Putting the Brakes on Tumorigenesis with Natural Products of Plant Origin: Insights into the Molecular Mechanisms of Actions and Immune Targets for Bladder Cancer Treatment
Abstract
:1. Introduction
2. Bladder Cancer Treatment
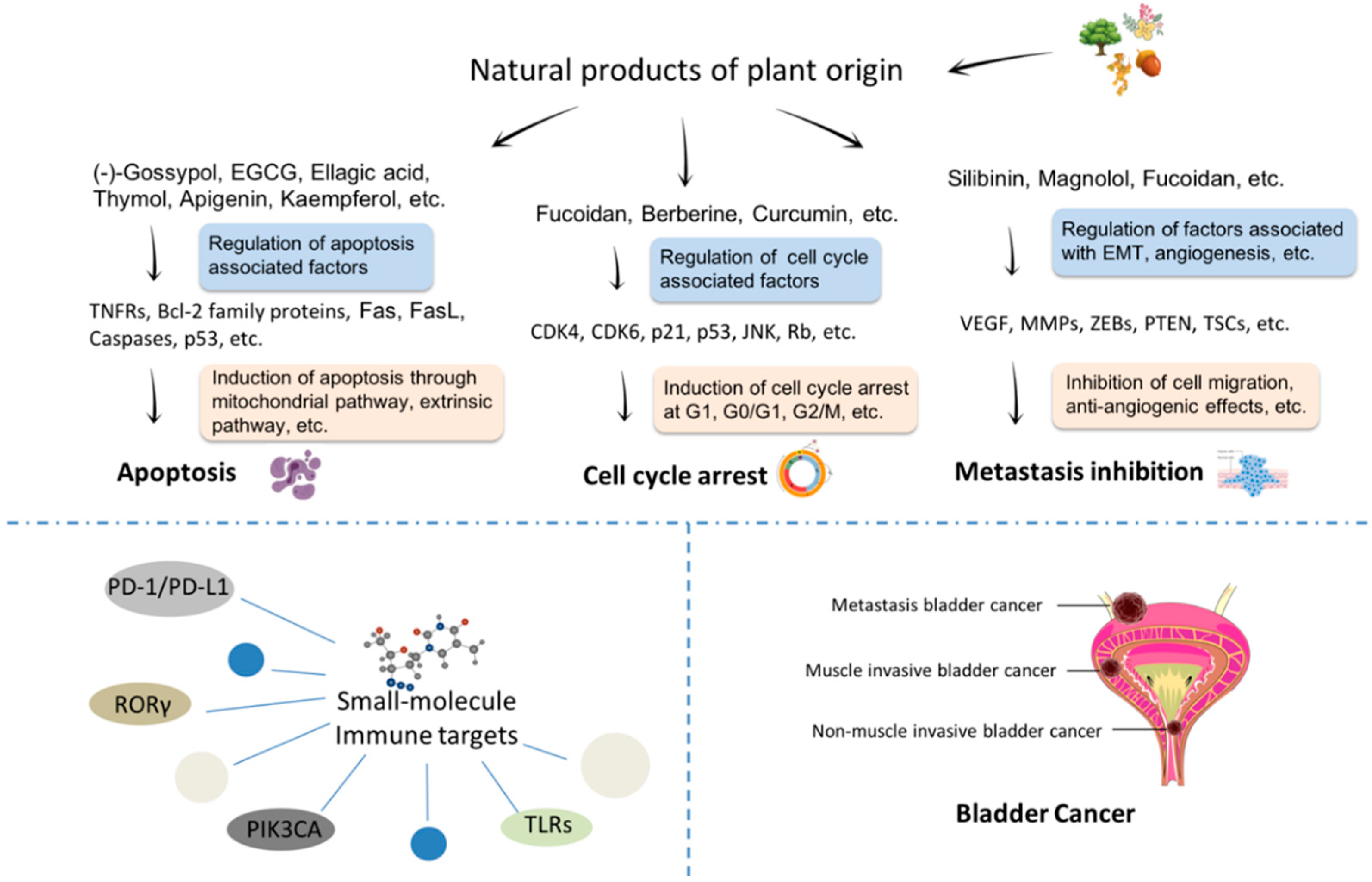
3. Plant-Origin Natural Products in Bladder Cancer Treatment
3.1. Plant-Origin Compounds Induce Apoptosis in Bladder Cancer
3.2. Plant-Origin Compounds Induce Cell Cycle Arrest in Bladder Cancer
3.3. Plant-Origin Compounds with Anti-Bladder Cancer Metastasis Activity
| Plant Origin Compounds | Source | Structure | Mechanisms | Related Factors | Ref. |
|---|---|---|---|---|---|
| (−)-Gossypol | Cotton plants |  | Inducing apoptosis through caspase-mediated signaling pathways | caspase-3, caspase-9, Bcl-xl, Mcl-1, Bim, and Puma | [33,34,35] |
| (−)-epigallocatechin-3-gallate (EGCG) | Green tea |  | Inducing apoptosis associated with the PI3K/Akt signaling pathway | Bcl-2, Bcl-xL, Bcl-2, Bcl-xL, caspase-3, and PARP | [36,37,38] |
| Ellagic acid | Fruits and some mushrooms |  | Inducing apoptosis through the mitochondrial pathway | caspase-3, caspase-9, Bax, and cytochrome c | [39,40,41] |
| Thymol | Thymus serpyllum L. |  | Triggering apoptosis via the intrinsic pathway | Bcl-2, Bcl-xl, Mcl-1, cytochrome c, and Smac/DIABLO | [42,43] |
| Apigenin | Fruits and vegetables, like parsley, celery, celeriac, and chamomile tea |  | Inducing apoptosis through the PI3K/Akt signaling pathway | Bax, Bad, Bak, Bcl-2, Bcl-XL, Mcl-1, caspase-3, 7, 9, and PARP | [45,46,47] |
| Kaempferol | Hamamelis mollis |  | Inducing apoptosis associated with the PI3K/Akt signaling pathway | AKT, Bid, Mcl-1, Bcl-xL, p53, p21, p38, Bax, and Bid | [48,49,50,51] |
| Baicalein | Scutellaria baicalensis |  | Inducing apoptosis and cell cycle arrest; inhibiting cell migration | p16, p21, p38, CDC2 Bcl-2, Bax, caspase-3, and caspase-9 | [52,84] |
| Curcumin | Curcuma spp. plants |  | Inducing apoptosis and cell cycle arrest | caspase-3, caspase-7, PARP, Bcl-2, p21, p27, cyclin D1 and cyclin E1 | [53,54,55,56] |
| Kazinol A | Broussonetia papyrifera |  | Inducing apoptosis associated with the AKT signaling pathway | p-AKT, p-Bid, Mcl-1, and Bcl-xL | [59] |
| Boldine | Peumus boldus |  | Inducing apoptosis mechanism associated with the ERK and AKT signaling pathway | p-AKT, p-ERK, and p-GSK-3β | [61,62] |
| Lycorine | Amaryllidaceae genera |  | Inducing apoptosis | p-ATK, caspase-3, and Bax | [63] |
| Tetrandrine | Stephania tetrandra |  | Inducing apoptosis associated with the mitochondrial pathway | caspase-3, caspase-9, and cytochrome c | [64] |
| 6’-hydroxy justicidin A | Justicia procumbens |  | Inducing apoptosis associated with the mitochondrial pathways | caspase-3 and caspase-9 | [65] |
