Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cell Cultures
2.3. Cytotoxicity Assay
2.4. Schedule of Single and Metronomic Treatments
2.5. Combination Assay and Analysis of Sesquiterpene-Drug Interactions
2.6. Intracellular Reactive Oxygen Species (ROS) Determination
2.7. Chromatographic Determination of Intracellular Glutathione Levels
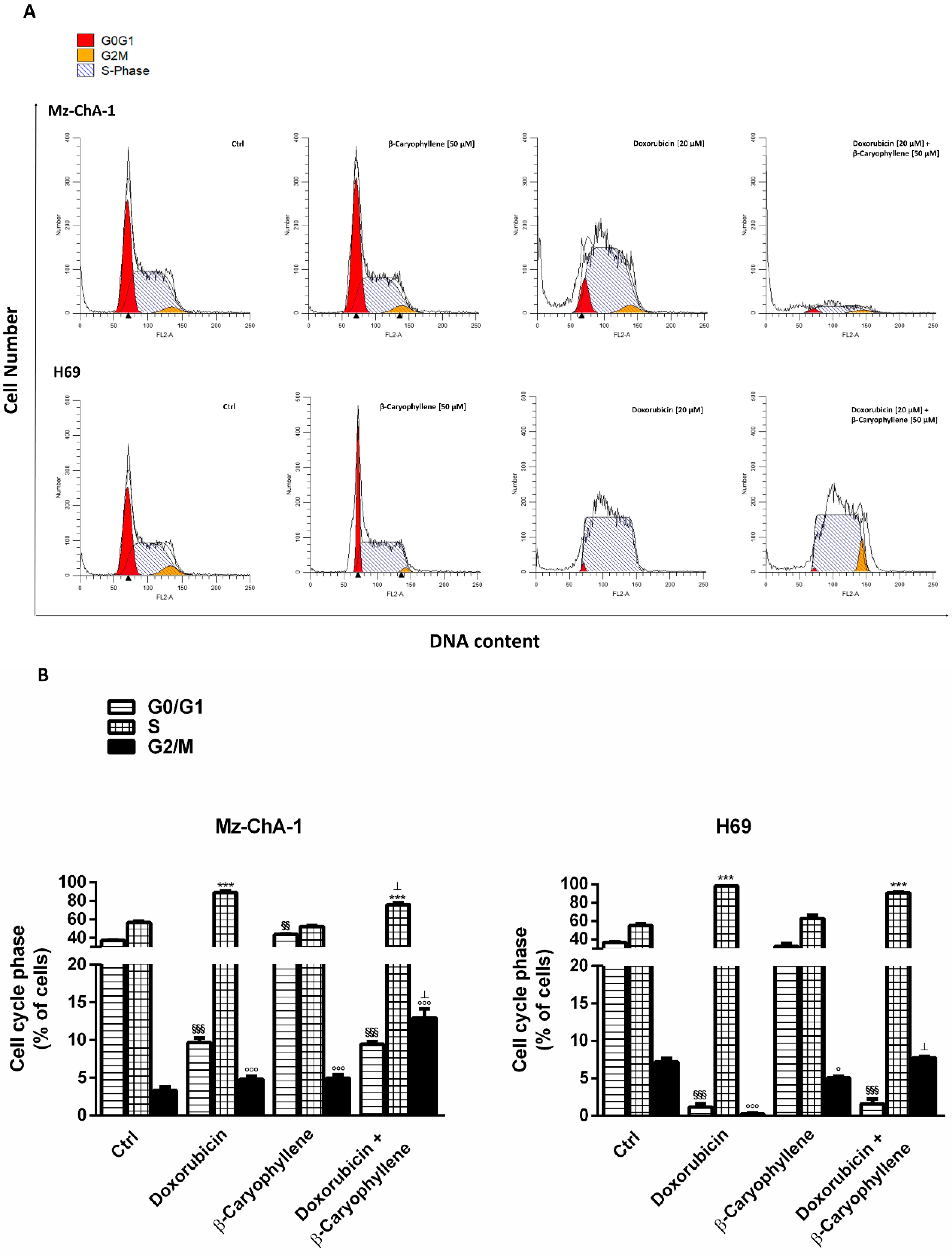
2.8. Cell Cycle Analysis
2.9. Detection of Phosphorylated Histone H2AX and STAT3 by Western Blotting Analysis
2.10. Immunofluorescence
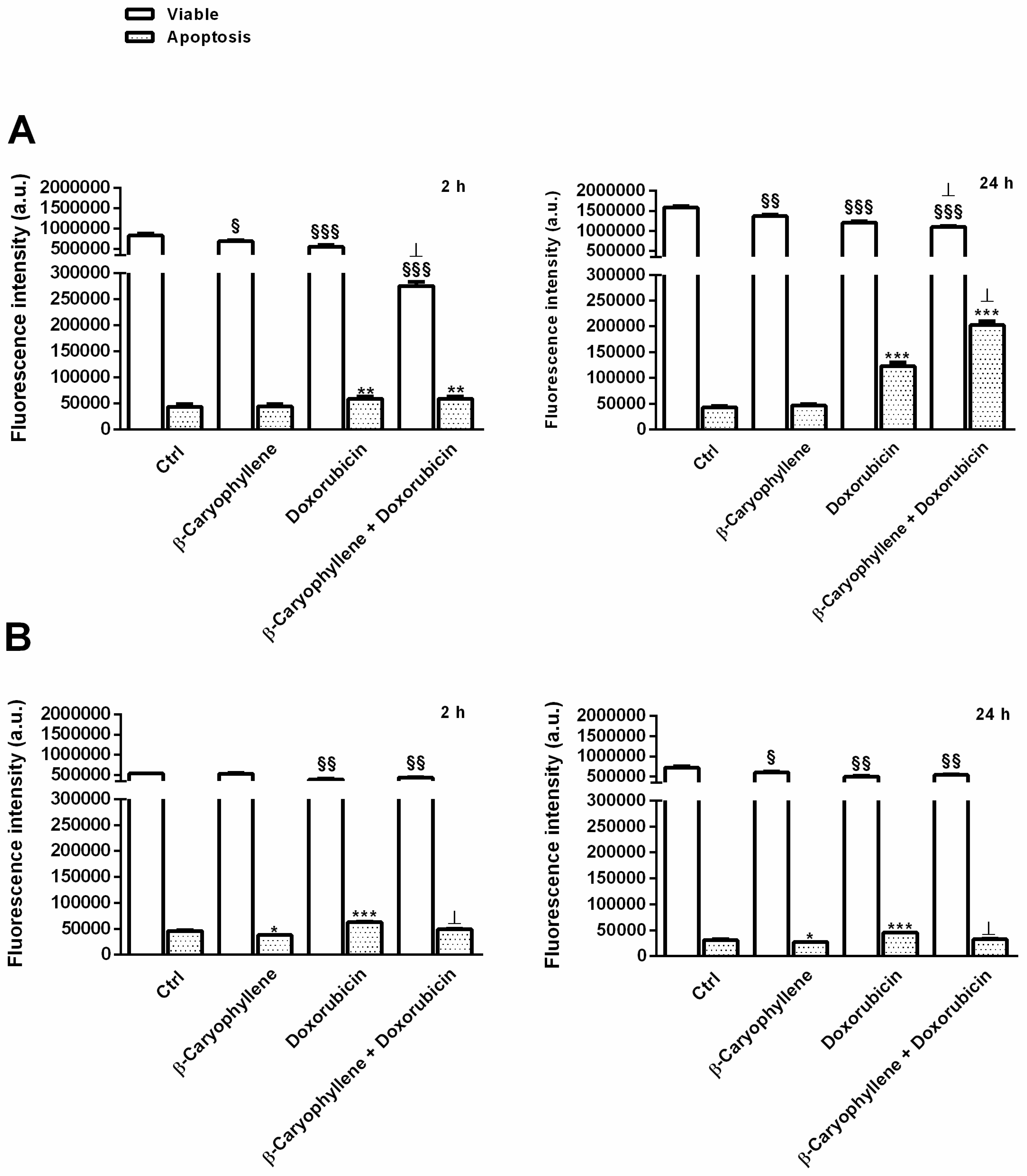
2.11. Apoptosis Detection
2.12. Statistical Analysis
3. Results
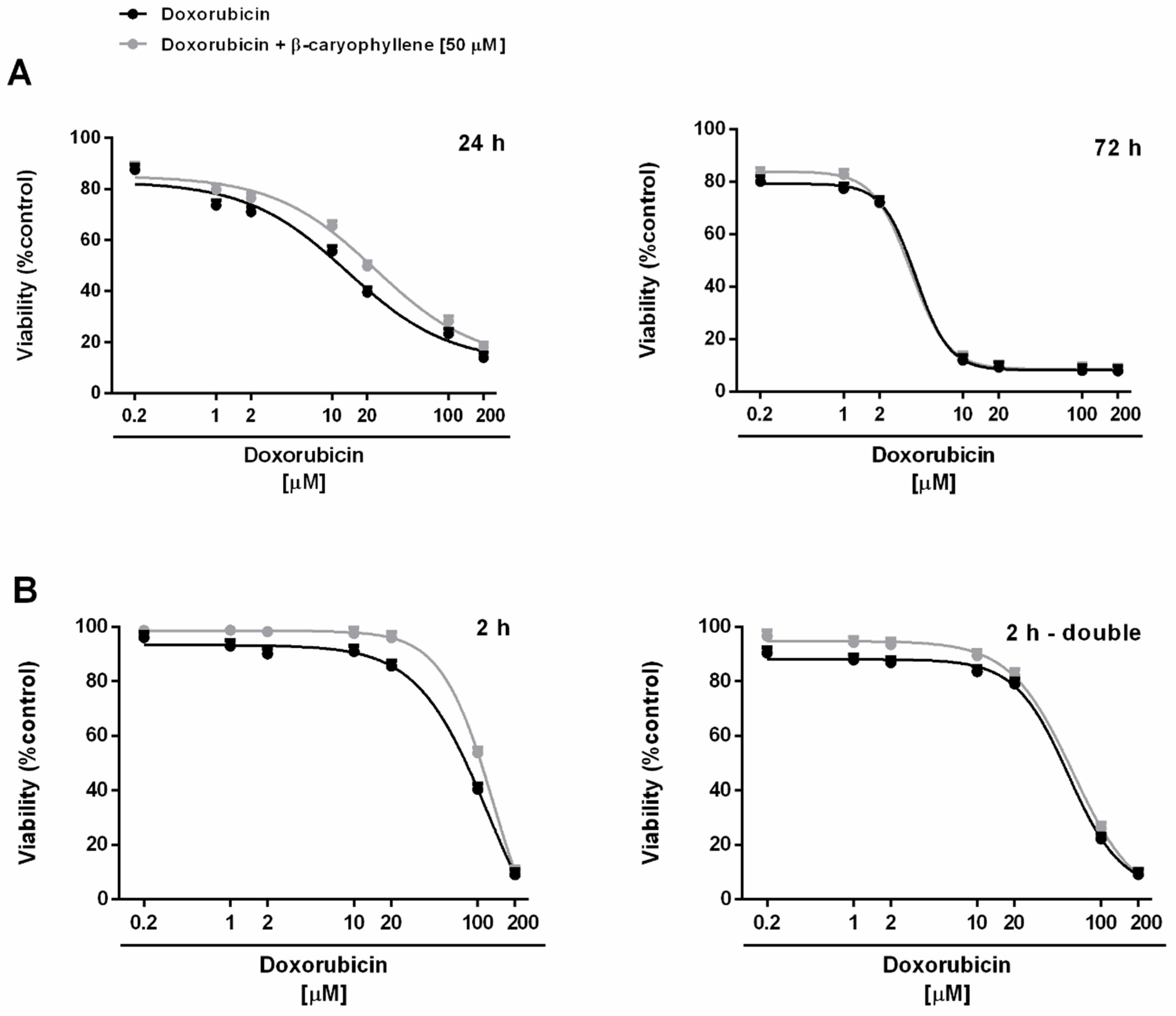
3.1. Cytotoxicity of β-Caryophyllene in Combination with Doxorubicin in Mz-ChA-1 Cholangiocarcinoma Cells and H69 Cholangiocytes
3.2. Genoprotective Effects of β-Caryophyllene in Combination with Doxorubicin
3.3. Effect of β-Caryophyllene in Combination with Doxorubicin on Cell Cycle Progression
3.4. Effect of β-Caryophyllene on the Intracellular Oxidative Stress
3.5. Effect of β-Caryophyllene in Combination with Doxorubicin on Cell Apoptosis
3.6. Effect of β-Caryophyllene in Combination with Doxorubicin on the STAT3 Signaling
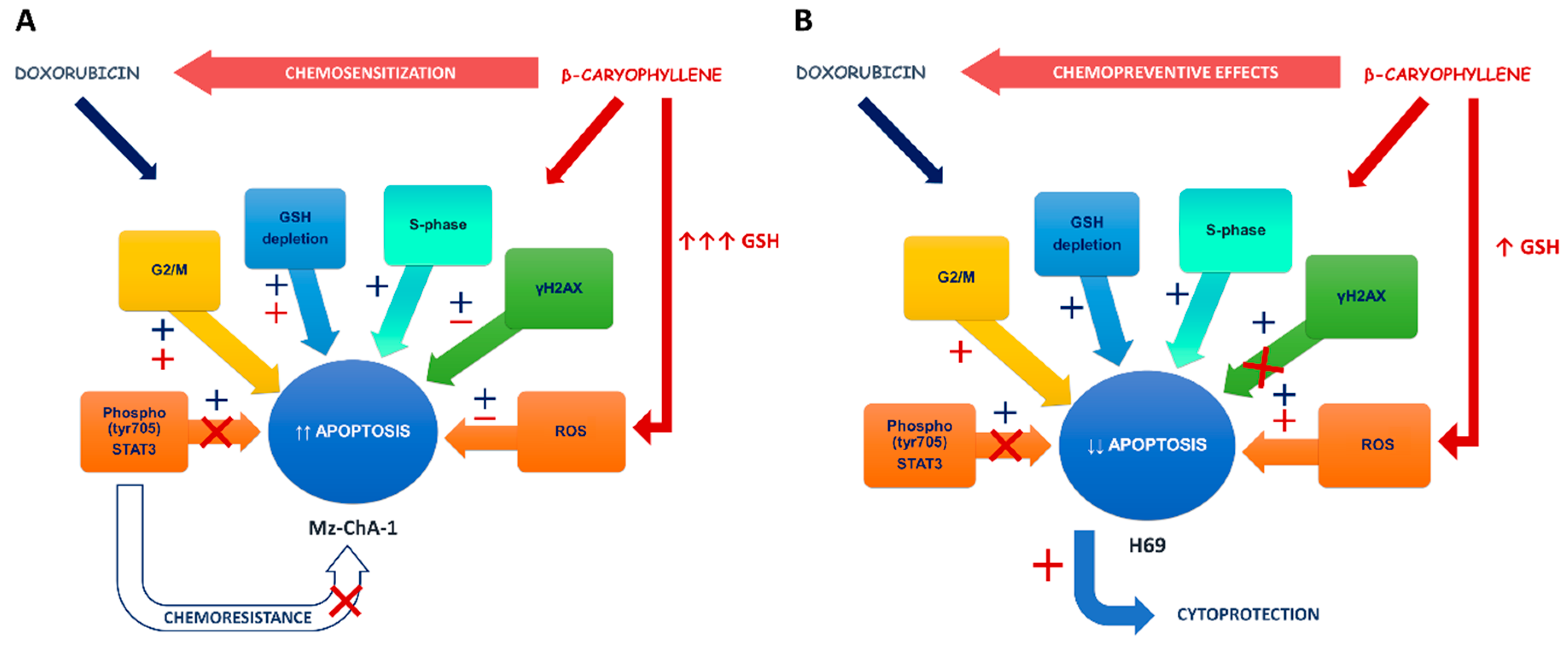
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [Green Version]
- Fava, G.; DeMorrow, S.; Gaudio, E.; Franchitto, A.; Onori, P.; Carpino, G.; Glaser, S.; Francis, H.; Coufal, M.; Marucci, L.; et al. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int. 2009, 29, 1031–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2017, 15, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeva, J.; Sangro, B.; Salati, M.; Edeline, J.; La Casta, A.; Bittoni, A.; Berardi, R.; Bruix, J.; Valle, J.W.; Berardi, R. Medical treatment for cholangiocarcinoma. Liver Int. 2019, 39, 123–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, E.; Macias, R.I.; Monte, M.J.; Asensio, M.; Del Carmen, S.; Sanchez-Vicente, L.; Alonso-Peña, M.; Al-Abdulla, R.; Munoz-Garrido, P.; Satriano, L.; et al. Causes of hOCT1-Dependent Cholangiocarcinoma Resistance to Sorafenib and Sensitization by Tumor-Selective Gene Therapy. Hepatology 2019, 70, 1246–1261. [Google Scholar] [CrossRef] [PubMed]
- Esnaola, N.F.; Meyer, J.; Karachristos, A.; Maranki, J.L.; Camp, E.R.; Denlinger, C.S. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer 2016, 122, 1349–1369. [Google Scholar] [CrossRef] [Green Version]
- Cadamuro, M.; Brivio, S.; Spirli, C.; Joplin, R.; Strazzabosco, M.; Fabris, L. Autocrine and Paracrine Mechanisms Promoting Chemoresistance in Cholangiocarcinoma. Int. J. Mol. Sci. 2017, 18, 149. [Google Scholar] [CrossRef]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef]
- Hanusova, V.; Boušová, I.; Skálová, L. Possibilities to increase the effectiveness of doxorubicin in cancer cells killing. Drug Metab. Rev. 2011, 43, 540–557. [Google Scholar] [CrossRef]
