Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity
Abstract
1. Introduction
2. Classification of Animals According to Body Temperature and Their Adaptation to Ambient Temperature
3. The Use of Experimental Mice as a Model in Human Research
3.1. Relationship Between Body Size and Physiological Temperature
3.2. Thermal Physiology and Thermoneutrality Zone
3.3. Thermal Variations in the Housing of Experimental Mice
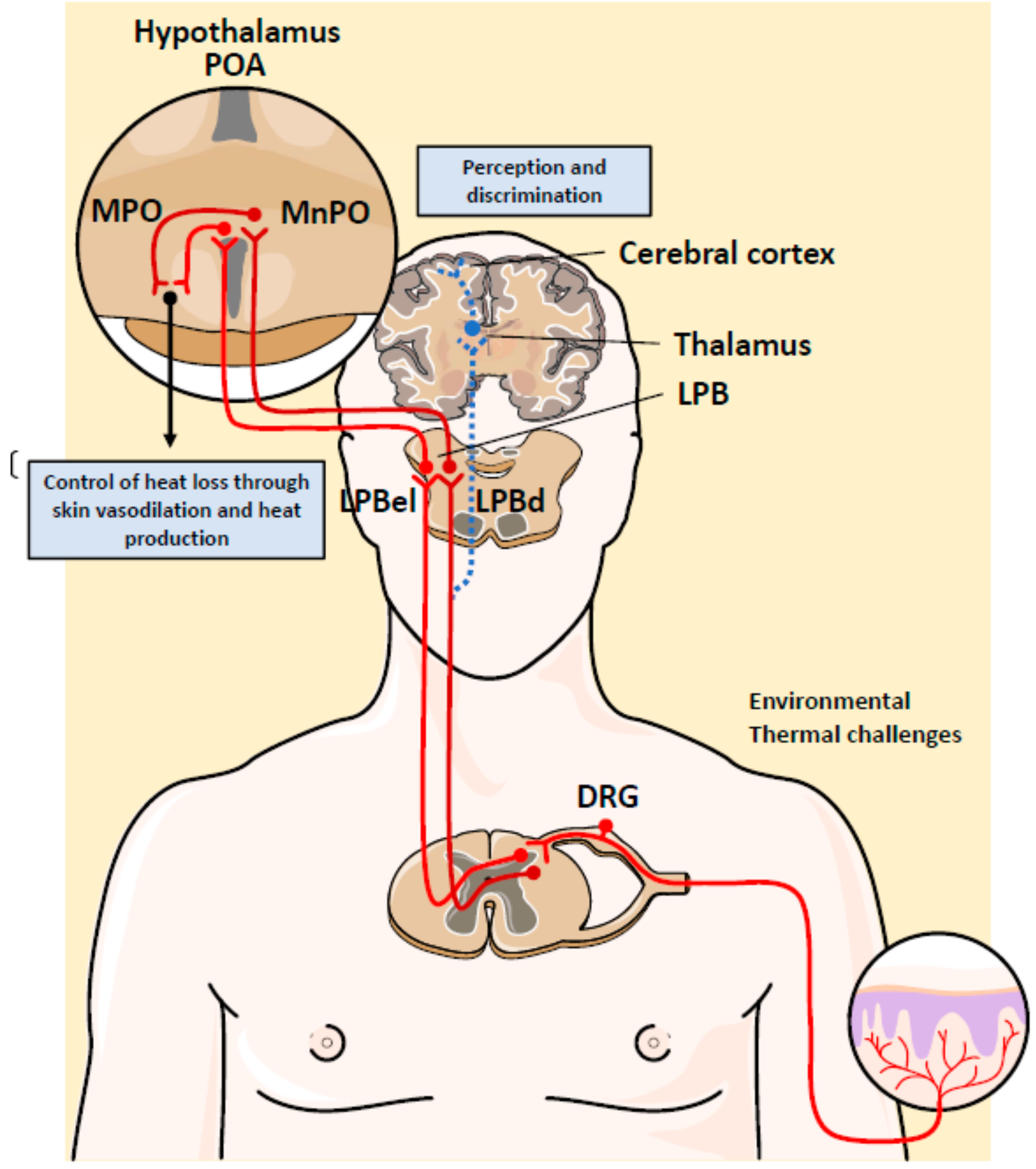
4. Neuronal Control of Body Temperature
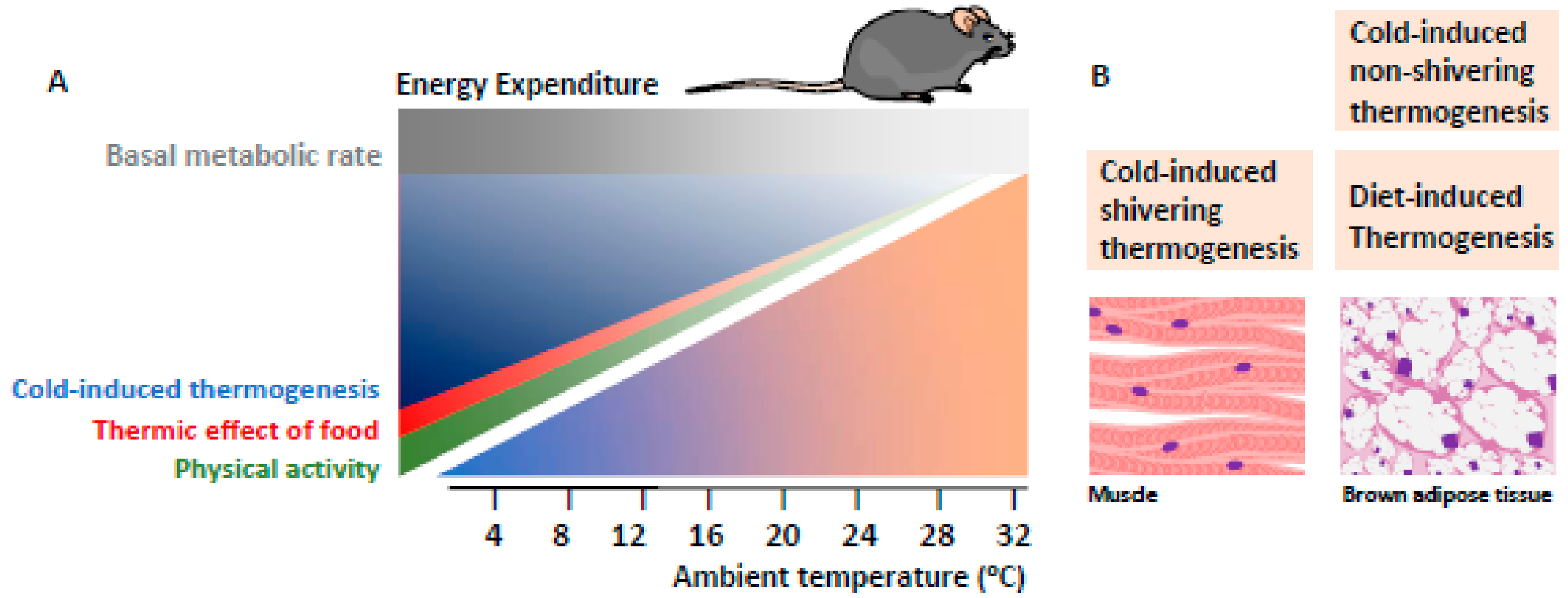
5. Adaptive Thermogenesis in Brown Adipose Tissue
6. Therapeutic Efficacy of Adaptive Thermogenesis in Obesity
6.1. Activating BAT to Treat Obesity
6.2. Role of Thermoneutrality in Obesity and Metabolic Studies: Chronic Cold vs. Thermoneutrality
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lovegrove, B.G. The evolution of endothermy in Cenozoic mammals: A plesiomorphic-apomorphic continuum. Biol. Rev. 2011, 87, 128–162. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Ruben, J. Endothermy and activity in vertebrates. Science 1979, 206, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O. Climate Variability and the Energetic Pathways of Evolution: The Origin of Endothermy in Mammals and Birds. Physiol. Biochem. Zoöl. 2004, 7, 959–981. [Google Scholar]
- Wu, C.-W.; Storey, K.B. Life in the cold: Links between mammalian hibernation and longevity. Biomol. Concepts 2016, 7, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bouma, H.R.; Verhaag, E.M.; Otis, J.P.; Heldmaier, G.; Swoap, S.J.; Strijkstra, A.M.; Henning, R.H.; Carey, H.V. Induction of torpor: Mimicking natural metabolic suppression for biomedical applications. J. Cell. Physiol. 2012, 227, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.K.; Fuller, A.; Mitchell, D.; Gordon, C.; Overton, J.M. Translating Animal Model Research: Does It Matter That Our Rodents Are Cold? Physiology 2014, 29, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Karp, C.L. Unstressing intemperate models: How cold stress undermines mouse modeling. J. Exp. Med. 2012, 209, 1069–1074. [Google Scholar] [CrossRef]
- Gordon, C. Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 2012, 37, 654–685. [Google Scholar] [CrossRef]
- Gordon, C.J. Temperature Regulation in Laboratory Rodents; Cambridge University Press (CUP): Cambridge, UK, 1993. [Google Scholar]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef]
- David, J.M.; Chatziioannou, A.F.; Taschereau, R.; Wang, H.; Stout, D.B. The Hidden Cost of Housing Practices: Using Noninvasive Imaging to Quantify the Metabolic Demands of Chronic Cold Stress of Laboratory Mice. Comp. Med. 2013, 63, 386–391. [Google Scholar]
- David, J.M.; Knowles, S.; Lamkin, D.M.; Stout, D.B. Individually Ventilated Cages Impose Cold Stress on Laboratory Mice: A Source of Systemic Experimental Variability. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 738–744. [Google Scholar] [PubMed]
