Urine-Derived Stem Cells: Applications in Regenerative and Predictive Medicine
Abstract
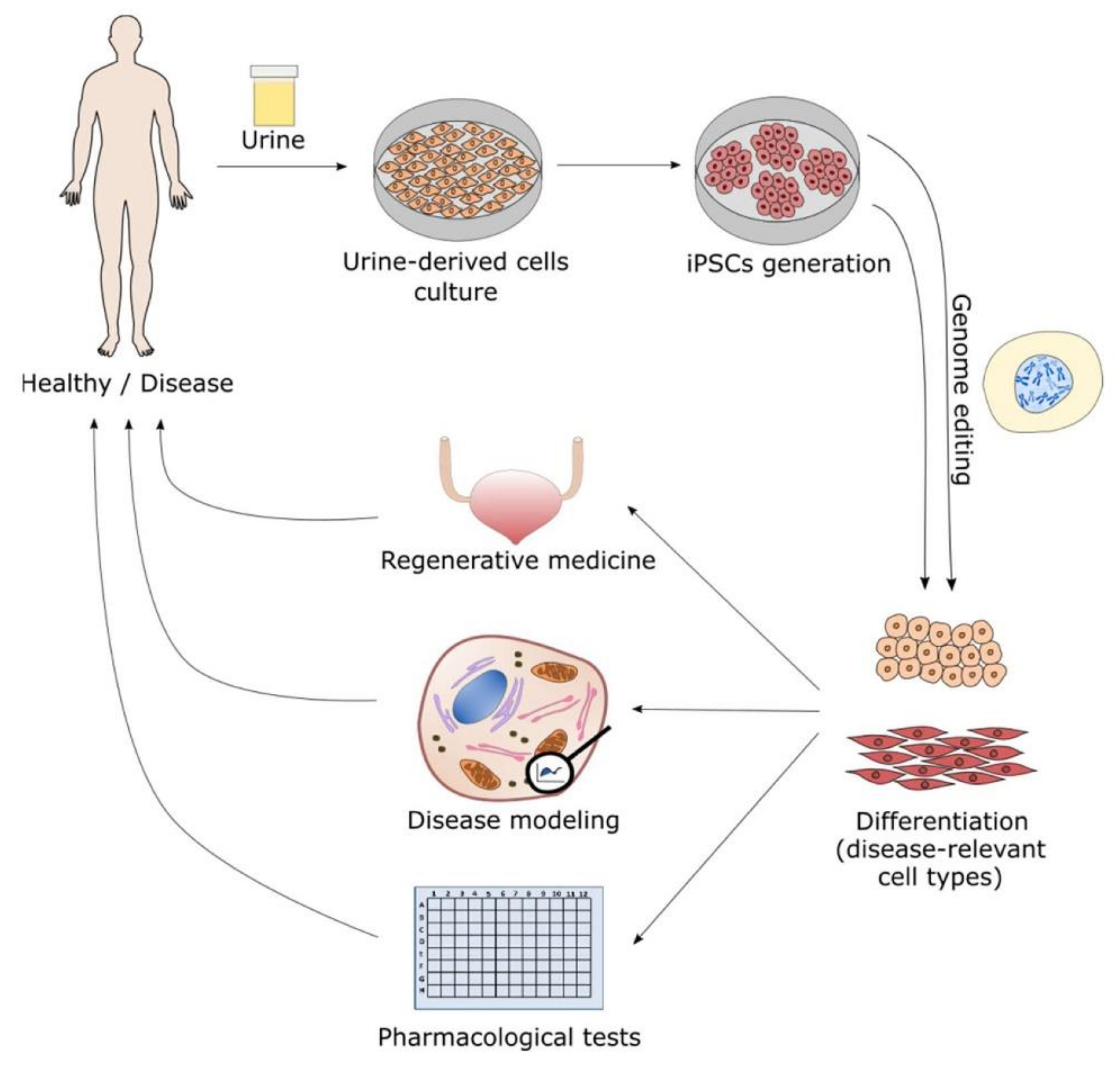
:1. Morphological Characterization and Early Applications of Urine-Derived Stem Cells
2. Generation of iPSC from Urine-Derived Stem Cells (UDSC)
3. Urine-Derived Stem Cells (UDSC) in Drug Discovery Therapeutics and Toxicology
4. Use of Urine-Derived Stem Cells (UDSC) for Regenerative Medicine
4.1. Urological Tissue Engineering
4.2. Bone Regeneration
4.3. Neuroregeneration
4.4. Dental Reconstruction Applications
4.5. Urine-Derived Stem Cells (UDSC) Extracellular Vesicles and Exosomes
5. Potential or Current Utilization of Urine-Derived Stem Cells as Disease Models
6. Advantages and Challenges of Urine-Derived Stem Cells (UDSC)
7. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Adamowicz, J.; Kloskowski, T.; Tworkiewicz, J.; Pokrywczynska, M.; Drewa, T. Urine is a highly cytotoxic agent: does it influence stem cell therapies in urology? Transplant. Proc. 2012, 44, 1439–1441. [Google Scholar] [CrossRef]
- Sutherland, G.R.; Bain, A.D. Culture of cells from the urine of newborn children. Nature 1972, 239, 231. [Google Scholar] [CrossRef] [PubMed]
- Linder, D. Culture of cells from the urine and bladder washings of adults. Somatic. Cell Genet. 1976, 2, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.S.; Littlefield, J.W. Urinary tract epithelial cells cultured from human urine. Int. Rev. Cytol. Suppl. 1979, 10, 11–23. [Google Scholar]
- Felix, J.S.; Littlefield, J.W. Human newborn urine as a source of epithelial cells. Birth Defects Orig. Artic. Ser. 1980, 16, 231–237. [Google Scholar]
- Felix, J.S.; Sun, T.T.; Littlefield, J.W. Human epithelial cells cultured from urine: growth properties and keratin staining. In Vitro 1980, 16, 866–874. [Google Scholar] [CrossRef]
- Herz, F.; Schermer, A.; Koss, L.G. Short-term culture of epithelial cells from urine of adults. Proc. Soc. Exp. Biol. Med. 1979, 161, 153–157. [Google Scholar] [CrossRef]
- Herz, F. Culture of urinary cells. Birth Defects Orig. Artic. Ser. 1980, 16, 85–93. [Google Scholar]
- Hintz, D.S.; Sens, M.A.; Jenkins, M.Q.; Sens, D.A. Tissue culture of epithelial cells from urine. I. Serum-free growth of cells from newborn infants. Pediatr Pathol. 1984, 2, 153–163. [Google Scholar] [CrossRef]
- Johnson, M.D.; Bryan, G.T.; Reznikoff, C.A. Serial cultivation of normal rat bladder epithelial cells in vitro. J. Urol. 1985, 133, 1076–1081. [Google Scholar] [CrossRef]
- Howlett, A.R.; Hodges, G.M.; Rowlatt, C. Epithelial-stromal interactions in the adult bladder: urothelial growth, differentiation, and maturation on culture facsimiles of bladder stroma. Dev. Biol. 1986, 118, 403–415. [Google Scholar] [CrossRef]
- Reznikoff, C.A.; Loretz, L.J.; Pesciotta, D.M.; Oberley, T.D.; Ignjatovic, M.M. Growth kinetics and differentiation in vitro of normal human uroepithelial cells on collagen gel substrates in defined medium. J. Cell Physiol. 1987, 131, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Southgate, J.; Hutton, K.A.; Thomas, D.F.; Trejdosiewicz, L.K. Normal human urothelial cells in vitro: Proliferation and induction of stratification. Lab. Invest. 1994, 71, 583–594. [Google Scholar] [PubMed]