| Fucoidan | Algae and seaweeds |  | Inducing apoptosis through the PI3K/Akt signaling pathway; inducing cell cycle arrest in the G1 phase; antiangiogenic and inhibiting cell migration and invasion | Fas, MMP, Bax, Bcl-2, cytochrome c, p21, CDK4, CDK6, p-RB, HIF-1α, and VEGF | [66,67,68,73,83] |
| Berberine | Berberis |  | G0/G1 cell cycle arrest and inducing apoptosis | caspase-3 and caspase-9 | [74] |
| Silibinin | Thistle |  | Inhibiting cell migration and invasion | mesenchymal markers, N-cadherin, vimentin, β-catenin, ZEB1, E-cadherin, COX-2, and MMP-2 | [80,81] |
| Magnolol | Magnolia officinalis |  | Inhibiting tumor angiogenesis | HIF-1a and VEGF | [82] |
4. Small-Molecule Immune Targets for Bladder Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.K.; Miller, M.J.; Agarwal, N.; Chang, S.M.; Chavez-MacGregor, M.; Cohen, E.; Cole, S.; Dale, W.; Diefenbach, C.S.M.; Disis, M.L.; et al. Clinical Cancer Advances 2019_Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2019, 37, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, J. Present and future of cancer immunotherapy: A tumor microenvironmental perspective. Oncol. Lett. 2018, 16, 4105–4113. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural Products in Drug Discovery. In Pharmacognosy; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Crabb, S.J.; Douglas, J. The latest treatment options for bladder cancer. Br. Med. Bull. 2018, 128, 85–95. [Google Scholar] [CrossRef]
- Woldu, S.L.; Bagrodia, A.; Lotan, Y. Guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2017, 119, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Abufaraj, M.; Dalbagni, G.; Daneshmand, S.; Horenblas, S.; Kamat, A.M.; Kanzaki, R.; Zlotta, A.R.; Shariat, S.F. The Role of Surgery in Metastatic Bladder Cancer: A Systematic Review. Eur. Urol. 2018, 73, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Selph, S.S.; Buckley, D.I.; Gustafson, K.S.; Griffin, J.C.; Grusing, S.E.; Gore, J.L. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer 2016, 122, 842–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gakis, G. Management of Muscle-invasive Bladder Cancer in the 2020s: Challenges and Perspectives. Eur. Urol. Focus 2020. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, G.; Sternberg, C.N. Systemic Chemotherapy in Muscle Invasive and Metastatic Bladder Cancer: Present and Future. Urol. J. 2017, 84, 130–141. [Google Scholar] [CrossRef]
- Davarpanah, N.N.; Yuno, A.; Trepel, J.B.; Apolo, A.B. Immunotherapy: A new treatment paradigm in bladder cancer. Curr. Opin. Oncol. 2017, 29, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Black, P.C.; Agarwal, P.K.; Dinney, C.P. Targeted therapies in bladder cancer--an update. Urol. Oncol. 2007, 25, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Casadei, C.; Dizman, N.; Schepisi, G.; Cursano, M.C.; Basso, U.; Santini, D.; Pal, S.K.; De Giorgi, U. Targeted therapies for advanced bladder cancer: New strategies with FGFR inhibitors. Adv. Med. Oncol. 