Plasma Membrane Transporters as Biomarkers and Molecular Targets in Cholangiocarcinoma
Abstract
:1. Introduction
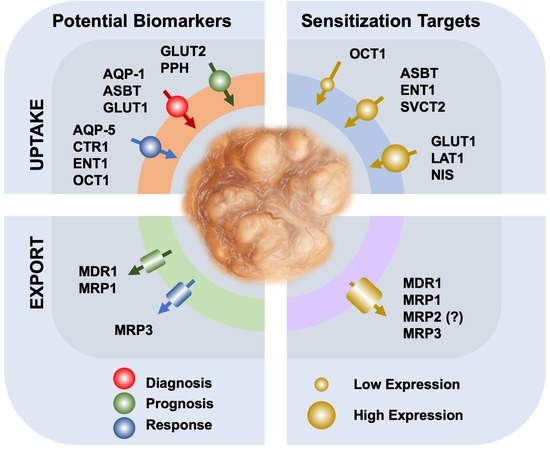
2. Carriers as Biomarkers
3. The Usefulness of Transporters in Drug Targeting
4. Overcoming Chemoresistance by Manipulation of Uptake Transporters
5. Strategies to Reduce Drug Efflux
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Marin, J.J.G.; Lozano, E.; Briz, O.; Al-Abdulla, R.; Serrano, M.A.; Macias, R.I.R. Molecular Bases of Chemoresistance in Cholangiocarcinoma. Curr. Drug Targets 2017, 18, 889–900. [Google Scholar] [CrossRef]
- Rau, S.; Autschbach, F.; Riedel, H.D.; Konig, J.; Kulaksiz, H.; Stiehl, A.; Riemann, J.F.; Rost, D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human cholangiocellular carcinomas. Eur. J. Clin. Investig. 2008, 38, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.; Macias, R.I.; Briz, O.; Banales, J.M.; Monte, M.J. Bile Acids in Physiology, Pathology and Pharmacology. Curr. Drug Metab. 2015, 17, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.G.; Briz, O.; Herraez, E.; Lozano, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Osorio-Padilla, L.M.; Santos-Llamas, A.I.; Serrano, M.A.; et al. Molecular bases of the poor response of liver cancer to chemotherapy. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 182–192. [Google Scholar] [CrossRef]
- Lozano, E.; Monte, M.J.; Briz, O.; Hernandez-Hernandez, A.; Banales, J.M.; Marin, J.J.; Macias, R.I. Enhanced antitumour drug delivery to cholangiocarcinoma through the apical sodium-dependent bile acid transporter (ASBT). J. Control Release 2015, 216, 93–102. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Jung, D.; Fried, M.; Grutzner, U.; Meier, P.J.; Kullak-Ublick, G.A. The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3beta in hepatocellular carcinoma. J. Hepatol. 2004, 40, 212–218. [Google Scholar] [CrossRef]
- Martinez-Becerra, P.; Vaquero, J.; Romero, M.R.; Lozano, E.; Anadon, C.; Macias, R.I.; Serrano, M.A.; Grane-Boladeras, N.; Munoz-Bellvis, L.; Alvarez, L.; et al. No correlation between the expression of FXR and genes involved in multidrug resistance phenotype of primary liver tumors. Mol. Pharm. 2012, 9, 1693–1704. [Google Scholar] [CrossRef]
- Pongmaneratanakul, S.; Tanasanvimon, S.; Pengsuparp, T.; Areepium, N. Prevalence of CTR1 and ERCC1 Polymorphisms and Response of Biliary Tract Cancer to Gemcitabine-Platinum Chemotherapy. Asian Pac. J. Cancer Prev. 2017, 18, 857–861. [Google Scholar]
- Brandi, G.; Deserti, M.; Vasuri, F.; Farioli, A.; Degiovanni, A.; Palloni, A.; Frega, G.; Barbera, M.A.; De Lorenzo, S.; Garajova, I.; et al. Membrane Localization of Human Equilibrative Nucleoside Transporter 1 in Tumor Cells May Predict Response to Adjuvant Gemcitabine in Resected Cholangiocarcinoma Patients. Oncologist 2016, 21, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, H.; Lee, J.C.; Kim, J.W.; Paik, W.H.; Lee, S.H.; Hwang, J.H.; Ryu, J.K.; Kim, Y.T. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS ONE 2018, 13, e0209104. [Google Scholar] [CrossRef]
- Grimm, D.; Lieb, J.; Weyer, V.; Vollmar, J.; Darstein, F.; Lautem, A.; Hoppe-Lotichius, M.; Koch, S.; Schad, A.; Schattenberg, J.M.; et al. Organic Cation Transporter 1 (OCT1) mRNA expression in hepatocellular carcinoma as a biomarker for sorafenib treatment. BMC Cancer 2016, 16, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geier, A.; Macias, R.I.; Bettinger, D.; Weiss, J.; Bantel, H.; Jahn, D.; Al-Abdulla, R.; Marin, J.J. The lack of the organic cation transporter OCT1 at the plasma membrane of tumor cells precludes a positive response to sorafenib in patients with hepatocellular carcinoma. Oncotarget 2017, 8, 15846–15857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, E.; Macias, R.I.R.; Monte, M.J.; Asensio, M.; Del Carmen, S.; Sanchez-Vicente, L.; Alonso-Pena, M.; Al-Abdulla, R.; Munoz-Garrido, P.; Satriano, L.; et al. Causes of hOCT1-Dependent Cholangiocarcinoma Resistance to Sorafenib and Sensitization by Tumor-Selective Gene Therapy. Hepatology 2019, 70, 1246–1261. [Google Scholar] [CrossRef] [PubMed]