- Swift, L.P.; Rephaeli, A.; Nudelman, A.; Phillips, D.R.; Cutts, S.M. Doxorubicin-DNA Adducts Induce a Non-Topoisomerase II–Mediated Form of Cell Death. Cancer Res. 2006, 66, 4863–4871. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E.; Cho, H.-J.; Kim, J.S.; Shim, C.-K.; Chung, S.-J.; Oak, M.-H.; Yoon, I.-S.; Kim, D.-D. The limited intestinal absorption via paracellular pathway is responsible for the low oral bioavailability of doxorubicin. Xenobiotica 2012, 43, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Capelôa, T.; Benyahia, Z.; Zampieri, L.X.; Blackman, M.C.N.M.; Sonveaux, P. Metabolic andnnon-metabolic pathways that control cancer resistance to anthracyclines. Semin. Cell Dev. Biol. 2019, 98, 181–191. [Google Scholar] [CrossRef]
- Fukuda, A.; Tahara, K.; Hane, Y.; Matsui, T.; Sasaoka, S.; Hatahira, H.; Motooka, Y.; Hasegawa, S.; Naganuma, M.; Abe, J.; et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS ONE 2017, 12, e0185654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, F.; Xiang, J.; Jiang, X.; Chen, J.; Zhang, J. Galactosylated alpha,beta-poly[(2-hydroxyethyl)-L-aspartamide]-bound doxorubicin: Improved antitumor activity against hepatocellular carcinoma with reduced hepatotoxicity. Anti-Cancer Drugs 2011, 22, 136–147. [Google Scholar] [CrossRef]
- Aliberti, C.; Carandina, R.; Sarti, D.; Pizzirani, E.; Ramondo, G.; Mulazzani, L.; Mattioli, G.M.; Fiorentini, G. Chemoembolization with Drug-eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer. Res. 2017, 37, 1859–1863. [Google Scholar]
- André, N.; Tsai, K.; Carré, M.; Pasquier, E. Metronomic Chemotherapy: Direct Targeting of Cancer Cells after all? Trends Cancer 2017, 3, 319–325. [Google Scholar] [CrossRef]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Ciliberto, G.; Buonaguro, F.M.; Buonaguro, L. Combinatorial immunotherapy strategies for hepatocellular carcinoma. Curr. Opin. Immunol. 2016, 39, 103–113. [Google Scholar] [CrossRef]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef]
- Wagner, H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia 2011, 82, 34–37. [Google Scholar] [CrossRef]
- Fouassier, L.; Marzioni, M.; Afonso, M.B.; Dooley, S.; Gaston, K.; Giannelli, G.; Rodrigues, C.M.P.; Lozano, E.; Mancarella, S.; Segatto, O.; et al. Signalling networks in cholangiocarcinoma: Molecular pathogenesis, targeted therapies and drug resistance. Liver Int. 2019, 39, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P.; Slív, D. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Kim, S.-H.; Sethi, G.; Ahn, K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015, 360, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jung, S.; Chinnathambi, A.; Alahmadi, T.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Attenuation of STAT3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing Properties of β-Caryophyllene and β-Caryophyllene Oxide in Combination with Doxorubicin in Human Cancer Cells. Anticancer. Res. 2017, 37, 1191–1196. [Google Scholar] [PubMed] [Green Version]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in HepG2 Cells: An in Vitro and in Silico Study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar]