2019, 11, 1758835919890285. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Protzel, C.; Hakenberg, O.W. Emerging apoptosis agonists for bladder cancer. Expert Opin. Emerg. Drugs 2009, 14, 607–618. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism- function and dysfunction of its modulators and targeted therapeutic strategies. AGING 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2013, 15, 49–63. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Cheng, L.; Davison, D.D.; Adams, J.; Lopez-Beltran, A.; Wang, L.; Montironi, R.; Zhang, S. Biomarkers in bladder cancer: Translational and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 73–111. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Mirza-Aghazadeh-Attari, M.; Ekrami, E.M.; Aghdas, S.A.M.; Mihanfar, A.; Hallaj, S.; Yousefi, B.; Safa, A.; Majidinia, M. Targeting PI3K/Akt/mTOR signaling pathway by polyphenols: Implication for cancer therapy. Life Sci. 1016. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38. [Google Scholar] [CrossRef]
- Keshmiri-Neghab, H.; Goliaei, B. Therapeutic potential of gossypol: An overview. Pharm. Biol. 2013, 52, 124–128. [Google Scholar] [CrossRef]
- Opydo-Chanek, M.; Gonzalo, O.; Marzo, I. Multifaceted anticancer activity of BH3 mimetics: Current evidence and future prospects. Biochem. Pharmacol. 2017, 136, 12–23. [Google Scholar] [CrossRef]
- Macoska, J.; Adsule, S.; Tantivejkul, K.; Wang, S.; Pienta, K.; Lee, C. −(−)Gossypol promotes the apoptosis of bladder cancer cells in vitro. Pharmacol. Res. 2008, 58, 323–331. [Google Scholar] [CrossRef]
- Mani, J.; Vallo, S.; Rakel, S.; Antonietti, P.; Gessler, F.; Blaheta, R.; Bartsch, G.; Michaelis, M.; Cinatl, J.; Haferkamp, A.; et al. Chemoresistance is associated with increased cytoprotective autophagy and diminished apoptosis in bladder cancer cells treated with the BH3 mimetic (−)-Gossypol (AT-101). BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea- A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Xie, L.-P.; Zheng, X.-Y.; Wang, Y.-B.; Bai, Y.; Shen, H.-F.; Li, L.-C.; Dahiya, R. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2007, 354, 852–857. [Google Scholar] [CrossRef]
- Luo, K.-W.; Lung, W.-Y.; Chun-Xie; Luo, X.-L.; Huang, W.-R. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K:AKT pathway. Oncotarget 2018, 9, 12261–12272. [Google Scholar] [CrossRef] [Green Version]
- Ceci, C.; Lacal, P.; Tentori, L.; De Martino, M.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.-C.; Huang, A.-C.; Yu, C.-S.; Lien, J.-C.; Wu, S.-H.; Huang, Y.-P.; Huang, H.-Y.; Kuo, J.-H.; Liao, W.-Y.; Yang, J.-S.; et al. Ellagic acid induces apoptosis in tsgh8301 human bladder cancer cells through the endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Environ. Toxicol. 2013. [Google Scholar] [CrossRef]
