The Role of miR-21 in Osteoblasts–Osteoclasts Coupling In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-osteoblastic Mouse Cell Line MC3T3
2.2. Transfection of miR-21 Inhibitor
2.3. Osteogenic Conditions
2.4. Co–culture with Pre-osteoclastic Cell Line 4B12
2.5. Evaluation of Extracellular Matrix Composition
2.6. Detection of Osteogenic Markers Using RT-qPCR
2.7. Western Blotting Detection of Osteopontin and Runx-2
2.8. Immunocytochemical Detection of Osteopontin and Tartrate-Resistant Acid Phosphatase (TRAP)
2.9. Statistical Analysis
3. Results
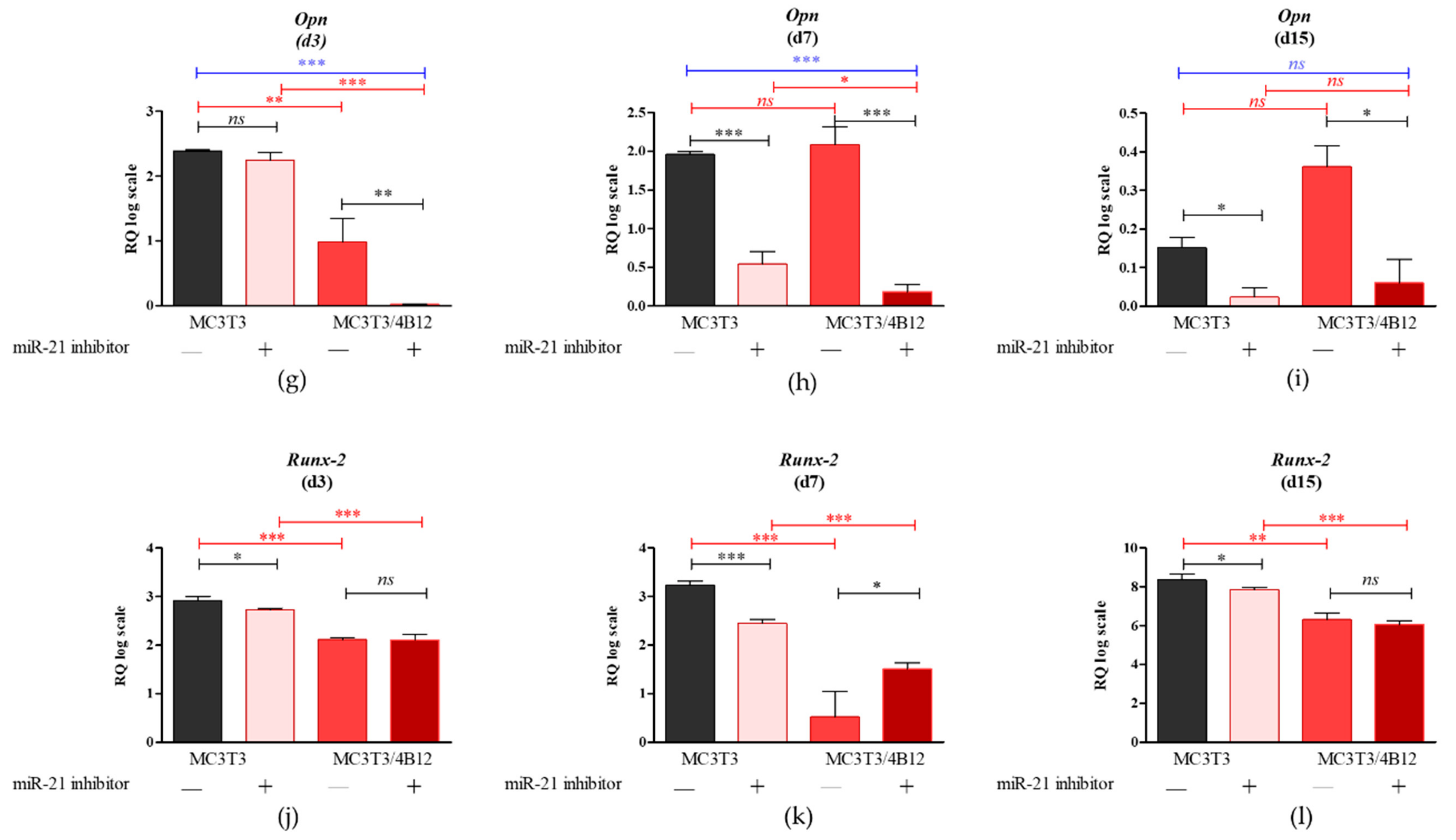
3.1. The Influence of miR-21 Inhibition on mRNA Expression of Osteogenic Markers
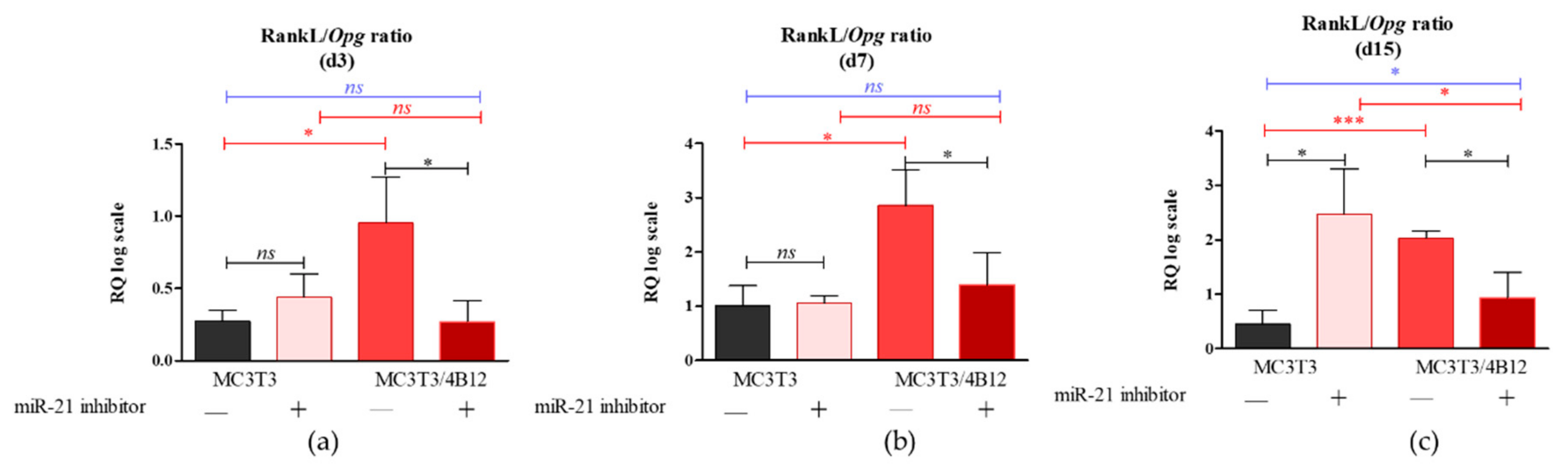
3.2. The Analysis of mRNA Transcripts of RANKL-OPG Axis
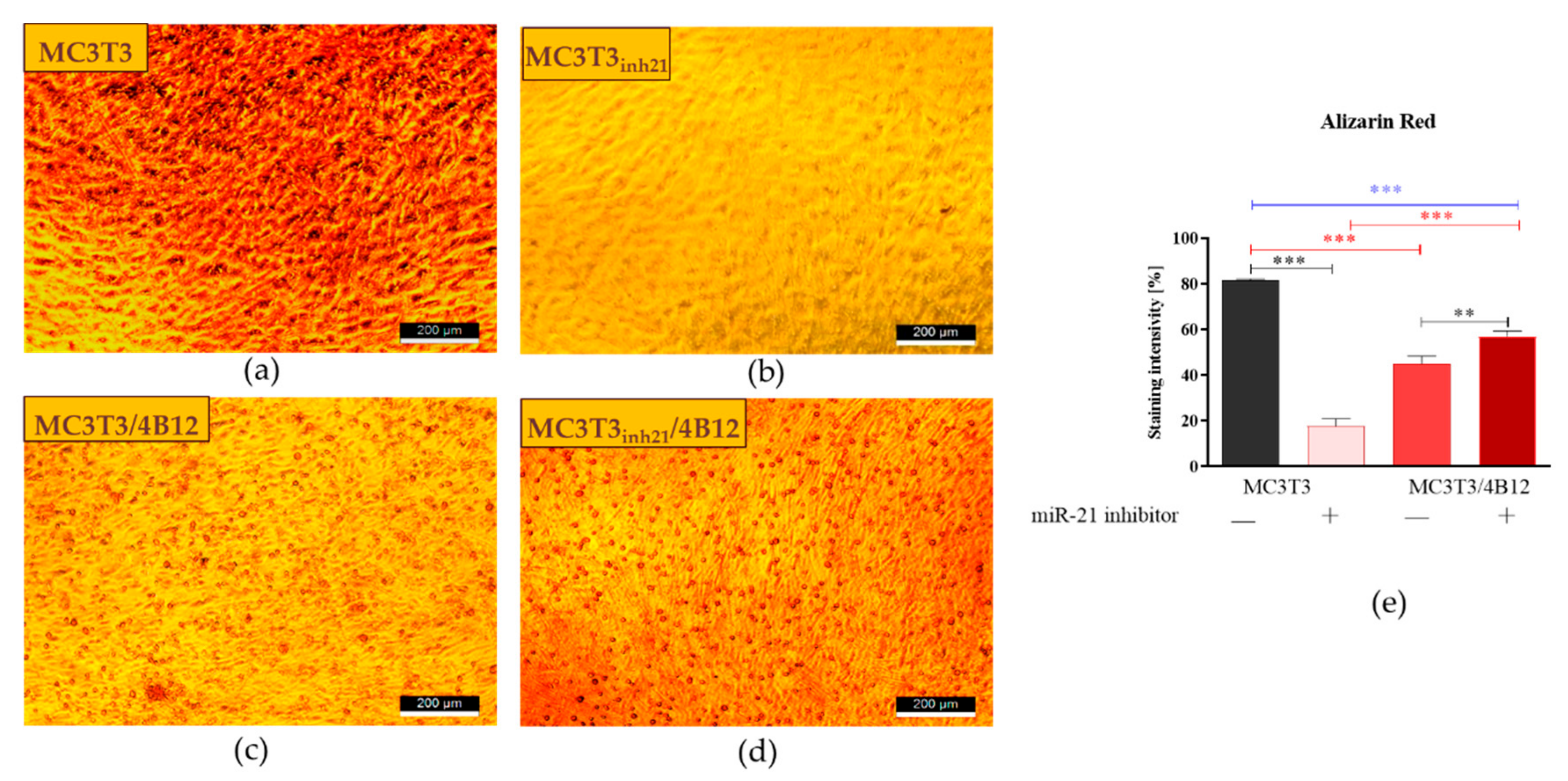
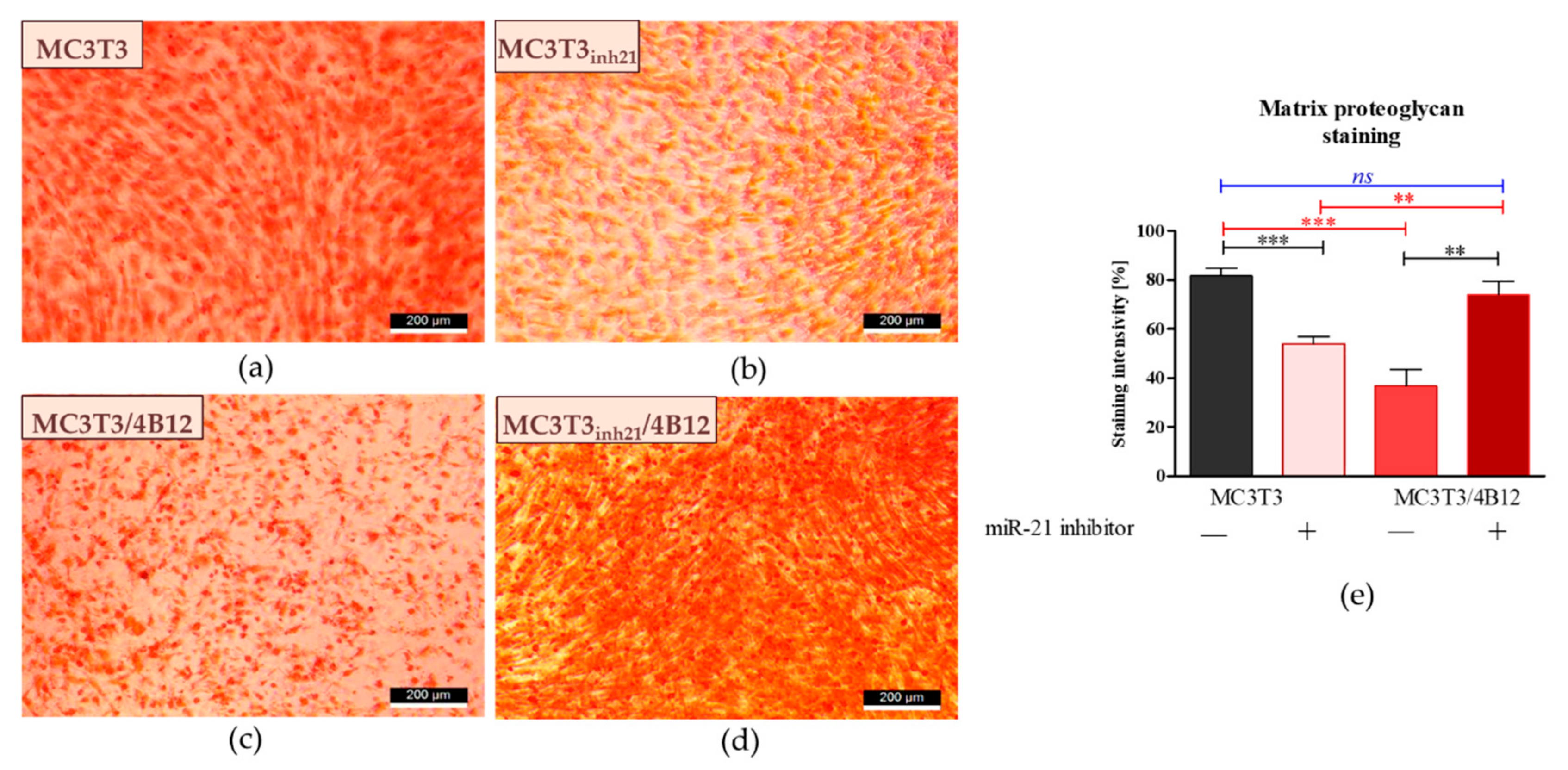
3.3. The Influence of miR-21 Inhibition on Extracellular Matrix Composition
3.4. The Influence of miR-21 Inhibition on Intracellular Accumulation of OPN and RUNX-2 in MC3T3 after 15 Days of Osteogenesis
3.5. The Analysis of Markers Associated with Differentiation and Bone Resorption Activity of Osteoclasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Li, X.; Zou, D.; Lian, Y.; Tian, S.; Dou, Z. Expression of microRNA-21 in osteoporotic patients and its involvement in the regulation of osteogenic differentiation. Exp. Ther. Med. 2019, 17, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.K.; Au, P.C.; Tan, K.C.; Cheung, C. MicroRNA and Human Bone Health. JBMR Plus 2018, 3, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zhang, Z.; Qiu, X.; Chu, H.; Sun, X.; Zheng, X.; Zhao, J.; Li, Q. Analysis of the miRNA and mRNA involved in osteogenesis of adipose-derived mesenchymal stem cells. Exp. Ther. Med. 2018, 16, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hassan, M.Q.; Jafferji, M.; Aqeilan, R.I.; Garzon, R.