- Gouveia, K.; Hurst, J.L. Reducing Mouse Anxiety during Handling: Effect of Experience with Handling Tunnels. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Greenberg, G.D.; Howerton, C.L.; Trainor, B.C. Fighting in the home cage: Agonistic encounters and effects on neurobiological markers within the social decision-making network of house mice (Mus musculus). Neurosci. Lett. 2014, 566, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Haemisch, A.; Gärtner, K. Effects of cage enrichment on territorial aggression and stress physiology in male laboratory mice. Acta Physiol. Scand. Suppl. 1997, 640, 73–76. [Google Scholar] [PubMed]
- Tabarés Seisdedos, R. Health effects of overweight and obesity in 195 countries over 25 years. New Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Pörtner, H.O.; Storch, D.; Heilmayer, O. Constraints and trade-offs in climate-dependent adaptation: Energy budgets and growth in a latitudinal cline. Sci. Mar. 2005, 69, 271–285. [Google Scholar] [CrossRef]
- Flouris, A.D.; Piantoni, C. Links between thermoregulation and aging in endotherms and ectotherms. Temperature 2015, 2, 73–85. [Google Scholar] [CrossRef]
- Terrien, J. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428. [Google Scholar] [CrossRef]
- Zhang, Y.; Proença, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Shutter, J.R.; Kinsey, A.C.; Scully, S.; Stark, K.L.; Graham, M.; Lüthy, R. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997, 11, 593–602. [Google Scholar] [CrossRef]
- Ollmann, M.M.; Wilson, B.D.; Yang, Y.-K.; Kerns, J.A.; Chen, Y.; Gantz, I.; Barsh, G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997, 278, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Boston, B.A.; Kesterson, R.A.; Hruby, V.J.; Cone, R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997, 385, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Farooqi, I.S.; O’Rahilly, S. Mutations in ligands and receptors of the leptin–melanocortin pathway that lead to obesity. Nat. Clin. Pr. Endocrinol. Metab. 2008, 4, 569–577. [Google Scholar] [CrossRef]
- Kleiber, M. The Fire of Life. An Introduction to Animal Energetics; John Wiley & Sons, Inc.: London, UK, 1961; p. 454. [Google Scholar]
- Schmidt-Nielsen, K.; Knut, S.N. Scaling: Why Is Animal Size so Important? Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Refinetti, R. The circadian rhythm of body temperature. Front. Biosci. 2010, 15, 564–594. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hock, R.; Walters, V.; Johnson, F.; Irving, L. Heat Regulation in Some Arctic and Tropical Mammals and Birds. Biol. Bull. 1950, 99, 237–258. [Google Scholar] [CrossRef]
- Abreu-Vieira, G.; Xiao, C.; Gavrilova, O.; Reitman, M.L. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol. Metab. 2015, 4, 461–470. [Google Scholar] [CrossRef]
- Brychta, R.J.; Chen, K.Y. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 2017, 71, 345–352. [Google Scholar] [CrossRef]
- Hill, R.W.; Muhich, T.E.; Humphries, M.M. City-Scale Expansion of Human Thermoregulatory Costs. PLoS ONE 2013, 8, e76238. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Hammel, H.T.; Andersen, K.L.; Andersen, K.L.; L⊘yning, Y. Metabolic Acclimation to Cold in Man. J. Appl. Physiol. 1958, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- E Wilkerson, J.; Raven, P.B.; Horvath, S.M. Critical temperature of unacclimatized male Caucasians. J. Appl. Physiol. 1972, 33, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.Y.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific Deletion of Stearoyl-CoA Desaturase-1 Alters Skin Lipid Composition and Protects Mice from High Fat Diet-induced Obesity. J. Biol. Chem. 2009, 284, 19961–19973. [Google Scholar] [CrossRef]