- Visentin, M.; Van Rosmalen, B.V.; Hiller, C.; Bieze, M.; Hofstetter, L.; Verheij, J.; Kullak-Ublick, G.A.; Koepsell, H.; Phoa, S.S.; Tamai, I.; et al. Impact of Organic Cation Transporters (OCT-SLC22A) on Differential Diagnosis of Intrahepatic Lesions. Drug Metab. Dispos. 2017, 45, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Meier, R.; Bi, C.; Gaedigk, R.; Heruth, D.P.; Ye, S.Q.; Leeder, J.S.; Fridley, B.L. Ontogeny-related pharmacogene changes in the pediatric liver transcriptome. Pharm. Genom. 2018, 28, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y.; Aishima, S.; Tanaka, Y.; Shindo, K.; Mizuuchi, Y.; Abe, K.; Shirabe, K.; Maehara, Y.; Honda, H.; Oda, Y. Different expression of glucose transporters in the progression of intrahepatic cholangiocarcinoma. Hum. Pathol. 2014, 45, 1610–1617. [Google Scholar] [CrossRef]
- Roh, M.S.; Jeong, J.S.; Kim, Y.H.; Kim, M.C.; Hong, S.H. Diagnostic utility of GLUT1 in the differential diagnosis of liver carcinomas. Hepatogastroenterology 2004, 51, 1315–1318. [Google Scholar]
- Alves, V.A.; Pinheiro, C.; Morais-Santos, F.; Felipe-Silva, A.; Longatto-Filho, A.; Baltazar, F. Characterization of monocarboxylate transporter activity in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 11780–11787. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.T.; Hsieh, Y.H.; Wu, C.C.; Tsai, J.H.; Hsieh, Y.S.; Huang, C.Y.; Liu, J.Y. Overexpression of anion exchanger 2 in human hepatocellular carcinoma. Chin. J. Physiol. 2006, 49, 192–198. [Google Scholar]
- Hohenester, S.; Wenniger, L.M.; Paulusma, C.C.; Van Vliet, S.J.; Jefferson, D.M.; Elferink, R.P.; Beuers, U. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology 2012, 55, 173–183. [Google Scholar] [CrossRef]
- Mazal, P.R.; Susani, M.; Wrba, F.; Haitel, A. Diagnostic significance of aquaporin-1 in liver tumors. Hum. Pathol. 2005, 36, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Shimada, Y.; Nagata, T.; Moriyama, M.; Omura, T.; Watanabe, T.; Hori, R.; Yoshioka, I.; Okumura, T.; Sawada, S.; et al. Prognostic significance of aquaporins in human biliary tract carcinoma. Oncol. Rep. 2012, 27, 1741–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Sun, M.; Hu, Y.; Zhang, H.; Wang, Z.; Zhou, N.; Yan, X. FXYD6 is a new biomarker of cholangiocarcinoma. Oncol. Lett. 2014, 7, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soini, Y.; Virkajarvi, N.; Raunio, H.; Paakko, P. Expression of P-glycoprotein in hepatocellular carcinoma: A potential marker of prognosis. J. Clin. Pathol. 1996, 49, 470–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srimunta, U.; Sawanyawisuth, K.; Kraiklang, R.; Pairojkul, C.; Puapairoj, A.; Titipungul, T.; Hahnvajanawong, C.; Tassaneeyakul, W.; Wongkham, C.; Wongkham, S.; et al. High expression of ABCC1 indicates poor prognosis in intrahepatic cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 125–130. [Google Scholar]
- Wang, B.L.; Zhai, H.Y.; Chen, B.Y.; Zhai, S.P.; Yang, H.Y.; Chen, X.P.; Zhao, W.T.; Meng, L. Clinical relationship between MDR1 gene and gallbladder cancer. Hepatobiliary Pancreat. Dis. Int. 2004, 3, 296–299. [Google Scholar] [PubMed]