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Biological Functions of miR-29b Contribute to Positive Regulation of Osteoblast Differentiation. J. Biol. Chem. 2009, 284, 15676–15684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, L.F. MicroRNAs at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration. Cells 2019, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Yang, S.; Guo, Q.; Zhang, X.; Ren, D.; Lv, T.; Xu, X. MicroRNA-21 regulates Osteogenic Differentiation of Periodontal Ligament Stem Cells by targeting Smad5. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res. Ther. 2019, 10, 65. [Google Scholar]
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Medina, P.P.; Nolde, M.; Slack, F.J. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature 2010, 467, 86–90. [Google Scholar] [CrossRef]
- Hu, X.; Li, L.; Lu, Y.; Yu, X.; Chen, H.; Yin, Q.; Zhang, Y. miRNA-21 inhibition inhibits osteosarcoma cell proliferation by targeting PTEN and regulating the TGF-β1 signaling pathway. Oncol. Lett. 2018, 16, 4337–4342. [Google Scholar] [CrossRef]
- Li, H.; Yang, F.; Wang, Z.; Fu, Q.; Liang, A. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol. Med. Rep. 2015, 12, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Liu, Y.; Su, Y.; Xie, Y.; Du, J.; Zhou, J.; Ding, G.; Wang, H.; Bai, Y.; et al. MicroRNA-21 promotes osteogenesis of bone marrow mesenchymal stem cells via the Smad7-Smad1/5/8-Runx2 pathway. Biochem. Biophys. Res. Commun. 2017, 493, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Dalle Carbonare, L.; Mottes, M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.-B.; Li, X.; Li, Z.-Y.; Zhao, J.; Yuan, X.-B.; Ren, Y.; Cui, Z.-D.; Liu, Y.-D.; Yang, X.-J. