat. Rec. 2011, 294, 533–549. [Google Scholar] [CrossRef]
- De Andrea, C.E.; Wiweger, M.; Prins, F.; Bovée, J.V.M.G.; Romeo, S.; Hogendoorn, P.C.W. Primary cilia organization reflects polarity in the growth plate and implies loss of polarity and mosaicism in osteochondroma. Lab. Investig. 2010, 90, 1091–1101. [Google Scholar] [CrossRef] [Green Version]
- Ascenzi, M.G.; Blanco, C.; Drayer, I.; Kim, H.; Wilson, R.; Retting, K.N.; Lyons, K.M.; Mohler, G. Effect of localization, length and orientation of chondrocytic primary cilium on murine growth plate organization. J. Theor. Biol. 2011, 285, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Delaval, B.; Bright, A.; Lawson, N.D.; Doxsey, S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 2011, 13, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.; Roper, V.C.; Foucher, I.; Qian, D.; Banizs, B.; Petit, C.; Yoder, B.K.; Chen, P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 2008, 40, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Canty, E.G.; Lu, Y.; Meadows, R.S.; Shaw, M.K.; Holmes, D.F.; Kadler, K.E. Coalignment of plasma membrane channels and protrusions (fibropositors) specifies the parallelism of tendon. J. Cell Biol. 2004, 165, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.H.; Starborg, T.; Lu, Y.; Humphries, S.M.; Meadows, R.S.; Kadler, K.E. Tendon Development Requires Regulation of Cell Condensation and Cell Shape via Cadherin-11-Mediated Cell-Cell Junctions. Mol. Cell. Biol. 2007, 27, 6218–6228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canty, E.G.; Starborg, T.; Lu, Y.; Humphries, S.M.; Holmes, D.F.; Meadows, R.S.; Huffman, A.; O’Toole, E.T.; Kadler, K.E. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J. Biol. Chem. 2006, 281, 38592–38598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapacee, Z.; Richardson, S.H.; Lu, Y.; Starborg, T.; Holmes, D.F.; Baar, K.; Kadler, K.E. Tension is required for fibripositor formation. Matrix Biol. 2008, 27, 371–375. [Google Scholar] [CrossRef]
- Kalson, N.S.; Starborg, T.; Lu, Y.; Mironov, A.; Humphries, S.M.; Holmes, D.F.; Kadler, K.E. Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane. Proc. Natl. Acad. Sci. USA 2013, 110, E4743–E4752. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, E.; Ascenzi, M.G.; Farnum, C. Primary cilia are highly oriented with respect to collagen direction and long axis of extensor tendon. J. Orthop. Res. 2010, 28, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Rowson, D.; Knight, M.M.; Screen, H.R.C. Zonal variation in primary cilia elongation correlates with localized biomechanical degradation in stress deprived tendon. J. Orthop. Res. 2016, 34, 2146–2153. [Google Scholar] [CrossRef] [Green Version]
- Rowson, D.T.; Shelton, J.C.; Screen, H.R.C.; Knight, M.M. Mechanical loading induces primary cilia disassembly in tendon cells via TGFβ and HDAC6. Sci. Rep. 2018, 8, 11107. [Google Scholar] [CrossRef]
- Butler, M.T.; Wallingford, J.B. Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 375–388. [Google Scholar] [CrossRef]
- Park, T.J.; Haigo, S.L.; Wallingford, J.B. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 2006, 38, 303–311. [Google Scholar] [CrossRef]
- Wallingford, J.B.; Mitchell, B. Strange as it may seem: The many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011, 25, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Goodyear, R.J.; Lu, X.; Deans, M.R.; Richardson, G.P. A tectorin-based matrix and planar cell polarity genes are required for normal collagen-fibril orientation in the developing tectorial membrane. Dev. 2017, 144, 3978–3989. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
I, C.; A.K.T, W. Regulation of the Extracellular Matrix by Ciliary Machinery. Cells 2020, 9, 278. https://doi.org/10.3390/cells9020278
I C, A.K.T W. Regulation of the Extracellular Matrix by Ciliary Machinery. Cells. 2020; 9(2):278. https://doi.org/10.3390/cells9020278
Chicago/Turabian StyleI, Collins, and Wann A.K.T. 2020. "Regulation of the Extracellular Matrix by Ciliary Machinery" Cells 9, no. 2: 278. https://doi.org/10.3390/cells9020278
APA StyleI, C., & A.K.T, W. (2020). Regulation of the Extracellular Matrix by Ciliary Machinery. Cells, 9(2), 278. https://doi.org/10.3390/cells9020278