- Ludwikowski, B.; Zhang, Y.Y.; Frey, P. The long-term culture of porcine urothelial cells and induction of urothelial stratification. Bju. Int. 1999, 84, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Chlapowski, F.J. Long term growth and maintenance of stratified rat urothelium in vitro. Cell Tissue Kinet. 1989, 22, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, J.L.; Leigh, I.M.; Duffy, P.G.; Masters, J.R. Culture and characterisation of human urothelium in vivo and in vitro. Urol. Res. 1994, 22, 67–74. [Google Scholar] [CrossRef]
- Gustafson, C.J.; Eldh, J.; Kratz, G. Culture of human urothelial cells on a cell-free dermis for autotransplantation. Eur. Urol. 1998, 33, 503–506. [Google Scholar] [CrossRef]
- Baer, P.C.; Nockher, W.A.; Haase, W.; Scherberich, J.E. Isolation of proximal and distal tubule cells from human kidney by immunomagnetic separation. Technical note. Kidney Int. 1997, 52, 1321–1331. [Google Scholar] [CrossRef] [Green Version]
- Dorrenhaus, A.; Muller, J.I.; Golka, K.; Jedrusik, P.; Schulze, H.; Follmann, W. Cultures of exfoliated epithelial cells from different locations of the human urinary tract and the renal tubular system. Arch. Toxicol. 2000, 74, 618–626. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132. [Google Scholar] [CrossRef]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef]
- Pavathuparambil Abdul Manaph, N.; Al-Hawwas, M.; Bobrovskaya, L.; Coates, P.T.; Zhou, X.F. Urine-derived cells for human cell therapy. Stem Cell Res. Ther. 2018, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Zhang, J.; Guo, S.; Zhu, H.; Zhu, Z.; Li, H.; Wang, Y.; Zhang, C.; Chang, J. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/beta-catenin signaling pathway. Biomaterials 2015, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Pareta, R.A.; Wu, R.; Shi, Y.; Zhou, X.; Liu, H.; Deng, C.; Sun, X.; Atala, A.; Opara, E.C.; et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials 2013, 34, 1311–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, S.Y.; Kim, H.T.; Lee, J.S.; Kim, M.J.; Kim, B.S.; Kim, B.W.; Kwon, T.G. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology 2012, 79, 1186.e1181–1187.e1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Ludwikowski, B.; Hurst, R.; Frey, P. Expansion and long-term culture of differentiated normal rat urothelial cells in vitro. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 419–429. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Frey, P. Growth of cultured human urothelial cells into stratified urothelial sheet suitable for autografts. Adv. Exp. Med. Biol. 2003, 539, 907–920. [Google Scholar]
- Fossum, M.; Lundberg, F.; Holmberg, K.; Schoumans, J.; Kratz, G.; Nordenskjold, A. Long-term culture of human urothelial cell--a qualitative analysis. Cells Tissues Organs 2005, 181, 11–22. [Google Scholar] [CrossRef]