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J. Orthop. Res. 2015, 33, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Molténi, A.; Modrowski, D.; Hott, M.; Marie, P.J. Alterations of matrix- and cell-associated proteoglycans inhibit osteogenesis and growth response to fibroblast growth factor-2 in cultured rat mandibular condyle and calvaria. Cell Tissue Res. 1999, 295, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Duroux-Richard, I.; Firat, H.; Schordan, E.; Apparailly, F. MicroRNAs: Key Regulators to Understand Osteoclast Differentiation? Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Udagawa, N.; Takahashi, N. Action of RANKL and OPG for osteoclastogenesis. Crit. Rev. Eukaryot. Gene Exp. 2009, 19, 61–72. [Google Scholar] [CrossRef]
- Amano, S.; Sekine, K.; Bonewald, L.; Ohmori, Y. A Novel Osteoclast Precursor Cell Line, 4B12, Recapitulates the Features of Primary Osteoclast Differentiation and Function: Enhanced Transfection Efficiency Before and After Differentiation. J. Cell Physiol. 2009, 221, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Amano, S.; Chang, Y.-T.; Fukui, Y. ERK5 Activation Is Essential for Osteoclast Differentiation. PLoS ONE 2015, 10, e0125054. [Google Scholar] [CrossRef]
- Androvic, P.; Valihrach, L.; Elling, J.; Sjoback, R.; Kubista, M. Two-tailed RT-qPCR: A novel method for highly accurate miRNA quantification. Nucleic Acids Res. 2017, 45, e144. [Google Scholar] [CrossRef]
- Androvic, P.; Romanyuk, N.; Urdzikova-Machova, L.; Rohlova, E.; Kubista, M.; Valihrach, L. Two-tailed RT-qPCR panel for quality control of circulating microRNA studies. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smieszek, A.; Kornicka, K.; Szłapka-Kosarzewska, J.; Androvic, P.; Valihrach, L.; Langerova, L.; Rohlova, E.; Kubista, M.; Marycz, K. Metformin Increases Proliferative Activity and Viability of Multipotent Stromal Stem Cells Isolated from Adipose Tissue Derived from