- Atta-ur-Rahman; Ahmad, V.U. Caryophyllane. In 13C-NMR of Natural Products; Springer: Boston, MA, USA, 1992; pp. 583–584. [Google Scholar]
- European Food Safety Authority (EFSA). Flavouring Group Evaluation 78 (FGE. 78)-Consideration of Aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromatic hydrocarbons evaluated by EFSA in FGE. 25-Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2009, 7, 931. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of eight compounds belonging to chemical group 31 (aliphatic and aromatic hydrocarbons) when used as flavourings for all animal species and categories. EFSA J. 2016, 14, 4339. [Google Scholar]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Castelli, F.; Sarpietro, M.G.; Mazzanti, G. Genotoxicity assessment of β-caryophyllene oxide. Regul. Toxicol. Pharmacol. 2013, 66, 264–268. [Google Scholar]
- Di Sotto, A.; Evandri, M.G.; Mazzanti, G. Antimutagenic and mutagenic activities of some terpenes in the bacterial reverse mutation assay. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 653, 130–133. [Google Scholar]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Inhibition by beta-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2010, 699, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jasso, D.; Álvarez-González, I.; Madrigal-Bujaidar, E. Clastogenicity of beta-caryophyllene in mouse. Boil. Pharm. Bull. 2009, 32, 520–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opdyke, D.L. Monographs on fragrance raw materials: Caryophyllene. Food Cosmet. Toxicol. 1973, 11, 1059–1060. [Google Scholar] [CrossRef]
- Schmitt, D.; Levy, R.; Carroll, B. Toxicological Evaluation of β-Caryophyllene Oil. Int. J. Toxicol. 2016, 35, 558–567. [Google Scholar] [CrossRef]
- Oliveira, G.L.D.S.; Machado, K.C.; Machado, K.C.; Silva, A.P.D.S.C.D.; Feitosa, C.M.; Almeida, F.R.D.C. Non-clinical toxicity of β -caryophyllene, a dietary cannabinoid: Absence of adverse effects in female Swiss mice. Regul. Toxicol. Pharmacol. 2018, 92, 338–346. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β -caryophyllene and β -caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Wang, G.F.; Ma, W.B.; Du, J.W. beta-Caryophyllene (BCP) ameliorates MPP plus induced cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef]
