A Peak of H3T3 Phosphorylation Occurs in Synchrony with Mitosis in Sea Urchin Early Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Handling of Gametes and Embryos
2.3. Quantification of Cleavage Rates and Phenotypic Analysis
2.4. Embryo Protein Extracts and Western Blot Analyses
2.5. Immunostaining and Confocal Microscopy
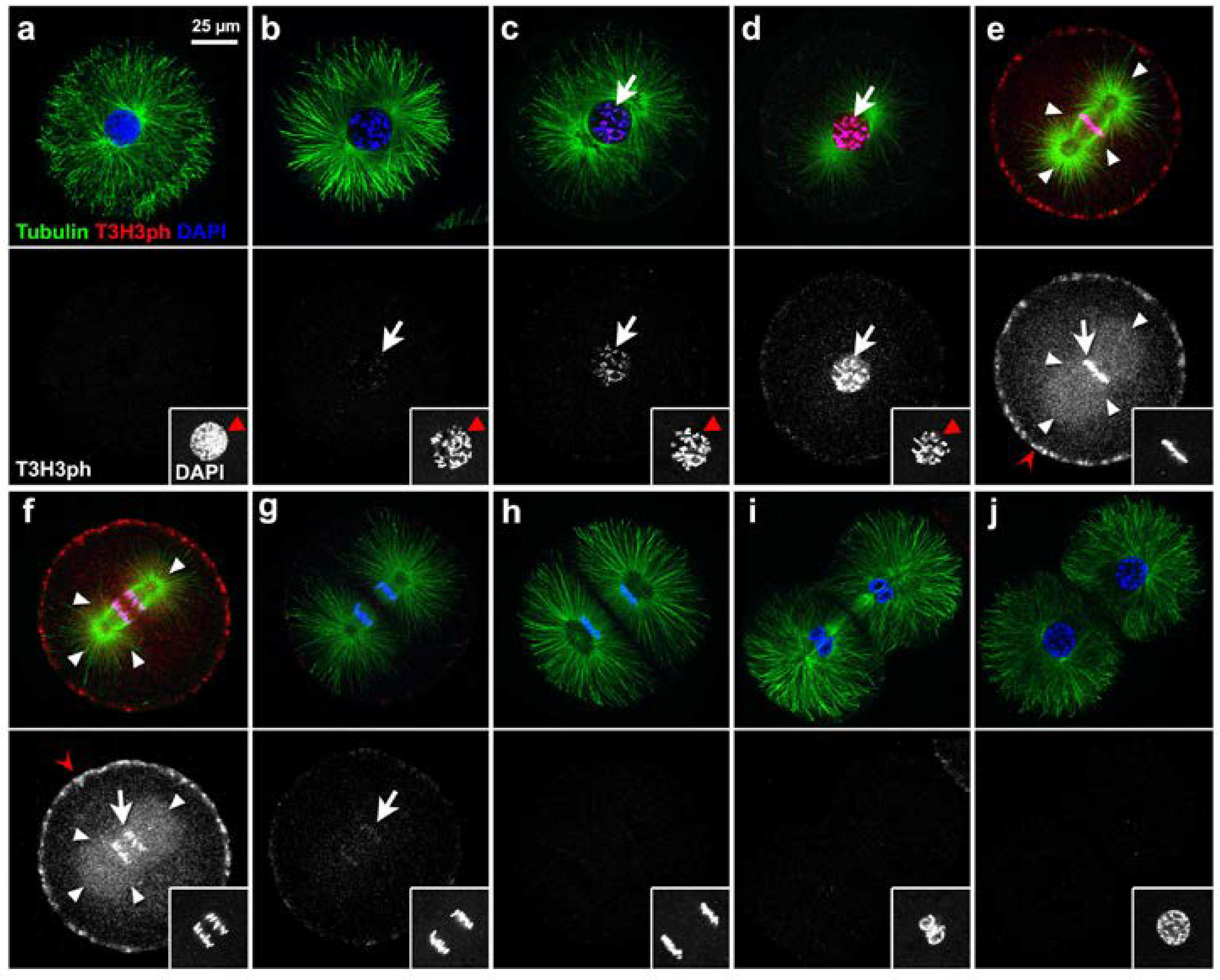
3. Results
3.1. The Levels of T3H3ph Cycle in Synchrony with Cell Division in Sea Urchin Early Embryos
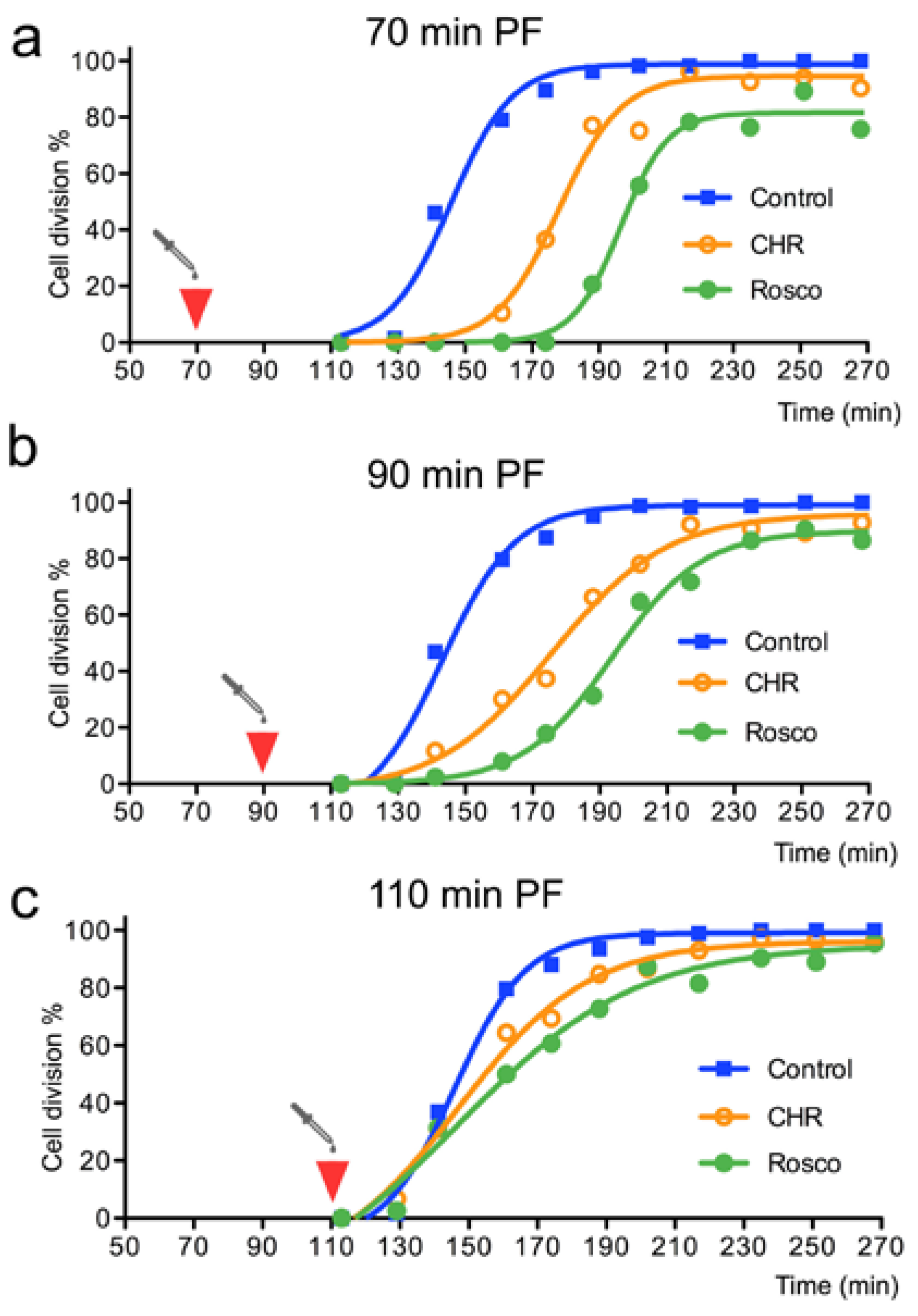
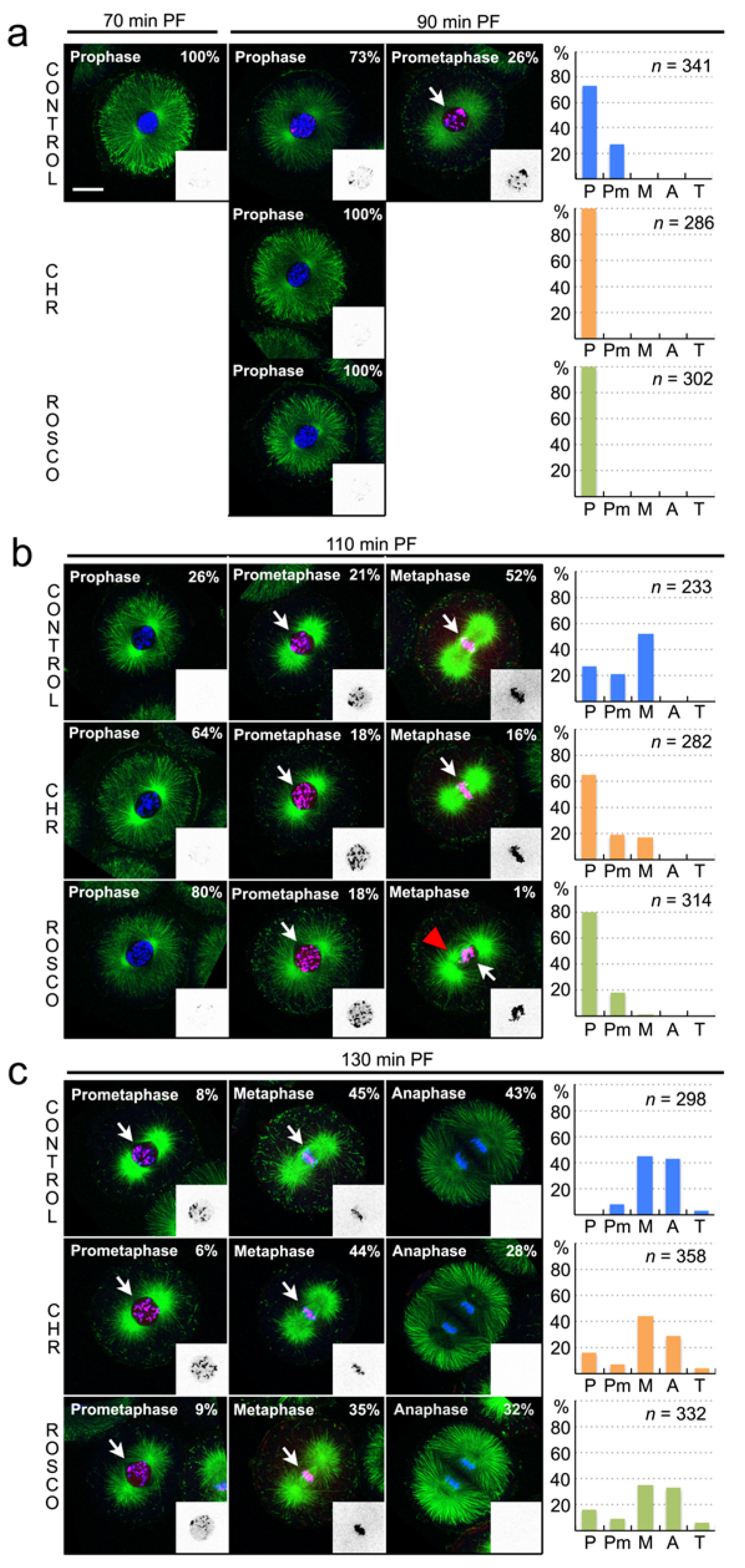
3.2. The Haspin Inhibitor CHR-6494 Delays Entry into Mitosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moundoyi, H.; Demouy, J.; Le Panse, S.; Morales, J.; Sarels, B.; Cormier, P. Toward Multiscale Modeling of Molecular and Biochemical Events Occurring at Fertilization Time in Sea Urchins. Results Probl. Cell Differ. 2018, 65, 69–89. [Google Scholar] [PubMed]
- Brandhorst, B.P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev. Biol. 1976, 52, 310–317. [Google Scholar] [CrossRef]
- Epel, D. Protein synthesis in sea urchin eggs: A “late” response to fertilization. Proc. Natl. Acad. Sci. USA 1967, 57, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubé, F. Effect of reduced protein synthesis on the cell cycle in sea urchin embryos. J. Cell. Physiol. 1988, 137, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, E.B. The timing of synthesis of proteins required for mitosis in the cell cycle of the sea urchin embryo. Exp. Cell Res. 1983, 144, 393–403. [Google Scholar] [CrossRef]
- Néant, I.; Charbonneau, M.; Guerrier, P. A requirement for protein phosphorylation in regulating the meiotic and mitotic cell cycles in echinoderms. Dev. Biol. 1989, 132, 304–314. [Google Scholar] [CrossRef]
- Nurse, P. A long twentieth century of the cell cycle and beyond. Cell 2000, 100, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Hayward, D.; Alfonso-Perez, T.; Gruneberg, U. Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1. FEBS Lett. 2019, 593, 2889–2907. [Google Scholar] [CrossRef]
