Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis in Aging Cells
Abstract
1. Introduction
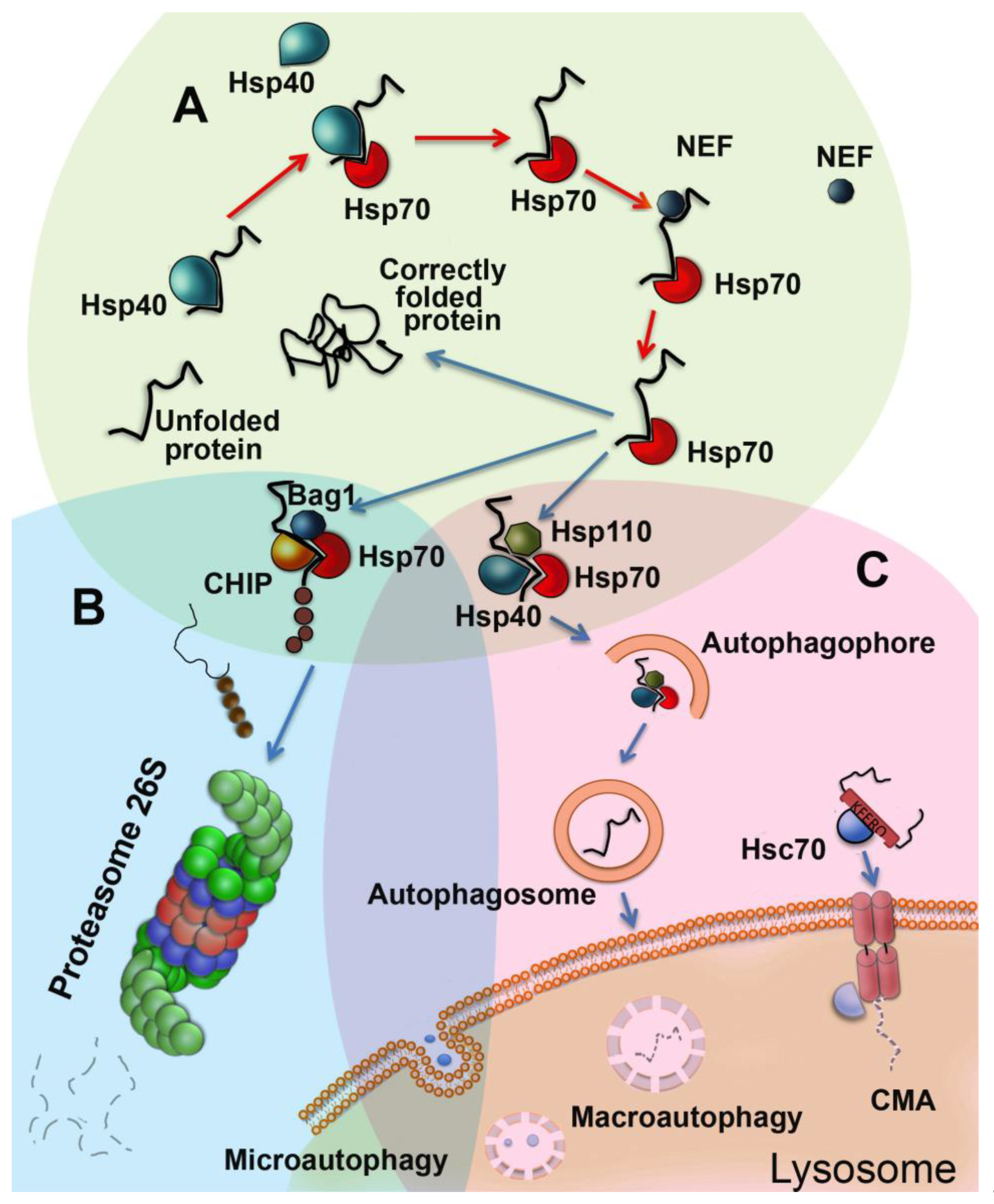
2. Functions of Chaperones in Aging Cells
3. Autophagy is a Process of Cell Upgrading
4. UPS is Necessary for Correct Aging
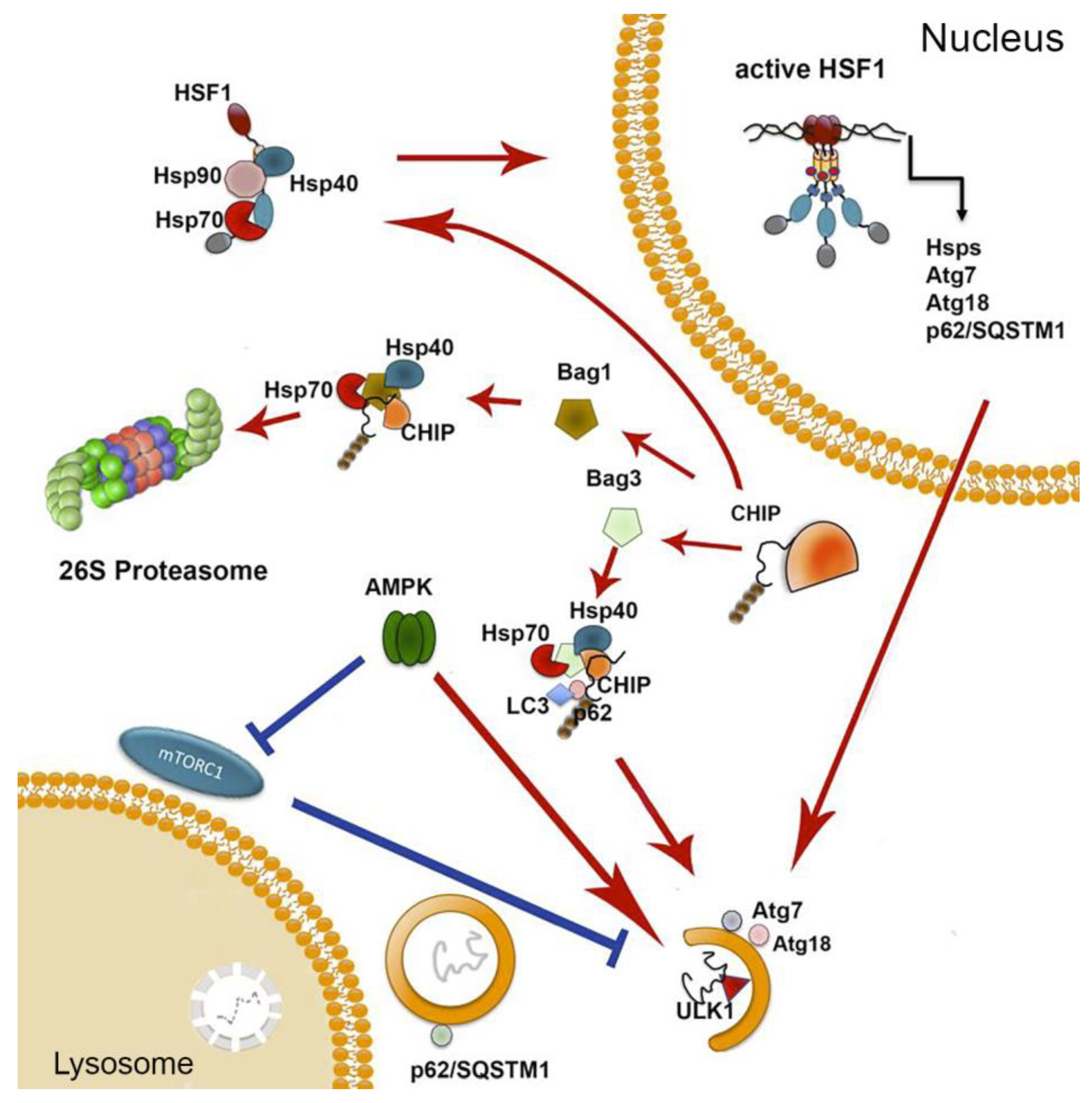
5. All Three Proteastasis Systems Cooperate In Aging
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kudryashova, K.S.; Burka, K.; Kulaga, A.Y.; Vorobyeva, N.S.; Kennedy, B. Aging Biomarkers: From Functional Tests to Multi-Omics Approaches. Proteomics 2020, 20, e1900408. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Rodríguez, M.S.; Matthiesen, R. Review and Literature Mining on Proteostasis Factors and Cancer. Adv. Struct. Saf. Stud. 2016, 1449, 71–84. [Google Scholar] [CrossRef]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Boil. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Kevei, E.; Pokrzywa, W.; Hoppe, T. Repair or destruction-an intimate liaison between ubiquitin ligases and molecular chaperones in proteostasis. FEBS Lett. 2017, 591, 2616–2635. [Google Scholar] [CrossRef]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, oxidative stress and aging. Redox Boil. 2017, 13, 550–567. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Boil. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Papaevgeniou, N.; Chondrogianni, N. UPS Activation in the Battle Against Aging and Aggregation-Related Diseases: An Extended Review. Adv. Struct. Saf. Stud. 2016, 1449, 1–70. [Google Scholar] [CrossRef]
- Frakes, A.E.; Dillin, A. The UPR ER: Sensor and Coordinator of Organismal Homeostasis. Mol. Cell 2017, 66, 761–771. [Google Scholar] [CrossRef]
- Dues, D.J.; Andrews, E.K.; Schaar, C.E.; Bergsma, A.L.; Senchuk, M.M.; Van Raamsdonk, J.M. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress response pathways. Aging 2016, 8, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Guerrero-Giménez, M.E.; Prince, T.L.; Ackerman, A.; Bonorino, C.; Calderwood, S.K. Heat Shock Proteins Are Essential Components in Transformation and Tumor Progression: Cancer Cell Intrinsic Pathways and Beyond. Int. J. Mol. Sci. 2019, 20, 4507. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Sekimoto, T.; Kurashima, K.; Fujimoto, M.; Nakai, A.; Yamashita, T. Acute HSF1 depletion induces cellular senescence through the MDM2-p53-p21 pathway in human diploid fibroblasts. J. Cell Sci. 2018, 131, jcs210724. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hunt, C.; Yaglom, J.A.; Gabai, V.; Sherman, M.Y. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene 2011, 30, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Bozaykut, P.; Sozen, E.; Kaga, E.; Ece, A.; Ozaltin, E.; Ek, B.; Ozer, N.K.; Grune, T.; Bergquist, J.; Karademir, B. The role of heat stress on the age related protein carbonylation. J. Proteom. 2013, 89, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef]
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Boil. 2017, 27, 895–905. [Google Scholar] [CrossRef]
- Mendillo, M.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 Drives a Transcriptional Program Distinct from Heat Shock to Support Highly Malignant Human Cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Santagata, S.; Mendillo, M.; Sholl, L.M.; Ben-Aharon, I.; Beck, A.H.; Dias-Santagata, R.; Koeva, M.; Stemmer, S.M.; Whitesell, L.; et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 2014, 158, 564–578. [Google Scholar] [CrossRef]
- Walker, G.A.; Thompson, F.J.; Brawley, A.; Scanlon, T.; Devaney, E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. FASEB J. 2003, 17, 1–19. [Google Scholar] [CrossRef]
- Xiao, X.; Zuo, X.; Davis, A.A.; McMillan, D.; Curry, B.B.; Richardson, J.A.; Benjamin, I.J. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. Embo J. 1999, 18, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Sigmond, T.; Hotzi, B.; Bohár, B.; Fazekas, D.; Deák, V.; Vellai, T.; Barna, J. HSF1Base: A Comprehensive Database of HSF1 (Heat Shock Factor 1) Target Genes. Int. J. Mol. Sci. 2019, 20, 5815. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.-L.; Murphy, C.T.; Kenyon, C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Iser, W.B.; Wilson, M.A.; Wood, W.H.; Becker, K.G.; Wolkow, C.A. Co-Regulation of the DAF-16 Target Gene, cyp-35B1/dod-13, by HSF-1 in C. elegans Dauer Larvae and daf-2 Insulin Pathway Mutants. PLoS ONE 2011, 6, e17369. [Google Scholar] [CrossRef]
- Gutsmann-Conrad, A.; Heydari, A.R.; You, S.; Richardson, A. The Expression of Heat Shock Protein 70 Decreases with Cellular Senescencein Vitroand in Cells Derived from Young and Old Human Subjects. Exp. Cell Res. 1998, 241, 404–413. [Google Scholar] [CrossRef]