- Viveros-Paredes, J.M.; Gonzalez-Castaneda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; Lopez-Roa, R.I.; Vazquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of beta-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s Disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Hurtado, P.; González-Castañeda, R.E.; Beas-Zarate, C.; Flores-Soto, M.E.; Viveros-Paredes, J.M. β-Caryophyllene Reduces DNA Oxidation and the Overexpression of Glial Fibrillary Acidic Protein in the Prefrontal Cortex and Hippocampus of d-Galactose-Induced Aged BALB/c Mice. J. Med. Food 2019. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Abete, L.; Cocchiola, R.; Mazzanti, G.; Eufemi, M.; Di Sotto, A. Caryophyllane sesquiterpenes inhibit DNA-damage by tobacco smoke in bacterial and mammalian cells. Food Chem. Toxicol. 2018, 111, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Mazzanti, G.; Di Sotto, A. Mutagenicity of cigarette butt waste in the bacterial reverse mutation assay: The protective effects of ?-caryophyllene and ?-caryophyllene oxide. Environ. Toxicol. 2015, 31, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.S.; Chun, H.S.; Kwon, S.H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [Green Version]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar]
- Ramachandhiran, D.; Sankaranarayanan, C.; Murali, R.; Babukumar, S.; Vinothkumar, V. β-Caryophyllene promotes oxidative stress and apoptosis in KB cells through activation of mitochondrial-mediated pathway—An in-vitro and in-silico study. Arch. Physiol. Biochem. 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Zhou, L.; Zhan, M.-L.; Tang, Y.; Xiao, M.; Li, M.; Li, Q.-S.; Yang, L.; Li, X.; Chen, W.-W.; Wang, Y. Effects of β-caryophyllene on arginine ADP-ribosyltransferase 1-mediated regulation of glycolysis in colorectal cancer under high-glucose conditions. Int. J. Oncol. 2018, 53, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, K.; Berneman, Z.N.; Van Bockstaele, D.R. Cell cycle and apoptosis. Cell Prolif. 2003, 36, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, S.P.; Townsend, P.A.; Knight, R.A.; Scarabelli, T.M.; Latchman, D.S.; Stephanou, A. STAT3 modulates the DNA damage response pathway. Int. J. Exp. Pathol. 2010, 91, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Farren, M.R.; Ahn, D.; Bekaii-Saab, T.; Lesinski, G.B. Signaling pathways as therapeutic targets in biliary tract cancer. Expert Opin. Ther. Targets 2017, 21, 485–498. [Google Scholar] [CrossRef]
- Dokduang, H.; Techasen, A.; Namwat, N.; Khuntikeo, N.; Pairojkul, C.; Murakami, Y.; Loilome, W.; Yongvanit, P. STATs profiling reveals predominantly-activated STAT3 in cholangiocarcinoma genesis and progression. J. Hepato-Biliary-Pancreatic Sci. 2014, 21, 767–776. [Google Scholar] [CrossRef]
- Lee, H.-J.; Zhuang, G.; Cao, Y.; Du, P.; Kim, H.-J.; Settleman, J. Drug Resistance via Feedback Activation of Stat3 in Oncogene-Addicted Cancer Cells. Cancer Cell 2014, 26, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Biological evaluation of medical devices Part 5: Tests for invitro cytotoxicity Geneva, ISO 10993–5:2009, 2nd ed.; International Organization for Standardization/ANSI: Geneva, Switzerland, 2009.