- Saurin, A.T. Kinase and Phosphatase Cross-Talk at the Kinetochore. Front. Cell Dev. Biol. 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Evans, T.; Rosenthal, E.T.; Youngblom, J.; Distel, D.; Hunt, T. Cyclin: A protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 1983, 33, 389–396. [Google Scholar] [CrossRef]
- Cerutti, H.; Casas-Mollano, J.A. Histone H3 phosphorylation: Universal code or lineage specific dialects? Epigenetics 2009, 4, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, M.L.; Higgins, J.M.G.; Seibert, M. Priming chromatin for segregation: Functional roles of mitotic histone modifications. Cell Cycle 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Green, G.R.; Collas, P.; Burrell, A.; Poccia, D.L. Histone phosphorylation during sea urchin development. Semin. Cell Biol. 1995, 6, 219–227. [Google Scholar] [CrossRef]
- Hendzel, M.J.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Ranalli, T.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef]
- Prigent, C.; Dimitrov, S. Phosphorylation of serine 10 in histone H3, what for? J. Cell Sci. 2003, 116, 3677–3685. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Yu, L.; Bowen, J.; Gorovsky, M.A.; Allis, C.D. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 1999, 97, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Sultan, S.; Taylor, S.S.; Higgins, J.M. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005, 19, 472–488. [Google Scholar] [CrossRef] [Green Version]
- Polioudaki, H.; Markaki, Y.; Kourmouli, N.; Dialynas, G.; Theodoropoulos, P.A.; Singh, P.B.; Georgatos, S.D. Mitotic phosphorylation of histone H3 at threonine 3. FEBS Lett. 2004, 560, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Escribá, M.C.; Goday, C. Histone H3 phosphorylation and elimination of paternal X chromosomes at early cleavages in sciarid flies. J. Cell. Sci. 2013, 126, 3214–3222. [Google Scholar] [CrossRef] [Green Version]