- Carnemolla, A.; Labbadia, J.P.; Lazell, H.; Neueder, A.; Moussaoui, S.; Bates, G. Contesting the dogma of an age-related heat shock response impairment: Implications for cardiac-specific age-related disorders. Hum. Mol. Genet. 2014, 23, 3641–3656. [Google Scholar] [CrossRef]
- Adachi, M.; Liu, Y.; Fujii, K.; Calderwood, S.K.; Nakai, A.; Imai, K.; Shinomura, Y. Oxidative Stress Impairs the Heat Stress Response and Delays Unfolded Protein Recovery. PLoS ONE 2009, 4, e7719. [Google Scholar] [CrossRef]
- Lechler, M.C.; Crawford, E.D.; Groh, N.; Widmaier, K.; Jung, R.; Kirstein, J.; Trinidad, J.C.; Burlingame, A.L.; David, D.C. Reduced Insulin/IGF-1 Signaling Restores the Dynamic Properties of Key Stress Granule Proteins during Aging. Cell Rep. 2017, 18, 454–467. [Google Scholar] [CrossRef]
- Blake, M.J.; Fargnoli, J.; Gershon, D.; Holbrook, N.J. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. Am. J. Physiol. Integr. Comp. Physiol. 1991, 260, R663–R667. [Google Scholar] [CrossRef]
- Pardue, S.; Groshan, K.; Raese, J.; Morrison-Bogorad, M. Hsp70 mRNA induction is reduced in neurons of aged rat hippocampus after thermal stress. Neurobiol. Aging 1992, 13, 661–672. [Google Scholar] [CrossRef]
- Heydari, A.R.; Wu, B.; Takahashi, R.; Strong, R.; Richardson, A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol. Cell. Boil. 1993, 13, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- A Moore, S.; Lopez, A.; Richardson, A.; A Pahlavani, M. Effect of age and dietary restriction on expression of heat shock protein 70 in rat alveolar macrophages. Mech. Ageing Dev. 1998, 104, 59–73. [Google Scholar] [CrossRef]
- Fonager, J.; Beedholm, R.; Clark, B.F.C.; Rattan, S. Mild stress-induced stimulation of heat-shock protein synthesis and improved functional ability of human fibroblasts undergoing aging in vitro. Exp. Gerontol. 2002, 37, 1223–1228. [Google Scholar] [CrossRef]
- Tandara, A.A.; Kloeters, O.; Kim, I.; Mogford, J.E.; Mustoe, T.A. Age Effect on HSP70: Decreased Resistance to Ischemic and Oxidative Stress in HDF. J. Surg. Res. 2006, 132, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Njemini, R.; Demanet, C.; Mets, T. Aging-related differences in basal heatshock protein 70 levels in lymphocytes are linked to altered frequencies of lymphocyte subsets. Aging Cell 2008, 7, 498–505. [Google Scholar] [CrossRef]