- Guan, J.J.; Niu, X.; Gong, F.X.; Hu, B.; Guo, S.C.; Lou, Y.L.; Zhang, C.Q.; Deng, Z.F.; Wang, Y. Biological characteristics of human-urine-derived stem cells: potential for cell-based therapy in neurology. Tissue Eng. Part A 2014, 20, 1794–1806. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.; Liu, G.; Shi, Y.; Bharadwaj, S.; Leng, X.; Zhou, X.; Liu, H.; Atala, A.; Zhang, Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS ONE 2013, 8, e53980. [Google Scholar] [CrossRef] [Green Version]
- Kloskowski, T.; Nowacki, M.; Pokrywczynska, M.; Drewa, T. Urine—A waste or the future of regenerative medicine? Med. Hypotheses 2015, 84, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856. [Google Scholar] [CrossRef]
- Sato, M.; Takizawa, H.; Nakamura, A.; Turner, B.J.; Shabanpoor, F.; Aoki, Y. Application of Urine-Derived Stem Cells to Cellular Modeling in Neuromuscular and Neurodegenerative Diseases. Front. Mol. Neurosci. 2019, 12, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef]
- Penny, J.; Harris, P.; Shakesheff, K.M.; Mobasheri, A. The biology of equine mesenchymal stem cells: phenotypic characterization, cell surface markers and multilineage differentiation. Front. Biosci. 2012, 17, 892–908. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, L.; Huang, Y.Z.; Huang, Y.; Parolini, O.; Zhong, Q.; Tian, X.; Deng, L. Comparison of the Proliferation and Differentiation Potential of Human Urine-, Placenta Decidua Basalis-, and Bone Marrow-Derived Stem Cells. Stem Cells Int. 2018, 2018, 7131532. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Xing, F.; Peng, J.; Peng, K.; Wang, Y.; Xiang, Z. Human Urine-Derived Stem Cells: Potential for Cell-Based Therapy of Cartilage Defects. Stem Cells Int. 2018, 2018, 4686259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, J.; Zhang, J.; Li, H.; Zhu, Z.; Guo, S.; Niu, X.; Wang, Y.; Zhang, C. Human Urine Derived Stem Cells in Combination with beta-TCP Can Be Applied for Bone Regeneration. PLoS ONE 2015, 10, e0125253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, G.; Li, P.; Zhou, X.; Zhang, Y. Urine-derived stem cells: A novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014, 1, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.S.; Choi, S.H.; Kim, B.S.; Choi, J.Y.; Park, G.B.; Kwon, T.G.; Chun, S.Y. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J. Korean Med. Sci. 2015, 30, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Gaignerie, A.; Lefort, N.; Rousselle, M.; Forest-Choquet, V.; Flippe, L.; Francois-Campion, V.; Girardeau, A.; Caillaud, A.; Chariau, C.; Francheteau, Q.; et al. Urine-derived cells provide a readily accessible cell type for feeder-free mRNA reprogramming. Sci. Rep. 2018, 8, 14363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.Y.; Chun, S.Y.; Ha, Y.S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Potency of Human Urine-Derived Stem Cells for Renal Lineage Differentiation. Tissue Eng. Regen. Med. 2017, 14, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, T.; Liu, Q.; Zhu, J.; Zhao, J.; Li, J.; Sun, B.; Ding, G.; Hu, X.; Yang, Z.; et al. Beneficial effects of urine-derived stem cells on fibrosis and apoptosis of myocardial, glomerular and bladder cells. Mol. Cell. Endocrinol. 2016, 427, 21–32. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, H.; Dong, X.; Liu, Q.; Wu, C.; Zhang, T.; Hu, X.; Zhang, Y.; Song, B.; Li, L. Therapeutic effect of urine-derived stem cells for protamine/lipopolysaccharide-induced interstitial cystitis in a rat model. Stem Cell Res. Ther. 2017, 8, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Han, P.; Jiang, D.; Yang, S.; Cui, Q.; Li, Z. Effects of the donor age on proliferation, senescence and osteogenic capacity of human urine-derived stem cells. Cytotechnology 2017, 69, 751–763. [Google Scholar] [CrossRef]