- Hirata, M.; Suzuki, M.; Ishii, R.; Satow, R.; Uchida, T.; Kitazumi, T.; Sasaki, T.; Kitamura, T.; Yamaguchi, H.; Nakamura, Y.; et al. Genetic Defect in Phospholipase Cδ1 Protects Mice From Obesity by Regulating Thermogenesis and Adipogenesis. Diabetes 2011, 60, 1926–1937. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fukami, K.; Yu, H.; Takenaka, K.; Kataoka, Y.; Shirakata, Y.; Nishikawa, S.; Hashimoto, K.; Yoshida, N.; Takenawa, T. Phospholipase Cδ1 is required for skin stem cell lineage commitment. EMBO J. 2003, 22, 2981–2991. [Google Scholar] [CrossRef]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; DeMay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef]
- Martin, B.; Ji, S.; Maudsley, S.; Mattson, M.P. “Control” laboratory rodents are metabolically morbid: Why it matters. Proc. Natl. Acad. Sci. USA 2010, 107, 6127–6133. [Google Scholar] [CrossRef]
- Overton, J.M. Phenotyping small animals as models for the human metabolic syndrome: Thermoneutrality matters. Int. J. Obes. 2010, 34, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, R.T.; Dauchy, E.M.; Hanifin, J.P.; Gauthreaux, S.L.; Mao, L.; Belancio, V.P.; Ooms, T.G.; Dupepe, L.M.; Jablonski, M.R.; Warfield, B.; et al. Effects of Spectral Transmittance through Standard Laboratory Cages on Circadian Metabolism and Physiology in Nude Rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 146–156. [Google Scholar] [PubMed]
- A Wren, M.; Dauchy, R.T.; Hanifin, J.P.; Jablonski, M.R.; Warfield, B.; Brainard, G.C.; E Blask, D.; Hill, S.M.; Ooms, T.G.; Bohm, R.P. Effect of Different Spectral Transmittances through Tinted Animal Cages on Circadian Metabolism and Physiology in Sprague–Dawley Rats. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 44–51. [Google Scholar] [PubMed]
- Horn, M.J.; Hudson, S.V.; A Bostrom, L.; Cooper, D.M. Effects of Cage Density, Sanitation Frequency, and Bedding Type on Animal Wellbeing and Health and Cage Environment in Mice and Rats. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 781–788. [Google Scholar] [PubMed]
- Leys, L.J.; McGaraughty, S.; Radek, R.J. Rats Housed on Corncob Bedding Show Less Slow-Wave Sleep. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 764–768. [Google Scholar] [PubMed]
- Royals, M.A.; Getzy, D.M.; Vandewoude, S. High Fungal Spore Load in Corncob Bedding Associated with Fungal-Induced Rhinitis in Two Rats. Contemp. Top. Lab. Anim. Sci. 1999, 38, 64–66. [Google Scholar] [PubMed]
- Smith, E.; Stockwell, J.D.; Schweitzer, I.; Langley, S.H.; Smith, A.L. Evaluation of cage micro-environment of mice housed on various types of bedding materials. Contemp. Top. Lab. Anim. Sci. 2004, 43, 12–17. [Google Scholar]
- Whiteside, T.E.; Thigpen, J.E.; Kissling, G.E.; Grant, M.G.; Forsythe, D.B. Endotoxin, Coliform, and Dust Levels in Various Types of Rodent Bedding. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 184–189. [Google Scholar]
- Kokolus, K.M.; Capitano, M.L.; Lee, C.-T.; Eng, J.W.-L.; Waight, J.D.; Hylander, B.L.; Sexton, S.; Hong, C.-C.; Gordon, C.J.; Abrams, S.I.; et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 20176–20181. [Google Scholar] [CrossRef]