- Di Giacomo, S.; Di Sotto, A.; El-Readi, M.Z.; Mazzanti, G.; Wink, M. α-Hexylcinnamaldehyde Synergistically Increases Doxorubicin Cytotoxicity Towards Human Cancer Cell Lines. Anticancer. Res. 2016, 36, 3347–3351. [Google Scholar]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and Antioxidant Activity of a Hydroalcoholic Extract from Humulus lupulus L. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, Antiglycation, and Cytoprotective Properties of a Phenol-Rich Extract From Waste Peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar]
- Mancinelli, R.; Olivero, F.; Carpino, G.; Overi, D.; Rosa, L.; Lepanto, M.S.; Cutone, A.; Franchitto, A.; Alpini, G.; Onori, P.; et al. Role of lactoferrin and its receptors on biliary epithelium. BioMetals 2018, 31, 369–379. [Google Scholar] [CrossRef]
- Wu, N.; Meng, F.; Invernizzi, P.; Bernuzzi, F.; Venter, J.; Standeford, H.; Onori, P.; Marzioni, M.; Alvaro, D.; Franchitto, A.; et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice. Hepatology 2016, 64, 865–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Francesco, E.M.; Rocca, C.; Scavello, F.; Amelio, D.; Pasqua, T.; Rigiracciolo, D.C.; Scarpelli, A.; Avino, S.; Cirillo, F.; Amodio, N.; et al. Protective Role of GPER Agonist G-1 on Cardiotoxicity Induced by Doxorubicin. J. Cell. Physiol. 2017, 232, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Spiotto, M.T.; Chung, T.D. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate 2000, 42, 88–98. [Google Scholar] [CrossRef]
- Jackson, N.M.; Ceresa, B.P. EGFR-mediated apoptosis via STAT3. Exp. Cell Res. 2017, 356, 93–103. [Google Scholar] [CrossRef]
- Marin, J.J.; Briz, O.; Herraez, E.; Lozano, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Osorio-Padilla, L.M.; Santos-Llamas, A.I.; Serrano, M.; et al. Molecular bases of the poor response of liver cancer to chemotherapy. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 182–192. [Google Scholar] [CrossRef]
- Martinez-Becerra, P.; Vaquero, J.; Romero, M.R.; Lozano, E.; Anadon, C.; Macias, R.I.; Serrano, M.; Grañé-Boladeras, N.; Muñoz-Bellvis, L.; Alvarez, L.; et al. No Correlation between the Expression of FXR and Genes Involved in Multidrug Resistance Phenotype of Primary Liver Tumors. Mol. Pharm. 2012, 9, 1693–1704. [Google Scholar] [CrossRef]
- Marin, J.J.; Lozano, E.; Herraez, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Briz, O.; Serrano, M.; Efferth, T.; Macias, R.I. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2018, 1864, 1444–1453. [Google Scholar] [CrossRef]
- Nakaoka, T.; Saito, H.; Saito, H. Aberrant DNA Methylation as a Biomarker and a Therapeutic Target of Cholangiocarcinoma. Int. J. Mol. Sci. 2017, 18, 1111. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Cho, S.K.; Kim, K.-D.; Nam, D.; Chung, W.-S.; Jang, H.-J.; Lee, S.-G.; Shim, B.S.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide potentiates TNFα-induced apoptosis and inhibits invasion through down-modulation of NF-κB-regulated gene products. Apoptosis 2013, 19, 708–718. [Google Scholar] [CrossRef]