- Fossum, M.; Skikuniene, J.; Orrego, A.; Nordenskjold, A. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr. 2012, 101, 755–760. [Google Scholar] [CrossRef]
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.; Li, X.; Li, Y.; Guo, X.; Cao, G.; Chen, S.; Hao, L.; et al. Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. 2011, 22, 1221–1228. [Google Scholar] [CrossRef]
- Jouni, M.; Si-Tayeb, K.; Es-Salah-Lamoureux, Z.; Latypova, X.; Champon, B.; Caillaud, A.; Rungoat, A.; Charpentier, F.; Loussouarn, G.; Baro, I.; et al. Toward Personalized Medicine: Using Cardiomyocytes Differentiated From Urine-Derived Pluripotent Stem Cells to Recapitulate Electrophysiological Characteristics of Type 2 Long QT Syndrome. J. Am. Heart Assoc. 2015, 4, e002159. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Wu, F.; Liu, H.; Gao, G.; Kou, S.; Yang, F.; Abbas, N.; Zhou, T.; Cai, X.; Zhang, H.; et al. Generation of non-integrated induced pluripotent stem cells from a 59-year-old female with multiple endocrine neoplasia type 1 syndrome. Stem Cell Res. 2017, 18, 64–66. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Li, S.; Xue, H.; Schmitt, K.; Hergenroeder, G.W.; Wu, J.; Zhang, Y.; Kim, D.H.; Cao, Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Strande, J.L. Generation of induced pluripotent stem cells from muscular dystrophy patients: Efficient integration-free reprogramming of urine derived cells. J. Vis. Exp. 2015, 18, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Y, M.L.; Zampieri, B.L.; Scott-McKean, J.J.; Johnson, M.W.; Costa, A.C.S. Generation of Integration-Free Induced Pluripotent Stem Cells from Urine-Derived Cells Isolated from Individuals with Down Syndrome. Stem Cells Transl. Med. 2017, 6, 1465–1476. [Google Scholar]
- Chen, Y.; Luo, R.; Xu, Y.; Cai, X.; Li, W.; Tan, K.; Huang, J.; Dai, Y. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol. Int. 2013, 33, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, X.; Sun, X.; Deng, C.; Atala, A.; Zhang, Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 2013, 34, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem cells From Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Cai, X.; Wang, L.; Liao, B.; Zhang, H.; Shan, Y.; Chen, Q.; Zhou, T.; Li, X.; Hou, J.; et al. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS ONE 2013, 8, e70573. [Google Scholar] [CrossRef]