- Caperta, A.D.; Rosa, M.; Delgado, M.; Karimi, R.; Demidov, D.; Viegas, W.; Houben, A. Distribution patterns of phosphorylated Thr 3 and Thr 32 of histone H3 in plant mitosis and meiosis. Cytogenet. Genome Res. 2008, 122, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, Y.; Honda, T.; Tanno, Y.; Watanabe, Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 2010, 330, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Higgins, J.M. Haspin: A mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle 2005, 4, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.M. Haspin-like proteins: A new family of evolutionarily conserved putative eukaryotic protein kinases. Protein Sci. 2001, 10, 1677–1684. [Google Scholar] [CrossRef]
- Higgins, J.M. Structure, function and evolution of haspin and haspin-related proteins, a distinctive group of eukaryotic protein kinases. Cell Mol. Life Sci. 2003, 60, 446–462. [Google Scholar] [CrossRef]
- Ashtiyani, R.K.; Moghaddam, A.M.; Schubert, V.; Rutten, T.; Fuchs, J.; Demidov, D.; Blattner, F.R.; Houben, A. AtHaspin phosphorylates histone H3 at threonine 3 during mitosis and contributes to embryonic patterning in Arabidopsis. Plant J. 2011, 68, 443–454. [Google Scholar] [CrossRef]
- Kurihara, D.; Matsunaga, S.; Omura, T.; Higashiyama, T.; Fukui, K. Identification and characterization of plant Haspin kinase as a histone H3 threonine kinase. BMC Plant Biol. 2011, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Feizbakhsh, O.; Place, M.; Fant, X.; Buron, F.; Routier, S.; Ruchaud, S. The Mitotic Protein Kinase Haspin and Its Inhibitors. In Protein Phosphorylation, 1st ed.; Prigent, C., Ed.; InTech: Rijeka, Croatia, 2017; pp. 31–47. [Google Scholar]
- Dai, J.; Sullivan, B.A.; Higgins, J.M. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev. Cell 2006, 11, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Kateneva, A.V.; Higgins, J.M. Studies of haspin-depleted cells reveal that spindle-pole integrity in mitosis requires chromosome cohesion. J. Cell Sci. 2009, 122, 4168–4176. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Park, Y.S.; Cho, D.-H.; Kim, J.-S.; Oh, J.S. Dynamics of histone H3 phosphorylation at threonine 3 during meiotic maturation in mouse oocytes. Biochem. Biophys. Res. Commun. 2015, 458, 280–286. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Gentilello, A.S.; Balboula, A.Z.; Shrivastava, V.; Ohring, J.; Schindler, K. Phosphorylation of threonine 3 on histone H3 by haspin kinase is required for meiosis I in mouse oocytes. J. Cell Sci. 2014, 127, 5066–5078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Hua, X.; Bryner, Y.H.; Liu, S.; Gitter, C.B.; Dai, J. Haspin inhibition delays cell cycle progression through interphase in cancer cells. J. Cell Physiol. 2020, 235, 4508–4519. [Google Scholar] [CrossRef] [PubMed]
- Maiolica, A.; de Medina-Redondo, M.; Schoof, E.M.; Chaikuad, A.; Villa, F.; Gatti, M.; Jeganathan, S.; Lou, H.J.; Novy, K.; Hauri, S.; et al. Modulation of the chromatin phosphoproteome by the Haspin protein kinase. Mol. Cell. Proteom. 2014, 13, 1724–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panigada, D.; Grianti, P.; Nespoli, A.; Rotondo, G.; Castro, D.G.; Quadri, R.; Piatti, S.; Plevani, P.; Muzi-Falconi, M. Yeast haspin kinase regulates polarity cues necessary for mitotic spindle positioning and is required to tolerate mitotic arrest. Dev. Cell 2013, 26, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassé, H.; Mulner-Lorillon, O.; Boulben, S.; Glippa, V.; Morales, J.; Cormier, P. Cyclin B Translation Depends on mTOR Activity after Fertilization in Sea Urchin Embryos. PLoS ONE 2016, 11, e0150318. [Google Scholar] [CrossRef] [Green Version]
- Strickland, L.; von Dassow, G.; Ellenberg, J.; Foe, V.; Lenart, P.; Burgess, D. Light microscopy of echinoderm embryos. Methods Cell Biol. 2004, 74, 371–409. [Google Scholar]
- Kwon, Y.G.; Lee, S.Y.; Choi, Y.; Greengard, P.; Nairn, A.C. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 2168–2173. [Google Scholar] [CrossRef] [Green Version]