- Skarkova, V.; Caltová, K.; Svobodová, H.; Ambroz, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans -nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakawa, Y.; Taira, Z.; Takemoto, T.; Ishida, T.; Kido, M.; Ichikawa, Y. X-Ray Crystal Structure Analysis of 14-Hydroxycaryophyllene Oxide, a New Metabolite of (—)-Caryophyllene, in Rabbits. J. Pharm. Sci. 1981, 70, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ishida, T.; Toyota, M.; Takemoto, T. Terpenoid biotransformation in mammals. IV Biotransformation of (+)-longifolene, (−)-caryophyllene, (−)-caryophyllene oxide, (−)-cyclocolorenone, (+)-nootkatone, (−)-elemol, (−)-abietic acid and (+)-dehydroabietic acid in rabbits. Xenobiotica 1986, 16, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Gazzano, E.; Gulino, G.R.; Volante, M.; Ghigo, D.; Kopecka, J. Two repeated low doses of doxorubicin are more effective than a single high dose against tumors overexpressing P-glycoprotein. Cancer Lett. 2015, 360, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Sterba, M.; Popelová, O.; Vávrová, A.; Jirkovsky, E.; Kovaříková, P.; Geršl, V.; Simunek, T. Oxidative Stress, Redox Signaling, and Metal Chelation in Anthracycline Cardiotoxicity and Pharmacological Cardioprotection. Antioxidants Redox Signal. 2013, 18, 899–929. [Google Scholar] [CrossRef] [Green Version]
- Cappetta, N.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Mallappa, S.; Neeli, P.K.; Karnewar, S.; Kotamraju, S. Doxorubicin induces prostate cancer drug resistance by upregulation of ABCG4 through GSH depletion and CREB activation: Relevance of statins in chemosensitization. Mol. Carcinog. 2019, 58, 1118–1133. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Di Sotto, A.; Accolla, M.L.; Castelli, F. Differential Scanning Calorimetry Study on the Interaction of β-Caryophyllene and β-Caryophyllene Oxide with Phospholipid Bilayers. Thermochim. Acta 2015, 600, 28–34. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, N.C.; Chae, K.H.; Kim, G.; Park, W.S.; Park, Y.-K.; Kim, Y.W. Expression of Multidrug Resistance-Associated Protein 2 in Human Gallbladder Carcinoma. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, S.; Autschbach, F.; Riedel, H.D.; König, J.; Kulaksiz, H.; Stiehl, A.; Riemann, J.F.; Rost, D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human cholangiocellular carcinomas. Eur. J. Clin. Investig. 2008, 38, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Shneider, B.L.; Hofmann, A.F. Bile Formation and the Enterohepatic Circulation. In Physiology of the Gastrointestinal Tract; Elsevier BV: Amsterdam, The Netherlands, 2006; pp. 1437–1462. [Google Scholar]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Deeley, R.G. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006, 27, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.H.; el-Naggar, A.K.; Priebe, W.; Perez-Soler, R. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol. Pharmacol. 1996, 49, 832–841. [Google Scholar] [PubMed]