- Lagana, S.M.; Salomao, M.; Remotti, H.E.; Knisely, A.S.; Moreira, R.K. Bile salt export pump: A sensitive and specific immunohistochemical marker of hepatocellular carcinoma. Histopathology 2015, 66, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Yamasaki, T.; Otani, K.; Kanzawa, M.; Fukumoto, T.; Ku, Y.; Hirose, T.; Itoh, T.; Zen, Y. BSEP and MDR3: Useful Immunohistochemical Markers to Discriminate Hepatocellular Carcinomas From Intrahepatic Cholangiocarcinomas and Hepatoid Carcinomas. Am. J. Surg. Pathol. 2016, 40, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Scheimann, A.O.; Strautnieks, S.S.; Knisely, A.S.; Byrne, J.A.; Thompson, R.J.; Finegold, M.J. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J. Pediatr. 2007, 150, 556–559. [Google Scholar] [CrossRef]
- Tougeron, D.; Fotsing, G.; Barbu, V.; Beauchant, M. ABCB4/MDR3 gene mutations and cholangiocarcinomas. J. Hepatol. 2012, 57, 467–468. [Google Scholar] [CrossRef] [Green Version]
- Cirqueira, C.S.; Felipe-Silva, A.S.; Wakamatsu, A.; Marins, L.V.; Rocha, E.C.; De Mello, E.S.; Alves, V.A.F. Immunohistochemical Assessment of the Expression of Biliary Transportation Proteins MRP2 and MRP3 in Hepatocellular Carcinoma and in Cholangiocarcinoma. Pathol. Oncol. Res. 2019, 25, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Tomonari, T.; Takeishi, S.; Taniguchi, T.; Tanaka, T.; Tanaka, H.; Fujimoto, S.; Kimura, T.; Okamoto, K.; Miyamoto, H.; Muguruma, N.; et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget 2016, 7, 7207–7215. [Google Scholar] [CrossRef] [Green Version]
- Larbcharoensub, N.; Sornmayura, P.; Sirachainan, E.; Wilasrusmee, C.; Wanmoung, H.; Janvilisri, T. Prognostic value of ABCG2 in moderately and poorly differentiated intrahepatic cholangiocarcinoma. Histopathology 2011, 59, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Molina-Arcas, M.; Casado, F.J.; Bellosillo, B.; Colomer, D.; Gil, J. Nucleoside transporters in chronic lymphocytic leukaemia. Leukemia 2004, 18, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Sueda, T. Concurrent analysis of human equilibrative nucleoside transporter 1 and ribonucleotide reductase subunit 1 expression increases predictive value for prognosis in cholangiocarcinoma patients treated with adjuvant gemcitabine-based chemotherapy. Br. J. Cancer 2014, 111, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Borbath, I.; Verbrugghe, L.; Lai, R.; Gigot, J.F.; Humblet, Y.; Piessevaux, H.; Sempoux, C. Human equilibrative nucleoside transporter 1 (hENT1) expression is a potential predictive tool for response to gemcitabine in patients with advanced cholangiocarcinoma. Eur. J. Cancer 2012, 48, 990–996. [Google Scholar] [CrossRef]
- Criado, J.J.; Macias, R.I.; Medarde, M.; Monte, M.J.; Serrano, M.A.; Marin, J.J. Synthesis and characterization of the new cytostatic complex cis-diammineplatinum(II)-chlorocholylglycinate. Bioconjug. Chem. 1997, 8, 453–458. [Google Scholar] [CrossRef]
- Criado, J.J.; Dominguez, M.F.; Medarde, M.; Fernandez, E.R.; Macias, R.I.; Marin, J.J. Structural characterization, kinetic studies, and in vitro biological activity of new cis-diamminebis-cholylglycinate(O,O’) Pt(II) and cis-diamminebis-ursodeoxycholate(O,O’) Pt(II) complexes. Bioconjug. Chem. 2000, 11, 167–174. [Google Scholar] [CrossRef]
- Dominguez, M.F.; Macias, R.I.; Izco-Basurko, I.; De La Fuente, A.; Pascual, M.J.; Criado, J.M.; Monte, M.J.; Yajeya, J.; Marin, J.J. Low in vivo toxicity of a novel cisplatin-ursodeoxycholic derivative (Bamet-UD2) with enhanced cytostatic activity versus liver tumors. J. Pharmacol. Exp. Ther. 2001, 297, 1106–1112. [Google Scholar]