Horses with Equine Metabolic Syndrome. Cells 2019, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smieszek, A.; Tomaszewski, K.A.; Kornicka, K.; Marycz, K. Metformin Promotes Osteogenic Differentiation of Adipose-Derived Stromal Cells and Exerts Pro-Osteogenic Effect Stimulating Bone Regeneration. J. Clin. Med. 2018, 7, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimoch-Korzycka, A.; Śmieszek, A.; Jarmoluk, A.; Nowak, U.; Marycz, K. Potential Biomedical Application of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges-Biocompatible Scaffolds Inducing Chondrogenic Differentiation of Human Adipose Derived Multipotent Stromal Cells. Polymers 2016, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. Hoboken 2013, 296, 378–381. [Google Scholar] [CrossRef]
- Śmieszek, A.; Stręk, Z.; Kornicka, K.; Grzesiak, J.; Weiss, C.; Marycz, K. Antioxidant and Anti-Senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels-An Ex Vivo Study. Int. J. Mol. Sci. 2017, 18, 872. [Google Scholar] [CrossRef]
- Śmieszek, A.; Szydlarska, J.; Mucha, A.; Chrapiec, M.; Marycz, K. Enhanced cytocompatibility and osteoinductive properties of sol-gel-derived silica/zirconium dioxide coatings by metformin functionalization. J. Biomater. Appl. 2017, 32, 570–586. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Smieszek, A.; Marycz, K.; Szustakiewicz, K.; Kryszak, B.; Targonska, S.; Zawisza, K.; Watras, A.; Wiglusz, R.J. New approach to modification of poly (l-lactic acid) with nano-hydroxyapatite improving functionality of human adipose-derived stromal cells (hASCs) through increased viability and enhanced mitochondrial activity. Mater. Sci. Eng. C 2019, 98, 213–226. [Google Scholar]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of Oxidative Stress and Mitophagy during Adipogenic Differentiation of Adipose-Derived Stem Cells Isolated from Equine Metabolic Syndrome (EMS) Horses. Stem Cells Int. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Sobierajska, P.; Smieszek, A.; Maredziak, M.; Wiglusz, K.; Wiglusz, R.J. Li+ activated nanohydroxyapatite doped with Eu3+ ions enhances proliferative activity and viability of human stem progenitor cells of adipose tissue and olfactory ensheathing cells. Further perspective of nHAP:Li+, Eu3+ application in theranostics. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, M.S.; Yang, H.S. Differentiated osteoblasts derived decellularized extracellular matrix to promote osteogenic differentiation. Biomater. Res. 2018, 22, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Ehnert, S.; Rouß, M.; Häussling, V.; Aspera-Werz, R.H.; Chen, T.; Nussler, A.K. From the Clinical Problem to the Basic Research—Co-Culture Models of Osteoblasts and Osteoclasts. Int. J. Mol. Sci. 2018, 19, 2284. [Google Scholar] [CrossRef] [Green Version]
- Janardhanan, S.; Wang, M.O.; Fisher, J.P. Coculture Strategies in Bone Tissue Engineering: The Impact of Culture Conditions on Pluripotent Stem Cell Populations. Tissue Eng. Part B Rev. 2012, 18, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, R.; Reilly, G.C. In vitro Models of Bone Remodelling and Associated Disorders. Front. Bioeng. Biotechnol. 2018, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.; Nelea, V.; Chicatun, F.; Chien, Y.; Tran-Khanh, N.; Buschmann, M.; Nazhat, S.; Kaartinen, M.; Vali, H.; Tecklenburg, M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef]
- Peng, J.; Huang, N.; Huang, S.; Li, L.; Ling, Z.; Jin, S.; Huang, A.; Lin, K.; Zou, X. Effect of miR-21 down-regulated by H2O2 on osteogenic differentiation of MC3T3-E1 cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 276–284. [Google Scholar]