- Klinakis, A.; Karagiannis, D.; Rampias, T. Targeting DNA repair in cancer: Current state and novel approaches. Cell. Mol. Life Sci. 2019, 77, 677–703. [Google Scholar] [CrossRef]
- Campos, A.; Clemente-Blanco, A. Cell Cycle and DNA Repair Regulation in the Damage Response: Protein Phosphatases Take Over the Reins. Int. J. Mol. Sci. 2020, 21, 446. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Barbash, O.; Diehl, J.A. Regulation of the Cell Cycle. In The Molecular Basis of Cancer (Fourth Edition); Mendelsohn, J., Gray, J.W., Howley, P.M., Israel, M.A., Thompson, C.B., Eds.; Content Repository Only: Philadelphia, PA, USA, 2015; pp. 165–178. [Google Scholar]
- Roos, W.; Thomas, A.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2015, 16, 20–33. [Google Scholar] [CrossRef]
- Shen, J.; Peng, Y.; Wei, L.; Zhang, W.; Yang, L.; Lan, L.; Kapoor, P.; Ju, Z.; Mo, Q.; Shih, I.-M.; et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015, 5, 752–767. [Google Scholar] [CrossRef] [Green Version]
- Irving, J.A.; Hall, A.G. Mismatch repair defects as a cause of resistance to cytotoxic drugs. Expert Rev. Anticancer. Ther. 2001, 1, 149–158. [Google Scholar] [CrossRef]
- De Flora, S. Mechanisms of inhibitors of mutagenesis and carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 151–158. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Fernándezcheca, J.C. Mitochondrial glutathione: Hepatocellular survival–death switch. J. Gastroenterol. Hepatol. 2006, 21, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.; Grandis, J.R. STAT3 SIGNALING: Anticancer Strategies and Challenges. Mol. Interv. 2011, 11, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, M. Cellular and molecular mechanisms of liver regeneration: Proliferation, growth, death and protection of hepatocytes. Semin. Cell Dev. Boil. 2020, 100, 62–73. [Google Scholar] [CrossRef]
- Trécul, A.; Morceau, F.; Dicato, M.; Diederich, M. Dietary compounds as potent inhibitors of the signal transducers and activators of transcription (STAT) 3 regulatory network. Genes Nutr. 2012, 7, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, C.; Lee, S.-G.; Sethi, G.; Ahn, K.S. Ophiopogonin D, a Steroidal Glycoside Abrogates STAT3 Signaling Cascade and Exhibits Anti-Cancer Activity by Causing GSH/GSSG Imbalance in Lung Carcinoma. Cancers 2018, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Talhi, O.; Jang, D.; Cerella, C.; Gaigneaux, A.; Kim, K.-W.; Lee, J.W.; Dicato, M.; Bachari, K.; Han, B.W.; et al. Cytostatic hydroxycoumarin OT52 induces ER/Golgi stress and STAT3 inhibition triggering non-canonical cell death and synergy with BH3 mimetics in lung cancer. Cancer Lett. 2018, 416, 94–108. [Google Scholar] [CrossRef]