- Brouwer, M.; Zhou, H.; Nadif Kasri, N. Choices for Induction of Pluripotency: Recent Developments in Human Induced Pluripotent Stem Cell Reprogramming Strategies. Stem Cell Rev. Rep. 2016, 12, 54–72. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012, 7, 2080–2089. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Guan, C.; Zhao, Z.; Li, Q.; Yang, J.; Mo, J.; Wang, B.; Wu, W.; Yang, X.; et al. Using low-risk factors to generate non-integrated human induced pluripotent stem cells from urine-derived cells. Stem Cell Res. Ther. 2017, 8, 245. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, J.C.; Sander, M.; Bus, J.S.; FutureTox Organizing, C. FutureTox: Building the road for 21st century toxicology and risk assessment practices. Toxicol. Sci. 2014, 137, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, T.B.; Keller, D.A.; Sander, M.; Carney, E.W.; Doerrer, N.G.; Eaton, D.L.; Fitzpatrick, S.C.; Hastings, K.L.; Mendrick, D.L.; Tice, R.R.; et al. FutureTox II: In vitro data and in silico models for predictive toxicology. Toxicol. Sci. 2015, 143, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Jiang, D.; Liu, W.; Li, H.; Li, Z. Urine-derived Stem Cells, a New Source of Seed Cells for Tissue Engineering. Curr. Stem Cell Res. Ther. 2016, 11, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Mack, D.L.; Moreno, C.M.; Strande, J.L.; Mathieu, J.; Shi, Y.; Markert, C.D.; Wang, Z.; Liu, G.; Lawlor, M.W.; et al. Dystrophin-deficient cardiomyocytes derived from human urine: New biologic reagents for drug discovery. Stem Cell Res. 2014, 12, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Djouad, F.; Bouffi, C.; Ghannam, S.; Noel, D.; Jorgensen, C. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 392–399. [Google Scholar] [CrossRef]
- Pei, M.; Li, J.; Zhang, Y.; Liu, G.; Wei, L.; Zhang, Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014, 356, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Chen, X.; Zheng, T.; Han, D.; Zhang, H.; Shi, Y.; Bian, J.; Sun, X.; Xia, K.; Liang, X.; et al. Transplantation of Human Urine-Derived Stem Cells Transfected with Pigment Epithelium-Derived Factor to Protect Erectile Function in a Rat Model of Cavernous Nerve Injury. Cell Transplant. 2016, 25, 1987–2001. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomedicine 2014, 9, 2469–2478. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Ma, Y.; Chen, F.; Wang, S.; Chen, B.; Shi, J. miR-133b and miR-199b knockdown attenuate TGF-beta1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur. J. Pharmacol. 2018, 837, 96–104. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Zhu, Z.; Niu, X.; Guo, S.; Wang, Y.; Zhang, C. Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res. Ther. 2015, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhang, Y.; Liu, P.; Chen, S.; Wu, X.; Sun, Y.; Li, A.; Huang, K.; Luo, R.; Wang, L.; et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen. 2013, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Rao, S.S.; Tan, Y.J.; Luo, M.J.; Hu, X.K.; Yin, H.; Huang, J.; Hu, Y.; Luo, Z.W.; Liu, Z.Z.; et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, B.; Xie, Y.; Zhang, C.; Deng, C.; Lv, L.; Yao, J.; Zhang, Y.; Liu, G.; Deng, J.; Deng, C. Extracellular Vesicles From Human Urine-Derived Stem Cells Ameliorate Erectile Dysfunction in a Diabetic Rat Model by Delivering Proangiogenic MicroRNA. Sex. Med. 2019, 7, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.Z.; Liu, Y.M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S.C.; Fan, Y.; Wang, Y.; Wang, N.S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.C.; Yip, H.K.; Shao, P.L.; Wu, S.C.; Chen, K.H.; Chen, Y.T.; Yang, C.C.; Sun, C.K.; Kao, G.S.; Chen, S.Y.; et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int. J. Cardiol. 