- Eswaran, J.; Patnaik, D.; Filippakopoulos, P.; Wang, F.; Stein, R.L.; Murray, J.W.; Higgins, J.M.G.; Knapp, S. Structure and functional characterization of the atypical human kinase haspin. Proc. Natl. Acad. Sci. USA 2009, 106, 20198–20203. [Google Scholar] [CrossRef] [Green Version]
- Villa, F.; Capasso, P.; Tortorici, M.; Forneris, F.; de Marco, A.; Mattevi, A.; Musacchio, A. Crystal structure of the catalytic domain of Haspin, an atypical kinase implicated in chromatin organization. Proc. Natl. Acad. Sci. USA 2009, 106, 20204–20209. [Google Scholar] [CrossRef] [Green Version]
- Huertas, D.; Soler, M.; Moreto, J.; Villanueva, A.; Martinez, A.; Vidal, A.; Charlton, M.; Moffat, D.; Patel, S.; McDermott, J.; et al. Antitumor activity of a small-molecule inhibitor of the histone kinase Haspin. Oncogene 2012, 31, 1408–1418. [Google Scholar] [CrossRef]
- Ghenoiu, C.; Wheelock, M.S.; Funabiki, H. Autoinhibition and Polo-dependent multisite phosphorylation restrict activity of the histone H3 kinase Haspin to mitosis. Mol. Cell 2013, 52, 734–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Azevedo, W.F.; Leclerc, S.; Meijer, L.; Havlicek, L.; Strnad, M.; Kim, S.H. Inhibition of cyclin-dependent kinases by purine analogues: Crystal structure of human cdk2 complexed with roscovitine. Eur. J. Biochem. 1997, 243, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Borgne, A.; Mulner, O.; Chong, J.P.; Blow, J.J.; Inagaki, N.; Inagaki, M.; Delcros, J.G.; Moulinoux, J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997, 243, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Raasholm, M.; Moosmann, A.; Campsteijn, C.; Thompson, E.M. Switching of INCENP paralogs controls transitions in mitotic chromosomal passenger complex functions. Cell Cycle 2019, 18, 2006–2025. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Tian, X.; Zhu, C.; Wang, F.; Higgins, J.M. Polo-like kinase-1 triggers histone phosphorylation by Haspin in mitosis. EMBO Rep. 2014, 15, 273–281. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Sun, Y.; Liu, S.; Dai, J. Anti-Melanoma Activities of Haspin Inhibitor CHR-6494 Deployed as a Single Agent or in a Synergistic Combination with MEK Inhibitor. J. Cancer 2017, 8, 2933–2943. [Google Scholar] [CrossRef]