- Ceci, C.; Tentori, L.; Atzori, M.; Lacal, P.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; de Martino, M.; Vespasiani, G.; et al. Ellagic Acid Inhibits Bladder Cancer Invasiveness and In Vivo Tumor Growth. Nutrients 2016, 8, 744. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.-M.; Du, C.-J.; Hu, S.-M.; Chen, J.-X.; Zhang, S.-G.; Zhang, N.; Gao, F.; Li, S.-J.; Mao, X.-W.; et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer activity of thymol: A literature-based review and docking study with Emphasis on its anticancer mechanisms. IUBMB Life 2019, 71, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm Res 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Shi, M.-D.; Shiao, C.-K.; Lee, Y.-C.; Shih, Y.-W. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef] [Green Version]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol – A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Jiang, L.; Song, H.; Guo, H.; Wang, C.; Lu, Z. Baicalein inhibits proliferation and migration of bladder cancer cell line T24 by down-regulation of microRNA-106. Biomed. Pharmacother. 2018, 107, 1583–1590. [Google Scholar] [CrossRef]
- Huminiecki, L.; Horbańczuk, J.; Atanasov, A.G. The functional genomic studies of curcumin. Semin. Cancer Biol. 2017, 46, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Zhang, R.; Dong, L.; Chen, H.; Bo, J.; Xue, W.; Huang, Y. Curcumin inhibits cell proliferation and motility via suppression of TROP2 in bladder cancer cells. Int. J. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Zhang, X.; Shi, T.; Li, H. Antitumor effects of curcumin in human bladder cancer in vitro. Oncol. Lett. 2017, 14, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Chadalapaka, G.; Jutooru, I.; Chintharlapalli, S.; Papineni, S.; Smith, R., 3rd; Li, X.; Safe, S. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008, 68, 5345–5354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wang, Y.; Jia, Z.; Gao, Y.; Zhao, C.; Yao, Y. Curcumin inhibits bladder cancer progression via regulation of β-catenin expression. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Fudhaili, A.; Oh, S.-S.; Lee, K.W.; Madhi, H.; Kim, D.-H.; Yoo, J.; Ryu, H.W.; Park, K.-H.; Kim, K.D. Cytotoxic effects of kazinol A derived from Broussonetia papyrifera on human bladder cancer cells, T24 and T24R2. Phytomedicine 2016, 23, 1462–1468. [Google Scholar] [CrossRef]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858. [Google Scholar] [CrossRef]