- Kanai, Y.; Segawa, H.; Miyamoto, K.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef] [Green Version]
- Janpipatkul, K.; Suksen, K.; Borwornpinyo, S.; Jearawiriyapaisarn, N.; Hongeng, S.; Piyachaturawat, P.; Chairoungdua, A. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell Signal. 2014, 26, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Yothaisong, S.; Dokduang, H.; Anzai, N.; Hayashi, K.; Namwat, N.; Yongvanit, P.; Sangkhamanon, S.; Jutabha, P.; Endou, H.; Loilome, W. Inhibition of l-type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment. Tumour Biol. 2017, 39, 1010428317694545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Mamede, A.C.; Laranjo, M.; Casalta-Lopes, J.E.; Goncalves, A.C.; Sarmento-Ribeiro, A.B.; Tralhao, J.G.; Botelho, M.F. New Approach for Treatment of Primary Liver Tumors: The Role of Quercetin. Nutr. Cancer 2016, 68, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, H.; Yang, W.; Li, T.; Fang, T.; Lv, G.; Han, Q.; Dong, L.; Jiang, T.; Jiang, B.; et al. SVCT-2 determines the sensitivity to ascorbate-induced cell death in cholangiocarcinoma cell lines and patient derived xenografts. Cancer Lett. 2017, 398, 1–11. [Google Scholar] [CrossRef]
- Liu, B.; Herve, J.; Bioulac-Sage, P.; Valogne, Y.; Roux, J.; Yilmaz, F.; Boisgard, R.; Guettier, C.; Cales, P.; Tavitian, B.; et al. Sodium iodide symporter is expressed at the preneoplastic stages of liver carcinogenesis and in human cholangiocarcinoma. Gastroenterology 2007, 132, 1495–1503. [Google Scholar] [CrossRef]
- Herraez, E.; Lozano, E.; Macias, R.I.; Vaquero, J.; Bujanda, L.; Banales, J.M.; Marin, J.J.; Briz, O. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology 2013, 58, 1065–1073. [Google Scholar] [CrossRef]
- Al-Abdulla, R.; Lozano, E.; Macias, R.I.R.; Monte, M.J.; Briz, O.; O’Rourke, C.J.; Serrano, M.A.; Banales, J.M.; Avila, M.A.; Martinez-Chantar, M.L.; et al. Epigenetic events involved in organic cation transporter 1-dependent impaired response of hepatocellular carcinoma to sorafenib. Br. J. Pharmacol. 2019, 176, 787–800. [Google Scholar] [CrossRef]
- Urtasun, N.; Boces-Pascual, C.; Boix, L.; Bruix, J.; Pastor-Anglada, M.; Perez-Torras, S. Role of drug-dependent transporter modulation on the chemosensitivity of cholangiocarcinoma. Oncotarget 2017, 8, 90185–90196. [Google Scholar] [CrossRef] [Green Version]
- Lagas, J.S.; Van Waterschoot, R.A.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. Cancer Ther. 2010, 9, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Tepsiri, N.; Chaturat, L.; Sripa, B.; Namwat, W.; Wongkham, S.; Bhudhisawasdi, V.; Tassaneeyakul, W. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J. Gastroenterol. 2005, 11, 2748–2753. [Google Scholar] [CrossRef]
- Polgar, O.; Bates, S.E. ABC transporters in the balance: Is there a role in multidrug resistance? Biochem. Soc. Trans. 2005, 33, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updat. 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.; Kang, M.; Meadows, B.; Bakke, S.; Choyke, P.; Merino, M.; Goldspiel, B.; Chico, I.; Smith, T.; Chen, C.; et al. A Phase I study of infusional vinblastine in combination with the P-glycoprotein antagonist PSC 833 (valspodar). Cancer 2001, 92, 1577–1590. [Google Scholar] [CrossRef]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Marienfeld, C.; Tadlock, L.; Yamagiwa, Y.; Patel, T. Inhibition of cholangiocarcinoma growth by tannic acid. Hepatology 2003, 37, 1097–1104. [Google Scholar] [CrossRef]