- Nagy, T.R.; Krzywanski, D.; Li, J.; Meleth, S.; Desmond, R. Effect of Group vs. Single Housing on Phenotypic Variance in C57BL/6J Mice. Obes. Res. 2002, 10, 412–415. [Google Scholar] [CrossRef]
- Nicholson, A.; Malcolm, R.D.; Russ, P.L.; Cough, K.; Touma, C.; Palme, R.; Wiles, M.V. The Response of C57BL/6J and BALB/cJ Mice to Increased Housing Density. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 740–753. [Google Scholar] [PubMed]
- Morgan, J.L.; Svenson, K.L.; Lake, J.P.; Zhang, W.; Stearns, T.M.; Marion, M.A.; Peters, L.L.; Paigen, B.; Donahue, L.R. Effects of Housing Density in Five Inbred Strains of Mice. PLoS ONE 2014, 9, e90012. [Google Scholar] [CrossRef] [PubMed]
- Paigen, B.; Svenson, K.L.; Von Smith, R.; Marion, M.A.; Stearns, T.; Peters, L.L.; Smith, A.L. Physiological effects of housing density on C57BL/6J mice over a 9-month period. J. Anim. Sci. 2012, 90, 5182–5192. [Google Scholar] [CrossRef] [PubMed]
- A Toth, L.; A Trammell, R.; Ilsley-Woods, M. Interactions Between Housing Density and Ambient Temperature in the Cage Environment: Effects on Mouse Physiology and Behavior. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 708–717. [Google Scholar] [PubMed]
- Aghajani, M.; Mahdavi, M.R.V.; Najafabadi, M.K.; Ghazanfari, T.; Azimi, A.; Soleymani, S.A.; Dust, S.M. Effects of Dominant/Subordinate Social Status on Formalin-Induced Pain and Changes in Serum Proinflammatory Cytokine Concentrations in Mice. PLoS ONE 2013, 8, e80650. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.S.; Laarakker, M.C.; Van Lith, H.A.; Van Der Staay, F.J.; Gieling, E.; Salomons, A.R.; Klooster, J.V.; Ohl, F. Individual housing of mice — Impact on behaviour and stress responses. Physiol. Behav. 2009, 97, 385–393. [Google Scholar] [CrossRef]
- Febinger, H.Y.; George, A.; Priestley, J.; A Toth, L.; Opp, M.R. Effects of Housing Condition and Cage Change on Characteristics of Sleep in Mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 29–37. [Google Scholar]
- Morrison, S.F.; Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104. [Google Scholar] [CrossRef]
- Ranson, S.W. The Hypothalamus as a Thermostat Regulating Body Temperature. Psychosom. Med. 1939, 1, 486–495. [Google Scholar] [CrossRef]
- Boulant, J.A. Role of the Preoptic-Anterior Hypothalamus in Thermoregulation and Fever. Clin. Infect. Dis. 2000, 31, 157. [Google Scholar] [CrossRef]
- Boulant, J.A.; Hardy, J.D. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J. Physiol. 1974, 240, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Cooke, E.K.; Leib, D.E.; Lin, Y.-C.; Daly, G.E.; Zimmerman, C.A.; Knight, Z.A. Warm-Sensitive Neurons that Control Body Temperature. Cell 2016, 167, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Jessen, C. Thermal afferents in the control of body temperature. Pharmacol. Ther. 1985, 28, 107–134. [Google Scholar] [CrossRef]
- Emery, E.C.; Ernfors, P. Dorsal Root Ganglion Neuron Types and Their Functional Specialization. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion Channels and Thermosensitivity: TRP, TREK, or Both? Int. J. Mol. Sci. 2019, 20, 2371. [Google Scholar] [CrossRef] [PubMed]