- Chai, X.; Zhang, W.; Chang, B.; Feng, X.; Song, J.; Li, L.; Yu, C.; Zhao, J.; Si, H. GPR39 agonist TC-G 1008 promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3569–3576. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, D.; Qin, Y.; Xu, M.; Zhou, L.; Xu, W.; Liu, X.; Ye, L.; Yue, S.; Zheng, Q.; et al. Astragalin Promotes Osteoblastic Differentiation in MC3T3-E1 Cells and Bone Formation in vivo. Front. Endocrinol. Lausanne 2019, 10, 228. [Google Scholar] [CrossRef]
- Sterner, R.M.; Kremer, K.N.; Dudakovic, A.; Westendorf, J.J.; van Wijnen, A.J.; Hedin, K.E. Tissue-Nonspecific Alkaline Phosphatase Is Required for MC3T3 Osteoblast–Mediated Protection of Acute Myeloid Leukemia Cells from Apoptosis. J. Immunol. 2018, 201, 1086–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Spevak, L.; Paschalis, E.; Doty, S.B.; McKee, M.D. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif. Tissue Int. 2002, 71, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Holm, E.; Gleberzon, J.S.; Liao, Y.; Sørensen, E.S.; Beier, F.; Hunter, G.K.; Goldberg, H.A. Osteopontin mediates mineralization and not osteogenic cell development in vitro. Biochem. J. 2014, 464, 355–364. [Google Scholar] [CrossRef]
- Rittling, S.R.; Matsumoto, H.N.; Mckee, M.D.; Nanci, A.; An, X.-R.; Novick, K.E.; Kowalski, A.J.; Noda, M.; Denhardt, D.T. Mice Lacking Osteopontin Show Normal Development and Bone Structure but Display Altered Osteoclast Formation In Vitro. J. Bone Mineral. Res. 1998, 13, 1101–1111. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Pregizer, S.; Baniwal, S.K.; Yan, X.; Borok, Z.; Frenkel, B. Progressive recruitment of Runx2 to genomic targets despite decreasing expression during osteoblast differentiation. J. Cell Biochem. 2008, 105, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar]