- Naus, P.J.; Henson, R.; Bleeker, G.; Wehbe, H.; Meng, F.; Patel, T. Tannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathways. J. Hepatol. 2007, 46, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.L.; Shen, D.Y.; Cai, C.F.; Zhang, Q.Y.; Ren, H.Y.; Chen, Q.X. beta-escin reverses multidrug resistance through inhibition of the GSK3beta/beta-catenin pathway in cholangiocarcinoma. World J. Gastroenterol. 2015, 21, 1148–1157. [Google Scholar] [CrossRef]
- Hahnvajanawong, C.; Wattanawongdon, W.; Chomvarin, C.; Anantachoke, N.; Kanthawong, S.; Sripa, B.; Reutrakul, V. Synergistic effects of isomorellin and forbesione with doxorubicin on apoptosis induction in human cholangiocarcinoma cell lines. Cancer Cell Int. 2014, 14, 68. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.; Feng, T.; Ke, Q.; Fan, N.; Li, L.; Li, Z.; Dong, C.; Wang, C.; Xu, F.; Li, Y.; et al. Metformin inhibits proliferation and enhances chemosensitivity of intrahepatic cholangiocarcinoma cell lines. Oncol. Rep. 2014, 31, 2611–2618. [Google Scholar] [CrossRef]
- Seeree, P.; Janvilisri, T.; Kangsamaksin, T.; Tohtong, R.; Kumkate, S. Downregulation of ABCA1 and ABCG1 transporters by simvastatin in cholangiocarcinoma cells. Oncol. Lett. 2019, 18, 5173–5184. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.H.; Ma, Y.L.; He, Y.P.; Zhang, P.; Zhou, Y.K.; Qin, H. Tamoxifen reverses the multi-drug-resistance of an established human cholangiocarcinoma cell line in combined chemotherapeutics. Mol. Biol. Rep. 2011, 38, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.L.; Tiwari, A.K.; Wu, C.P.; Su, X.D.; Wang, S.R.; Liu, D.G.; Ashby, C.R., Jr.; Huang, Y.; Robey, R.W.; Liang, Y.J.; et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008, 68, 7905–7914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, A.K.; Sodani, K.; Wang, S.R.; Kuang, Y.H.; Ashby, C.R., Jr.; Chen, X.; Chen, Z.S. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem. Pharmacol. 2009, 78, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Peng, X.X.; Kim, I.W.; Shukla, S.; Si, Q.S.; Robey, R.W.; Bates, S.E.; Shen, T.; Ashby, C.R., Jr.; Fu, L.W.; et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007, 67, 11012–11102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, J.T.; Ganguly, S.S.; Bennett, H.; Friend, J.W.; Tepe, J.; Plattner, R. Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-kappaB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS ONE 2013, 8, e55509. [Google Scholar] [CrossRef] [Green Version]
- Seubwai, W.; Vaeteewoottacharn, K.; Kraiklang, R.; Umezawa, K.; Okada, S.; Wongkham, S. Inhibition of NF-kappaB Activity Enhances Sensitivity to Anticancer Drugs in Cholangiocarcinoma Cells. Oncol. Res. 2016, 23, 21–28. [Google Scholar] [CrossRef]
- Wang, C.; Ye, H.; Zhang, L.; Cheng, Y.; Xu, S.; Zhang, P.; Zhang, Z.; Bai, J.; Meng, F.; Zhong, L.; et al. Enhanced expression of ten-eleven translocation 1 reverses gemcitabine resistance in cholangiocarcinoma accompanied by a reduction in P-glycoprotein expression. Cancer Med. 2019, 8, 990–1003. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Xia, X.; Ji, J.; Ma, J.; Tao, L.; Mo, L.; Chen, W. MiR-199a-3p enhances cisplatin sensitivity of cholangiocarcinoma cells by inhibiting mTOR signaling pathway and expression of MDR1. Oncotarget 2017, 8, 33621–33630. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.Y.; Zhang, W.; Zeng, X.; Liu, C.Q. Inhibition of Wnt/beta-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013, 104, 1303–1308. [Google Scholar] [CrossRef]
| Usefulness | Gene | Protein | Levels in CCA | Levels in HCC | Potential Interest/Evidences |
|---|---|---|---|---|---|
| Diagnosis | SLC10A2 | ASBT | Mild | N.D. | To distinguish CCA and HCC/in vitro/in vivo/IHC |
| SLC2A1 | GLUT1 | High | Low | To distinguish CCA and HCC/IHC | |