- Karanika, E.; Soupsana, K.; Christogianni, A.; Stellas, D.; Klinakis, A.; Politou, A.S.; Georgatos, S. Haspin-dependent and independent effects of the kinase inhibitor 5-Iodotubercidin on self-renewal and differentiation. Sci. Rep. 2020, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.H.; Park, D.Y.; Choi, Y.H.; Kim, K.J.; Yoon, H.S.; Kim, K.T. Mitotic histone H3 phosphorylation by vaccinia-related kinase 1 in mammalian cells. Mol Cell Biol. 2007, 27, 8533–8546. [Google Scholar] [CrossRef] [Green Version]
- Tu, Q.; Cameron, R.A.; Worley, K.C.; Gibbs, R.A.; Davidson, E.H. Gene structure in the sea urchin Strongylocentrotus purpuratus based on transcriptome analysis. Genome Res. 2012, 22, 2079–2087. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feizbakhsh, O.; Pontheaux, F.; Glippa, V.; Morales, J.; Ruchaud, S.; Cormier, P.; Roch, F. A Peak of H3T3 Phosphorylation Occurs in Synchrony with Mitosis in Sea Urchin Early Embryos. Cells 2020, 9, 898. https://doi.org/10.3390/cells9040898
Feizbakhsh O, Pontheaux F, Glippa V, Morales J, Ruchaud S, Cormier P, Roch F. A Peak of H3T3 Phosphorylation Occurs in Synchrony with Mitosis in Sea Urchin Early Embryos. Cells. 2020; 9(4):898. https://doi.org/10.3390/cells9040898
Chicago/Turabian StyleFeizbakhsh, Omid, Florian Pontheaux, Virginie Glippa, Julia Morales, Sandrine Ruchaud, Patrick Cormier, and Fernando Roch. 2020. "A Peak of H3T3 Phosphorylation Occurs in Synchrony with Mitosis in Sea Urchin Early Embryos" Cells 9, no. 4: 898. https://doi.org/10.3390/cells9040898
APA StyleFeizbakhsh, O., Pontheaux, F., Glippa, V., Morales, J., Ruchaud, S., Cormier, P., & Roch, F. (2020). A Peak of H3T3 Phosphorylation Occurs in Synchrony with Mitosis in Sea Urchin Early Embryos. Cells, 9(4), 898. https://doi.org/10.3390/cells9040898