- Kamm, G.B.; Siemens, J. The TRPM2 channel in temperature detection and thermoregulation. Temperature 2016, 4, 21–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wetsel, W.C. Sensing hot and cold with TRP channels. Int. J. Hyperth. 2011, 27, 388–398. [Google Scholar] [CrossRef]
- Ran, C.; Chen, X. Probing the coding logic of thermosensation using spinal cord calcium imaging. Exp. Neurol. 2019, 318, 42–49. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Palkar, R.; Lippoldt, E.K.; McCoy, D.D.; Baluch, F.; Chen, J.; McKemy, D.D. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 2013, 33, 2837–2848. [Google Scholar] [CrossRef]
- Craig, A.D.; Bushnell, M.C.; Zhang, E.-T.; Blomqvist, A. A thalamic nucleus specific for pain and temperature sensation. Nature 1994, 372, 770–773. [Google Scholar] [CrossRef]
- Yahiro, T.; Kataoka, N.; Nakamura, Y.; Nakamura, K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci. Rep. 2017, 7, 5031. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2007, 11, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, L.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2015, 5, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Metab. 2007, 293, 444–452. [Google Scholar] [CrossRef]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; I Tromsdorf, U.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef]
- Krauss, S.; Zhang, C.-Y.; Lowell, B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005, 6, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Klingenspor, M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp. Physiol. 2003, 88, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.S. Rate of Gain and Loss of Cold Resistance in Mice. Can. J. Zool. 1953, 31, 112–116. [Google Scholar] [CrossRef]
- Kaplan, H.M.; Foster, H. The Mouse in Biomedical Research. Physiology 1983, 111, 248–292. [Google Scholar]
- Bartelt, A.; John, C.; Schaltenberg, N.; Berbée, J.F.P.; Worthmann, A.; Cherradi, M.L.; Schlein, C.; Piepenburg, J.; Boon, M.R.; Rinninger, F.; et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 2017, 8, 15010. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Widenmaier, S.B.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgul, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Drong, A.; Lehne, B.; Loh, M.; Scott, W.R.; Kunze, S.; Tsai, P.C.; Ried, J.S.; Zhang, W.; Yang, Y.; et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017, 541, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. A role for brown adipose tissue in diet-induced thermogenesis. Obes. Res. 1997, 5, 650–656. [Google Scholar] [CrossRef]
- Lowell, B.B.; S-Susulic, V.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.-E.; Kozak, L.P.; Enerb, S. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997, 387, 90–94. [Google Scholar]
- Liu, X.; Rossmeisl, M.; McClaine, J.; Kozak, L.P. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J. Clin. Investig. 2003, 111, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 Ablation Induces Obesity and Abolishes Diet-Induced Thermogenesis in Mice Exempt from Thermal Stress by Living at Thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.M.; Sun, W.; Pires, N.D.; Frontini, A.; Balaz, M.; Jespersen, N.Z.; Feizi, A.; Petrovic, K.; Fischer, A.W.; Bokhari, M.H.; et al. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat. Metab. 2019, 1, 830–843. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 2017, 13, 458–465. [Google Scholar] [CrossRef]