| AQP1 | AQP-1 | High | Low | To distinguish CCA and HCC/IHC | |
| Response to chemotherapy | SLC29A1 | ENT1 | Variable | Variable | Prediction of response to nucleoside analogues/Expression associated with gemcitabine response in patients |
| SLC22A1 | OCT1 | Low | Low | Prediction of response to sorafenib/Expression and location associated with sorafenib response in patients | |
| SLC31A1 | CTR1 | Low | Variable | Prediction of response to Pt derivatives/Expression related with drug response | |
| AQP5 | AQP-5 | High | High | Prognosis and drug sensitivity to gemcitabine/IHC/Expression related with drug response | |
| ABCC3 | MRP3 | High | Low | Biomarker of drug resistance to sorafenib/in vitro evidences | |
| Prognosis | SLC2A2 | GLUT2 | High | High | Marker of high-grade biliary tumors/IHC |
| FXYD6 | PPH | High | High | Biomarker for favorable outcome in CCA/IHC | |
| ABCB1 | MDR1 | High | High | Biomarker of bad prognosis/IHC | |
| ABCC1 | MRP1 | High | High | Biomarker of bad prognosis/IHC |
| Gene | Protein | Levels a | Substrates | Role | Modulation |
|---|---|---|---|---|---|
| SLC102 | ASBT | Mild | Bile acid derivatives | Drug uptake | in vitro and in vivo evidences |
| SLC22A1 | OCT1 | Low | Sorafenib | Drug uptake | Decitabine and cisplatin temporarily induce its expression. In vitro and in vivo evidences |
| SLC29A1 | ENT1 | Variable | Nucleoside analogs | Drug uptake | Cisplatin temporarily induces its expression. Associated to gemcitabine response in patients |
| SLC7A5 | LAT1 | High | Neutral amino acids | Suppress CCA invasion and migration | JPH203 inhibits its expression in vitro |
| SLC2A1 | GLUT1 | High | Glucose | Inhibition of GLUT1 reduces tumor metabolic activity | Quercetin inhibits GLUT1 in vitro |
| SLC23A2 | SVCT2 | Mild | L-Ascorbic acid | Uptake of L-ascorbic acid induces cytotoxicity | in vitro and in vivo evidences |
| SLC5A5 | NIS | High | 131I | Drug uptake | in vitro and in vivo evidences |
| ABCB1 | MDR1 | High | Doxorubicin, etoposide, paclitaxel, vinblastine, sorafenib | Drug efflux | Expression modulated by verapamil, cyclosporine A, quinine, TKIs, and others. In vitro and in vivo evidences |
| ABCC1 | MRP1 | High | Mitomycin C, gemcitabine, doxorubicine, sorafenib, 5-FU | Drug efflux | Expression modulated by tannic acid, isomorellin and metformin in vitro |
| ABCC2 | MRP2 | Unclear | Mitomycin C, gemcitabine, 5-FU | Drug efflux | In vitro evidences. Expression modulated by tannic acid. |
| ABCC3 | MRP3 | High | Sorafenib | Drug efflux | In vitro evidences |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, J.J.G.; Macias, R.I.R.; Cives-Losada, C.; Peleteiro-Vigil, A.; Herraez, E.; Lozano, E. Plasma Membrane Transporters as Biomarkers and Molecular Targets in Cholangiocarcinoma. Cells 2020, 9, 498. https://doi.org/10.3390/cells9020498
Marin JJG, Macias RIR, Cives-Losada C, Peleteiro-Vigil A, Herraez E, Lozano E. Plasma Membrane Transporters as Biomarkers and Molecular Targets in Cholangiocarcinoma. Cells. 2020; 9(2):498. https://doi.org/10.3390/cells9020498
Chicago/Turabian StyleMarin, Jose J.G., Rocio I.R. Macias, Candela Cives-Losada, Ana Peleteiro-Vigil, Elisa Herraez, and Elisa Lozano. 2020. "Plasma Membrane Transporters as Biomarkers and Molecular Targets in Cholangiocarcinoma" Cells 9, no. 2: 498. https://doi.org/10.3390/cells9020498
APA StyleMarin, J. J. G., Macias, R. I. R., Cives-Losada, C., Peleteiro-Vigil, A., Herraez, E., & Lozano, E. (2020). Plasma Membrane Transporters as Biomarkers and Molecular Targets in Cholangiocarcinoma. Cells, 9(2), 498. https://doi.org/10.3390/cells9020498