- Chung, L.; Ng, Y.-C. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2006, 1762, 103–109. [Google Scholar] [CrossRef]
- Vasilaki, A.; Jackson, M.J.; McArdle, A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve 2002, 25, 902–905. [Google Scholar] [CrossRef]
- Nardai, G.; Csermely, P.; Söti, C. Chaperone function and chaperone overload in the aged. A preliminary analysis. Exp. Gerontol. 2002, 37, 1257–1262. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, J.H.; Choi, J.H.; Yoo, K.-Y.; Ryu, P.D.; Won, M.-H. Heat shock protein 90 and its cochaperone, p23, are markedly increased in the aged gerbil hippocampus. Exp. Gerontol. 2011, 46, 768–772. [Google Scholar] [CrossRef]
- Karvinen, S.M.; Silvennoinen, M.; Vainio, P.; Sistonen, L.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Effects of intrinsic aerobic capacity, aging and voluntary running on skeletal muscle sirtuins and heat shock proteins. Exp. Gerontol. 2016, 79, 46–54. [Google Scholar] [CrossRef]
- Piec, I.; Listrat, A.; Alliot, J.; Chambon, C.; Taylor, R.G.; Bechet, D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005, 19, 1143–1145. [Google Scholar] [CrossRef]
- Brehme, M.; Voisine, C.; Rolland, T.; Wachi, S.; Soper, J.H.; Zhu, Y.; Orton, K.; Villella, A.; Garza, D.; Vidal, M.; et al. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 2014, 9, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 2013, 38, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Taldone, T.; Ochiana, S.O.; Patel, P.D.; Chiosis, G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol. Sci. 2014, 35, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Bascos, N.A.D.; Landry, S. A History of Molecular Chaperone Structures in the Protein Data Bank. Int. J. Mol. Sci. 2019, 20, 6195. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, P.; Picard, D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2010, 1803, 641–649. [Google Scholar] [CrossRef] [PubMed]
- McArdle, A.; Dillmann, W.H.; Mestril, R.; Faulkner, J.A.; Jackson, M.J. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2003, 18, 1–12. [Google Scholar] [CrossRef]
- Prodromou, C. Mechanisms of Hsp90 regulation. Biochem. J. 2016, 473, 2439–2452. [Google Scholar] [CrossRef]