- Rodríguez-Arce, E.; Cancino, P.; Arias-Calderón, M.; Silva-Matus, P.; Saldías, M. Oxoisoaporphines and Aporphines: Versatile Molecules with Anticancer Effects. Molecules 2019, 25, 108. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, D.; Bertola, G.; Dietrich, F.; Figueiró, F.; Zanotto-Filho, A.; Moreira Fonseca, J.C.; Morrone, F.B.; Barrios, C.H.; Battastini, A.M.O.; Salbego, C.G. Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 36.e31–36.e39. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Li, X.; Jin, Z.; Xu, P.; Xu, N.; Xu, A.; Xu, Y.; Zheng, S.; Zheng, J.; et al. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade. Biochem. Biophys. Res. Commun. 2017, 483, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, B.; Liu, R.; Wu, D.; He, D. Tetrandrine Induces Apoptosis and Triggers Caspase Cascade in Human Bladder Cancer Cells. J. Surg. Res. 2011, 166, e45–e51. [Google Scholar] [CrossRef] [PubMed]
- He, X.-L.; Zhang, P.; Dong, X.-Z.; Yang, M.-H.; Chen, S.-L.; Bi, M.-G. JR6, a new compound isolated from Justicia procumbens, induces apoptosis in human bladder cancer EJ cells through caspase-dependent pathway. J. Ethnopharmacol. 2012, 144, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.H.; Lee, D.-S.; Jeong, J.-W.; Hong, S.-H.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Park, C.; Kim, G.-Y.; et al. Fucoidan Induces ROS-Dependent Apoptosis in 5637 Human Bladder Cancer Cells by Downregulating Telomerase Activity via Inactivation of the PI3K/Akt Signaling Pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.-M.; Kim, W.-J.; Moon, S.-K. AKT signaling is involved in fucoidan-induced inhibition of growth and migration of human bladder cancer cells. Food Chem. Toxicol. 2014, 64, 344–352. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharm. Res 2019, 139, 471–488. [Google Scholar] [CrossRef]
- Jingwen, B.; Yaochen, L.; Guojun, Z. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14. [Google Scholar] [CrossRef]
- Yun, S.J.; Moon, S.K.; Kim, W.J. Investigational cell cycle inhibitors in clinical trials for bladder cancer. Expert. Opin. Investig. Drugs 2013, 22, 369–377. [Google Scholar] [CrossRef]
- Bonelli, P.; Tuccillo, F.M.; Borrelli, A.; Schiattarella, A.; Buonaguro, F.M. CDK/CCN and CDKI Alterations for Cancer Prognosis and Therapeutic Predictivity. Biomed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Park, H.Y.; Choi, I.-W.; Kim, G.-Y.; Kim, B.W.; Kim, W.-J.; Choi, Y.H. Fucoidan induces G1 arrest of the cell cycle in EJ human bladder cancer cells through down-regulation of pRB phosphorylation. Rev. Bras. De Farmacogn. 2015, 25, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Zhang, C.; Feng, J.; Hou, L.; Yan, L.; Zhou, Z.; Liu, Z.; Liu, C.; Fan, Y.; Zheng, B.; et al. Induction of G1 cell cycle arrest and apoptosis by berberine in bladder cancer cells. Eur. J. Pharm. 2011, 661, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2018, 16, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Chou, R.; Selph, S.; Buckley, D.I.; Fu, R.; Griffin, J.C.; Grusing, S.; Gore, J.L. Intravesical Therapy for the Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2017, 197, 1189–1199. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.net/cancer-types/bladder-cancer/statistics (accessed on 14 April 2020).