2016, 216, 173–185. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Wang, R.; Lin, M.; Li, L.; Li, L.; Qi, G.; Rong, R.; Xu, M.; Zhu, T. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi 2014, 94, 3298–3303. [Google Scholar]
- Eirin, A.; Zhu, X.Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, H.G.; Kim, B.S.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, J.E.; Kim, H.J.; Ha, S.K.; Park, H.C. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res. Ther. 2015, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. The J. Clin. Invest. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Wang, M.; Chen, F.; Zhou, J. Urine-Derived Stem Cells: The Present and the Future. Stem Cells Int. 2017, 2017, 4378947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, D.L.; Guan, X.; Wagoner, A.; Walker, S.J.; Childers, M.K. Disease-in-a-dish: The contribution of patient-specific induced pluripotent stem cell technology to regenerative rehabilitation. Am. J. Phys. Med. Rehabil. 2014, 93, S155–S168. [Google Scholar] [CrossRef] [PubMed]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170–182. [Google Scholar] [CrossRef]
- Jia, B.; Chen, S.; Zhao, Z.; Liu, P.; Cai, J.; Qin, D.; Du, J.; Wu, C.; Chen, Q.; Cai, X.; et al. Modeling of hemophilia A using patient-specific induced pluripotent stem cells derived from urine cells. Life Sci. 2014, 108, 22–29. [Google Scholar] [CrossRef]
- Shi, L.; Cui, Y.; Luan, J.; Zhou, X.; Han, J. Urine-derived induced pluripotent stem cells as a modeling tool to study rare human diseases. Intractable Rare Dis. Res. 2016, 5, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Park, C.Y.; Kim, D.H.; Son, J.S.; Sung, J.J.; Lee, J.; Bae, S.; Kim, J.H.; Kim, D.W.; Kim, J.S. Functional Correction of Large Factor VIII Gene Chromosomal Inversions in Hemophilia A Patient-Derived iPSCs Using CRISPR-Cas9. Cell Stem Cell 2015, 17, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Billings, P.C.; Fiori, J.L.; Bentwood, J.L.; O’Connell, M.P.; Jiao, X.; Nussbaum, B.; Caron, R.J.; Shore, E.M.; Kaplan, F.S. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J. Bone Miner. Res. 2008, 23, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, L.; Rossbach, B.; Kuhnen, P.; Gossen, M.; Kurtz, A.; Reinke, P.; Seemann, P.; Stachelscheid, H. Generation of integration free induced pluripotent stem cells from fibrodysplasia ossificans progressiva (FOP) patients from urine samples. Stem Cell Res. 2016, 16, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Hei, F.; Ju, Z.; Yu, J.; Yang, S.; Chen, M. Differentiation of Urine-Derived Human Induced Pluripotent Stem Cells to Alveolar Type II Epithelial Cells. Cell. Reprogram. 