- Lee, J.; Rangappa, S.; Mohan, C.; Sethi, G.; Lin, Z.; Rangappa, K.S.; Ahn, K.S. Brusatol, a Nrf2 Inhibitor Targets STAT3 Signaling Cascade in Head and Neck Squamous Cell Carcinoma. Biomolecules 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Li, F.; Manu, K.A.; Shanmugam, M.K.; Loo, S.Y.; Kumar, A.P.; Sethi, G. γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br. J. Pharmacol. 2011, 163, 283–298. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Ong, T.H.; Chen, L.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; et al. Suppression of Signal Transducer and Activator of Transcription 3 Activation by Butein Inhibits Growth of Human Hepatocellular Carcinoma In Vivo. Clin. Cancer Res. 2010, 17, 1425–1439. [Google Scholar] [CrossRef] [Green Version]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.-F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakobwong, S.; Gupta, S.C.; Kim, J.H.; Sung, B.; Pinlaor, P.; Hiraku, Y.; Wongkham, S.; Sripa, B.; Pinlaor, S.; Aggarwal, B.B. Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinog. 2011, 32, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Al-Taee, H.; Azimullah, S.; Meeran, M.N.; Almheiri, M.K.A.; Al Jasmi, R.A.; Tariq, S.; Ab Khan, M.; Adeghate, E.; Ojha, S.K.; Almehiri, M.K.A. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef]

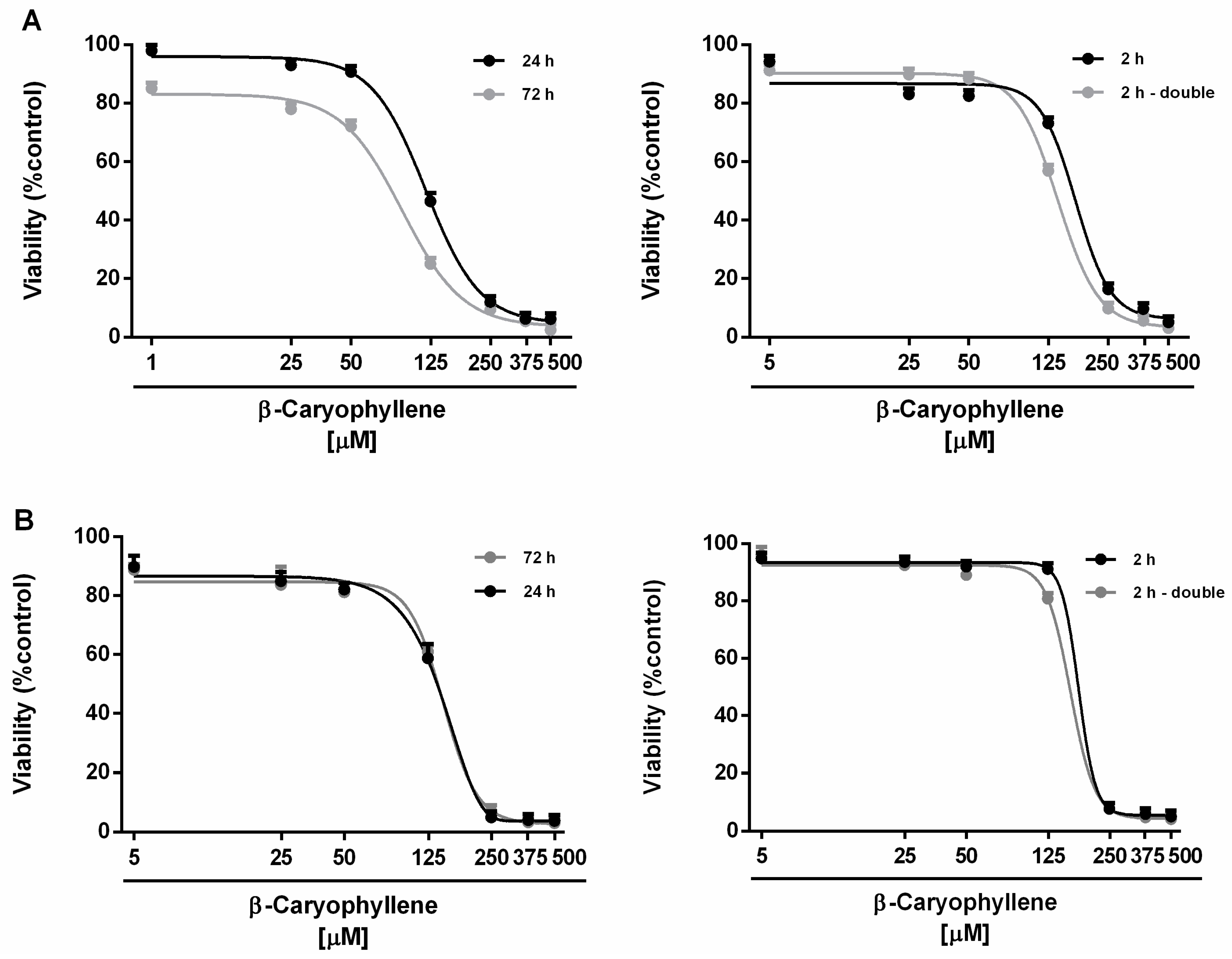





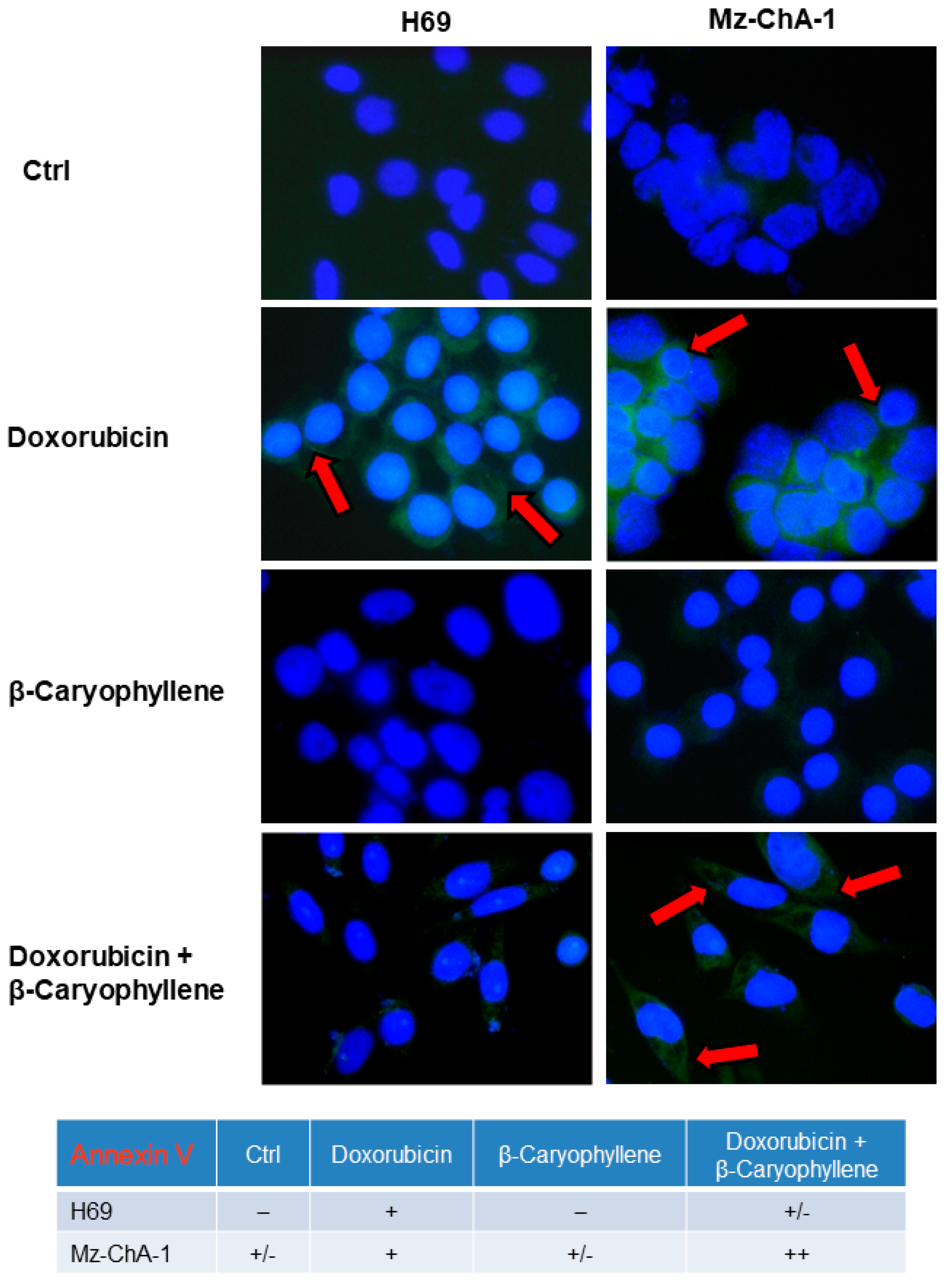

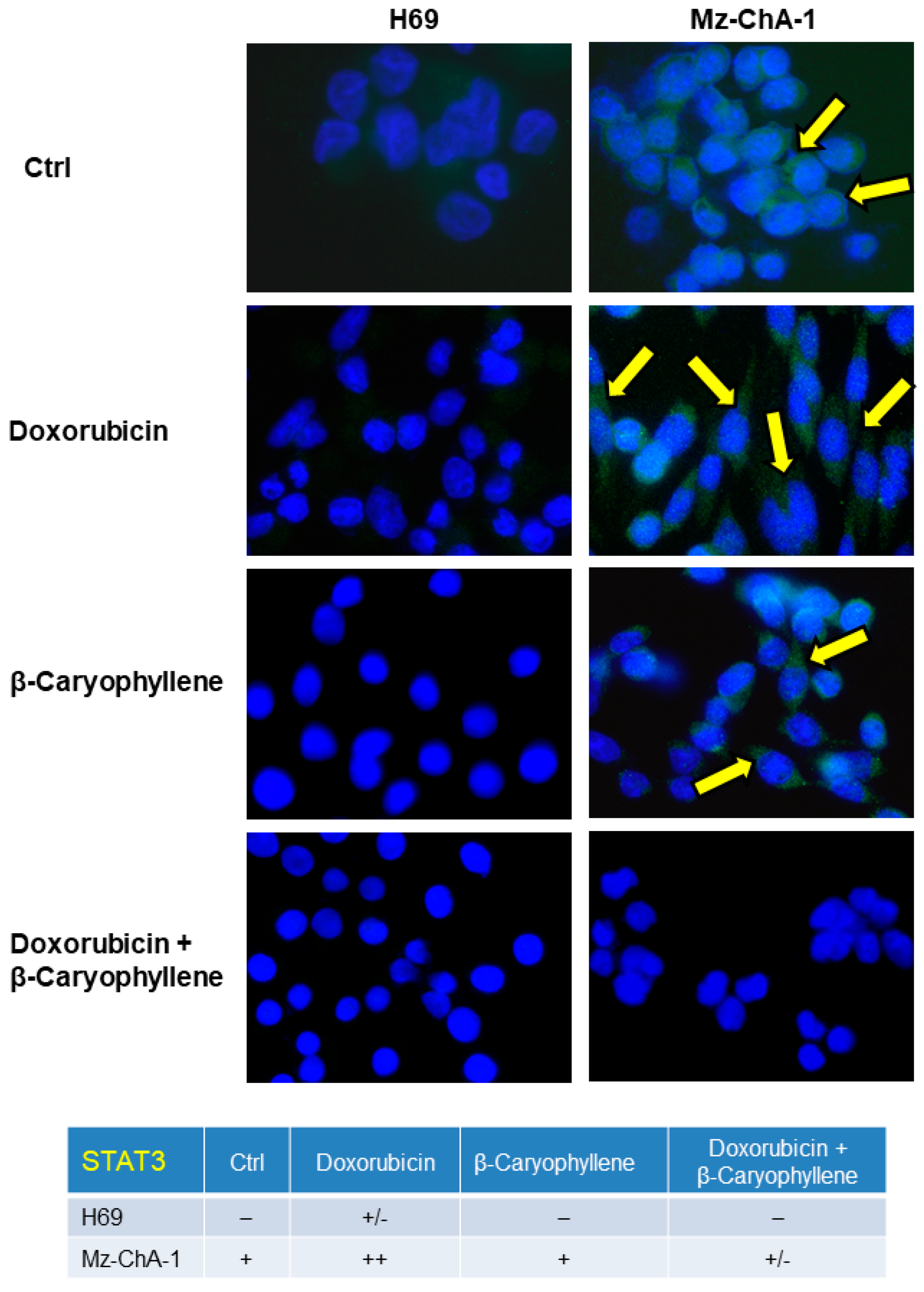
| Time Exposure | β-Caryophyllene IC50 [µM] (CL) | |
|---|---|---|
| Mz-ChA-1 | H69 | |
| 24 h | 124.0 (105.0–176.2) * | 147.6 (124.5–175.7) |
| 72 h | 90.0 (73.1–111.5) §* | 146.0 (98.7–211.5) |
| 2 h | 171.5 (80.5–213.5) | 176.6 (153.7–202.9) |
| 2 h double | 139.5 (90.0–173.0) * | 161.2 (131.4–197.6) |
| Time Exposure | IC50 [µM] (CL a)RR b | |||
|---|---|---|---|---|
| Mz-ChA-1 | H69 | |||
| Doxorubicin | Doxorubicin + β-Caryophyllene | Doxorubicin | Doxorubicin + β-Caryophyllene | |
| 24 h | 27.8 (18.0–42.4) | 11.2 (7.4–16.6) °° | 13.6 (5.8–31.2) | 25.5 (12.0–45.0) |
| 2.5 | 0.5 | |||
| 72 h | 3.8 (2.2–6.4) §** | 3.2 (1.2–8.6) §** | 4.0 (3.4–4.6) § | 3.6 (3.2–4.0) |
| 1.2 | 1.1 | |||
| 2 h | 19.8 (8.2–45.4) § | 8.8 (2.6–18.2) §°° | 81.8 (69.8–126.2) | 122.6 (50.0–301.0) |
| 2.3 | 0.7 | |||
| 2 h - double | 11.6 (4.0–33.2) § * | 5.8 (0.4–25.0) §**°° | 54.4 (39.4–75.0) | 59.2 (46.2–76.0) |
| 2 | 0.9 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gulli, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention. Cells 2020, 9, 858. https://doi.org/10.3390/cells9040858
Di Sotto A, Di Giacomo S, Rubini E, Macone A, Gulli M, Mammola CL, Eufemi M, Mancinelli R, Mazzanti G. Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention. Cells. 2020; 9(4):858. https://doi.org/10.3390/cells9040858
Chicago/Turabian StyleDi Sotto, Antonella, Silvia Di Giacomo, Elisabetta Rubini, Alberto Macone, Marco Gulli, Caterina Loredana Mammola, Margherita Eufemi, Romina Mancinelli, and Gabriela Mazzanti. 2020. "Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention" Cells 9, no. 4: 858. https://doi.org/10.3390/cells9040858