- Castillo, M.; Hall, J.A.; Correa-Medina, M.; Ueta, C.; Kang, H.W.; Cohen, D.E.; Bianco, A.C. Disruption of Thyroid Hormone Activation in Type 2 Deiodinase Knockout Mice Causes Obesity With Glucose Intolerance and Liver Steatosis Only at Thermoneutrality. Diabetes 2011, 60, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Santry, H.P.; Madore, J.C.; Collins, C.E.; Ayturk, M.D.; Velmahos, G.C.; Britt, L.; Kiefe, C.I. Variations in the implementation of acute care surgery: Results from a national survey of university-affiliated hospitals. J. Trauma Acute Care Surg. 2015, 78, 60–68. [Google Scholar] [CrossRef]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- E Smith, R.; A Horwitz, B. Brown fat and thermogenesis. Physiol. Rev. 1969, 49, 330–425. [Google Scholar] [CrossRef]
- McMaster, M.L.; Kristinsson, S.Y.; Turesson, I.; Björkholm, M.; Landgren, O. Novel aspects pertaining to the relationship of Waldenström’s macroglobulinemia, IgM monoclonal gammopathy of undetermined significance, polyclonal gammopathy, and hypoglobulinemia. Clin. Lymphoma Myeloma 2009, 9, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Lichtenbelt, W.D.V.M.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-Activated Brown Adipose Tissue in Healthy Men. New Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, M.C.; Crosta, F.; Vitali, A.; Guerrieri, M.; Frontini, A.; Cannon, B.; Nedergaard, J.; Cinti, S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009, 23, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger prevents obesity through regulation of energy metabolism and activation of browning in high-fat diet-induced obese mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Hsu, Y.-J.; Chien, Y.-W.; Huang, C.-C.; Huang, W.-C.; Chiu, W.-C. Tea Seed Oil Prevents Obesity, Reduces Physical Fatigue, and Improves Exercise Performance in High-Fat-Diet-Induced Obese Ovariectomized Mice. Molecules 2019, 24, 980. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef]
- Song, N.-J.; Choi, S.; Rajbhandari, P.; Chang, S.-H.; Kim, S.; Vergnes, L.; Kwon, S.-M.; Yoon, J.-H.; Lee, S.-C.; Ku, J.-M.; et al. Prdm4 induction by the small molecule butein promotes white adipose tissue browning. Nat. Methods 2016, 12, 479–481. [Google Scholar] [CrossRef]
- Joo, J.I.; Kim, N.H.; Choi, J.-W.; Yun, J.W. Proteomic Analysis for Antiobesity Potential of Capsaicin on White Adipose Tissue in Rats Fed with a High Fat Diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef]
- Choi, H.; Kim, C.-S.; Yu, R. Quercetin Upregulates Uncoupling Protein 1 in White/Brown Adipose Tissues through Sympathetic Stimulation. J. Obes. Metab. Syndr. 2018, 27, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Su, H.; Song, M.; Zheng, J.; Liu, F.; Yuan, C.; Fu, Q.; Chen, S.; Zhu, X.; Wang, L.; et al. Calcium Supplementation Alleviates High-Fat Diet-Induced Estrous Cycle Irregularity and Subfertility Associated with Concomitantly Enhanced Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. J. Agric. Food Chem. 2019, 67, 7073–7081. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Zhang, H.; Ye, R.; Dong, M.; Lin, J.; Zhou, H.; Huang, Y.; Chen, L.; Jiang, X.; Nagaoka, K.; et al. Fluvastatin Sodium Ameliorates Obesity through Brown Fat Activation. Int. J. Mol. Sci. 2019, 20, 1622. [Google Scholar] [CrossRef] [PubMed]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- Otero-Díaz, B.; Rodríguez-Flores, M.; Sánchez-Muñoz, V.; Monraz-Preciado, F.; Ordoñez-Ortega, S.; Becerril-Elias, V.; Baay-Guzmán, G.; Obando-Monge, R.; García-García, E.; Palacios-González, B.; et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Carrière, A.; Jeanson, Y.; Berger-Müller, S.; André, M.; Chenouard, V.; Arnaud, E.; Barreau, C.; Walther, R.; Galinier, A.; Wdziekonski, B.; et al. Browning of White Adipose Cells by Intermediate Metabolites: An Adaptive Mechanism to Alleviate Redox Pressure. Diabetes 2014, 63, 3253–3265. [Google Scholar] [CrossRef]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- De Souza, C.J.; Hirshman, M.F.; Horton, E.S. CL-316,243, a β3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes 1997, 46, 1257–1263. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Korstanje, C. β3-Adrenoceptor agonists for overactive bladder syndrome: Role of translational pharmacology in a repositioning clinical drug development project. Pharmacol. Ther. 2016, 159, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Rosell, M.; Gonzalez, F.J.; Giralt, M.; Iglesias, R.; Villarroya, F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.J.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; Van Der Lans, A.A.; Cheng, C.C.; Adams, A.C.; Lichtenbelt, W.D.V.M.; Schrauwen, P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015, 5, 10275. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat involvement of PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef]
- Okla, M.; Ha, J.H.; Temel, R.E.; Chung, S. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids 2015, 50, 111–120. [Google Scholar] [CrossRef]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, N.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef]
- Hoffmann, J.M.; Grünberg, J.R.; Church, C.; Elias, I.; Pálsdóttir, V.; Jansson, J.-O.; Bosch, F.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. BMP4 Gene Therapy in Mature Mice Reduces BAT Activation but Protects from Obesity by Browning Subcutaneous Adipose Tissue. Cell Rep. 2017, 20, 1038–1049. [Google Scholar] [CrossRef]
- Digby, J.E.; Montague, C.T.; Sewter, C.P.; Sanders, L.; Wilkison, W.O.; O’Rahilly, S.; Prins, J.B. Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 1998, 47, 138–141. [Google Scholar] [CrossRef]
- Fayyad, A.M.; Khan, A.A.; Abdallah, S.H.; Alomran, S.S.; Bajou, K.; Khattak, M.N.K. Rosiglitazone Enhances Browning Adipocytes in Association with MAPK and PI3-K Pathways During the Differentiation of Telomerase-Transformed Mesenchymal Stromal Cells into Adipocytes. Int. J. Mol. Sci. 2019, 20, 1618. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Vidal-Puig, A. Beyond the Sympathetic Tone: The New Brown Fat Activators. Cell Metab. 2013, 17, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Liu, D.; Amri, E.Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Gnad, T.; Scheibler, S.; Von Kügelgen, I.; Schéele, C.; Kilic, A.; Glöde, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, O.; Funaba, M.; Sekiyama, K.; Doi, S.; Shindo, D.; Satoh, R.; Itoi, H.; Oiwa, H.; Morita, M.; Suzuki, C.; et al. Activin E Controls Energy Homeostasis in Both Brown and White Adipose Tissues as a Hepatokine. Cell Rep. 2018, 25, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, K.; Ushiro, Y.; Kurisaki, A.; Funaba, M.; Hashimoto, O. Activin E enhances insulin sensitivity and thermogenesis by activating brown/beige adipocytes. J. Veter. Med. Sci. 2019, 81, 646–652. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017, 13, 26. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Peijs, L.; Nielsen, S.; Bayarri-Olmos, R.; Larsen, T.J.; Jespersen, N.Z.; Hattel, H.; Holst, B.; Garred, P.; Tang-Christensen, M.; et al. Proteomics-based comparative mapping of the human brown and white adipocyte secretome reveals EPDR1 as a novel batokine. bioRxiv 2018, 402867. [Google Scholar] [CrossRef]
- Cereijo, R.; Gavaldà-Navarro, A.; Cairó, M.; Quesada-López, T.; Villarroya, J.; Morón-Ros, S.; Sánchez-Infantes, D.; Peyrou, M.; Iglesias, R.; Mampel, T.; et al. CXCL14, a Brown Adipokine that Mediates Brown-Fat-to-Macrophage Communication in Thermogenic Adaptation. Cell Metab. 2018, 28, 750–763.e6. [Google Scholar] [CrossRef]
- Soler-Vázquez, M.C.; Mera, P.; Zagmutt, S.; Serra, D.; Herrero, L. New approaches targeting brown adipose tissue transplantation as a therapy in obesity. Biochem. Pharmacol. 2018, 155, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Viana, M. Mechanisms of Impaired Brown Adipose Tissue Recruitment in Obesity. Front. Physiol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Reitman, M.L. Of mice and men - environmental temperature, body temperature, and treatment of obesity. FEBS Lett. 2018, 592, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- West, D.B.; Boozer, C.N.; Moody, D.L.; Atkinson, R.L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. Integr. Comp. Physiol. 1992, 262, R1025–R1032. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef]
- Kodela, E.; Moysidou, M.; Karaliota, S.; Koutmani, Y.; Tsakanikas, P.; Kodella, K.; A Karavia, E.; E Kypreos, K.; Kostomitsopoulos, N.; Karalis, K.P. Strain-specific Differences in the Effects of Lymphocytes on the Development of Insulin Resistance and Obesity in Mice. Comp. Med. 2018, 68, 15–24. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastías-Pérez, M.; Zagmutt, S.; Soler-Vázquez, M.C.; Serra, D.; Mera, P.; Herrero, L. Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells 2020, 9, 316. https://doi.org/10.3390/cells9020316
Bastías-Pérez M, Zagmutt S, Soler-Vázquez MC, Serra D, Mera P, Herrero L. Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells. 2020; 9(2):316. https://doi.org/10.3390/cells9020316
Chicago/Turabian StyleBastías-Pérez, Marianela, Sebastián Zagmutt, M Carmen Soler-Vázquez, Dolors Serra, Paula Mera, and Laura Herrero. 2020. "Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity" Cells 9, no. 2: 316. https://doi.org/10.3390/cells9020316
APA StyleBastías-Pérez, M., Zagmutt, S., Soler-Vázquez, M. C., Serra, D., Mera, P., & Herrero, L. (2020). Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells, 9(2), 316. https://doi.org/10.3390/cells9020316