- O’Brien, C.A. Control of RANKL Gene Expression. Bone 2010, 46, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Pitari, M.R.; Rossi, M.; Amodio, N.; Botta, C.; Morelli, E.; Federico, C.; Gullà, A.; Caracciolo, D.; Di Martino, M.T.; Arbitrio, M.; et al. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget 2015, 6, 27343–27358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, K.A.; Rogers, H.L.; Tosh, D.; Rogers, M.J. RANKL increases the level of Mcl-1 in osteoclasts and reduces bisphosphonate-induced osteoclast apoptosis in vitro. Arthritis Res. Ther. 2009, 11, R58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.-H.; Sui, B.-D.; Du, F.-Y.; Shuai, Y.; Zheng, C.-X.; Zhao, P.; Yu, X.-R.; Jin, Y. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luukkonen, J.; Hilli, M.; Nakamura, M.; Ritamo, I.; Valmu, L.; Kauppinen, K.; Tuukkanen, J.; Lehenkari, P. Osteoclasts secrete osteopontin into resorption lacunae during bone resorption. Histochem. Cell Biol. 2019, 151, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ek-Rylander, B.; Andersson, G. Osteoclast migration on phosphorylated osteopontin is regulated by endogenous tartrate-resistant acid phosphatase. Exp. Cell Res. 2010, 316, 443–451. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smieszek, A.; Marcinkowska, K.; Pielok, A.; Sikora, M.; Valihrach, L.; Marycz, K. The Role of miR-21 in Osteoblasts–Osteoclasts Coupling In Vitro. Cells 2020, 9, 479. https://doi.org/10.3390/cells9020479
Smieszek A, Marcinkowska K, Pielok A, Sikora M, Valihrach L, Marycz K. The Role of miR-21 in Osteoblasts–Osteoclasts Coupling In Vitro. Cells. 2020; 9(2):479. https://doi.org/10.3390/cells9020479
Chicago/Turabian StyleSmieszek, Agnieszka, Klaudia Marcinkowska, Ariadna Pielok, Mateusz Sikora, Lukas Valihrach, and Krzysztof Marycz. 2020. "The Role of miR-21 in Osteoblasts–Osteoclasts Coupling In Vitro" Cells 9, no. 2: 479. https://doi.org/10.3390/cells9020479