2016, 18, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Page, P.; Dellefave-Castillo, L.M.; McNally, E.M.; Wyatt, E.J. Direct reprogramming of urine-derived cells with inducible MyoD for modeling human muscle disease. Skelet. Muscle 2016, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzarano, M.S.; D’Amario, D.; Siracusano, A.; Massetti, M.; Amodeo, A.; La Neve, F.; Maroni, C.R.; Mercuri, E.; Osman, H.; Scotton, C.; et al. Duchenne Muscular Dystrophy Myogenic Cells from Urine-Derived Stem Cells Recapitulate the Dystrophin Genotype and Phenotype. Hum. Gene Ther. 2016, 27, 772–783. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Zhang, S.; Gu, Y.; Yu, L.; Wu, J.; Gao, T.; Chen, F. Generation and characterization of human cryptorchid-specific induced pluripotent stem cells from urine. Stem Cells Dev. 2013, 22, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Si-Tayeb, K.; Idriss, S.; Champon, B.; Caillaud, A.; Pichelin, M.; Arnaud, L.; Lemarchand, P.; Le May, C.; Zibara, K.; Cariou, B. Urine-sample-derived human induced pluripotent stem cells as a model to study PCSK9-mediated autosomal dominant hypercholesterolemia. Dis. Model. Mech. 2016, 9, 81–90. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Zhu, W.; Cao, Q.; Shen, Y.; Zhou, Q.; Yu, P.; Liu, X.; Ma, J.; Li, Y.; Hong, K. Generation of Mesenchymal-Like Stem Cells From Urine in Pediatric Patients. Transplant. Proc. 2016, 48, 2181–2185. [Google Scholar] [CrossRef]
- Niemietz, C.J.; Sauer, V.; Stella, J.; Fleischhauer, L.; Chandhok, G.; Guttmann, S.; Avsar, Y.; Guo, S.; Ackermann, E.J.; Gollob, J.; et al. Evaluation of Therapeutic Oligonucleotides for Familial Amyloid Polyneuropathy in Patient-Derived Hepatocyte-Like Cells. PLoS ONE 2016, 11, e0161455. [Google Scholar] [CrossRef] [Green Version]
- Schosserer, M.; Reynoso, R.; Wally, V.; Jug, B.; Kantner, V.; Weilner, S.; Buric, I.; Grillari, J.; Bauer, J.W.; Grillari-Voglauer, R. Urine is a novel source of autologous mesenchymal stem cells for patients with epidermolysis bullosa. BMC Res. Notes 2015, 8, 767. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Zhao, R.; Doi, A.; Ng, K.; Unternaehrer, J.; Cahan, P.; Huo, H.; Loh, Y.H.; Aryee, M.J.; Lensch, M.W.; et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1117–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 2015, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, G.; Shafigullina, A.K.; Rizvanov, A.A.; Sardão, V.A.; Macedo, M.P.; Oliveira, P.J. Urine-Derived Stem Cells: Applications in Regenerative and Predictive Medicine. Cells 2020, 9, 573. https://doi.org/10.3390/cells9030573
Bento G, Shafigullina AK, Rizvanov AA, Sardão VA, Macedo MP, Oliveira PJ. Urine-Derived Stem Cells: Applications in Regenerative and Predictive Medicine. Cells. 2020; 9(3):573. https://doi.org/10.3390/cells9030573
Chicago/Turabian StyleBento, Guida, Aygul K. Shafigullina, Albert A. Rizvanov, Vilma A. Sardão, Maria Paula Macedo, and Paulo J. Oliveira. 2020. "Urine-Derived Stem Cells: Applications in Regenerative and Predictive Medicine" Cells 9, no. 3: 573. https://doi.org/10.3390/cells9030573
APA StyleBento, G., Shafigullina, A. K., Rizvanov, A. A., Sardão, V. A., Macedo, M. P., & Oliveira, P. J. (2020). Urine-Derived Stem Cells: Applications in Regenerative and Predictive Medicine. Cells, 9(3), 573. https://doi.org/10.3390/cells9030573