- Liu, S.T.; Hui, G.; Mathis, C.; Chamie, K.; Pantuck, A.J.; Drakaki, A. The Current Status and Future Role of the Phosphoinositide 3 Kinase/AKT Signaling Pathway in Urothelial Cancer: An Old Pathway in the New Immunotherapy Era. Clin. Genitourin. Cancer 2018, 16, e269–e276. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Reis, S.; Lobo, J.; Henrique, R.; Jerónimo, C. Epigenetic Mechanisms Influencing Epithelial to Mesenchymal Transition in Bladder Cancer. Int. J. Mol. Sci. 2019, 20, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Sun, Y.; Jia, J.; Yang, C.; Tang, X.; Jin, B.; Wang, K.; Guo, P.; Ma, Z.; Chen, Y.; et al. Silibinin attenuates TGF-β1-induced migration and invasion via EMT suppression and is associated with COX-2 downregulation in bladder transitional cell carcinoma. Oncol. Rep. 2018. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Ning, Z.; Zeng, J.; Fan, J.; Zhou, J.; Zhang, T.; Zhang, L.; Chen, Y.; Gao, Y.; Wang, B.; et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial–mesenchymal transition and stemness. Cell. Signal. 2013, 25, 2625–2633. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lee, C.-F.; Huang, W.-H.; Chou, T.-C. Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells. Biochem. Pharmacol. 2013, 85, 1278–1287. [Google Scholar] [CrossRef]
- Chen, M.C.; Hsu, W.L.; Hwang, P.A.; Chou, T.C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef]
- Chao, J.I.; Su, W.C.; Liu, H.F. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol. Cancer 2007, 6, 3039–3048. [Google Scholar] [CrossRef] [Green Version]
- Dhanak, D.; Edwards, J.P.; Nguyen, A.; Tummino, P.J. Small-Molecule Targets in Immuno-Oncology. Cell Chem. Biol. 2017, 24, 1148–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinmann, H. Cancer Immunotherapy: Selected Targets and Small-Molecule Modulators. ChemMedChem 2016, 11, 450–466. [Google Scholar] [CrossRef]
- Cheng, B.; Yuan, W.-E.; Su, J.; Liu, Y.; Chen, J. Recent advances in small molecule based cancer immunotherapy. Eur. J. Med. Chem. 2018, 157, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.F.; Li, Y. Small-Molecule Targets in Tumor Immunotherapy. Nat. Prod. Bioprospect 2018, 8, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Tian, H. Development of small-molecule immune checkpoint inhibitors of PD-1/PD-L1 as a new therapeutic strategy for tumour immunotherapy. J. Drug Target. 2019, 27, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, P.G.; Ramachandra, M. Small-Molecule Immune Checkpoint Inhibitors Targeting PD-1/PD-L1 and Other Emerging Checkpoint Pathways. BioDrugs 2018, 32, 481–497. [Google Scholar] [CrossRef]
- Rutz, S.; Eidenschenk, C.; Kiefer, J.R.; Ouyang, W. Post-translational regulation of RORγt—A therapeutic target for the modulation of interleukin-17-mediated responses in autoimmune diseases. Cytokine Growth Factor Rev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Liu, X.; Moisan, J.; Wang, Y.; Lesch, C.A.; Spooner, C.; Morgan, R.W.; Zawidzka, E.M.; Mertz, D.; Bousley, D.; et al. Synthetic RORγ agonists regulate multiple pathways to enhance antitumor immunity. OncoImmunology 2016, 5. [Google Scholar] [CrossRef]
- Ohadian Moghadam, S.; Nowroozi, M.R. Toll-like receptors: The role in bladder cancer development, progression and immunotherapy. Scand. J. Immunol. 2019, 90, e12818. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, J.S.; Massi, D.; Teng, M.W.L.; Mandala, M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin. Cancer Biol. 2018, 48, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Houédé, N.; Pourquier, P. Targeting the genetic alterations of the PI3K–AKT–mTOR pathway: Its potential use in the treatment of bladder cancers. Pharmacol. Ther. 2015, 145, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borcoman, E.; De La Rochere, P.; Richer, W.; Vacher, S.; Chemlali, W.; Krucker, C.; Sirab, N.; Radvanyi, F.; Allory, Y.; Pignot, G.; et al. Inhibition of PI3K pathway increases immune infiltrate in muscle-invasive bladder cancer. Oncoimmunology 2019, 8, e1581556. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Wong, J.P.C.; Kwok, H.F. Putting the Brakes on Tumorigenesis with Natural Products of Plant Origin: Insights into the Molecular Mechanisms of Actions and Immune Targets for Bladder Cancer Treatment. Cells 2020, 9, 1213. https://doi.org/10.3390/cells9051213
Wu Q, Wong JPC, Kwok HF. Putting the Brakes on Tumorigenesis with Natural Products of Plant Origin: Insights into the Molecular Mechanisms of Actions and Immune Targets for Bladder Cancer Treatment. Cells. 2020; 9(5):1213. https://doi.org/10.3390/cells9051213
Chicago/Turabian StyleWu, Qiushuang, Janet P. C. Wong, and Hang Fai Kwok. 2020. "Putting the Brakes on Tumorigenesis with Natural Products of Plant Origin: Insights into the Molecular Mechanisms of Actions and Immune Targets for Bladder Cancer Treatment" Cells 9, no. 5: 1213. https://doi.org/10.3390/cells9051213



_Kwok.png)