- Neckers, L.M.; Blagg, B.; Haystead, T.; Trepel, J.B.; Whitesell, L.; Picard, D. Methods to validate Hsp90 inhibitor specificity, to identify off-target effects, and to rethink approaches for further clinical development. Cell Stress Chaperones 2018, 23, 467–482. [Google Scholar] [CrossRef]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef]
- Janssens, G.E.; Lin, X.-X.; Millan-Ariño, L.; Kavšek, A.; Sen, I.; Seinstra, R.I.; Stroustrup, N.; Nollen, E.A.; Riedel, C.G. Transcriptomics-Based Screening Identifies Pharmacological Inhibition of Hsp90 as a Means to Defer Aging. Cell Rep. 2019, 27, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.-P. Mammalian HspB1 (Hsp27) is a molecular sensor linked to the physiology and environment of the cell. Cell Stress Chaperones 2017, 22, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Carra, S.; Alberti, S.; Arrigo, P.A.; Benesch, J.L.; Benjamin, I.J.; Boelens, W.; Bartelt-Kirbach, B.; Brundel, B.J.J.M.; Buchner, J.; Bukau, B.; et al. The growing world of small heat shock proteins: From structure to functions. Cell Stress Chaperones 2017, 22, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Zoubeidi, A.; Gleave, M. Small heat shock proteins in cancer therapy and prognosis. Int. J. Biochem. Cell Boil. 2012, 44, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Arai, H.; Katayama, N.; Ishikawa, T.; Kikumoto, K.; Atomi, Y. Age-related increase of insoluble, phosphorylated small heat shock proteins in human skeletal muscle. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2007, 62, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Morris, J.K.; Zhang, H.; Bomhoff, G.L.; Geiger, P.C.; Stanford, J.A. Age-related changes in HSP25 expression in basal ganglia and cortex of F344/BN rats. Neurosci. Lett. 2010, 472, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Der Perng, M.; Wen, S.F.; Ijssel, P.V.D.; Prescott, A.R.; Quinlan, R.A. Desmin Aggregate Formation by R120G αB-Crystallin Is Caused by Altered Filament Interactions and Is Dependent upon Network Status in Cells. Mol. Boil. Cell 2004, 15, 2335–2346. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Connell, P.; Christians, E.S.; Yan, L.-J.; Taylor, R.P.; Orosz, A.; Zhang, X.Q.; Stevenson, T.J.; Peshock, R.; Leopold, J.A.; et al. Human αB-Crystallin Mutation Causes Oxido-Reductive Stress and Protein Aggregation Cardiomyopathy in Mice. Cell 2007, 130, 427–439. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Pedro, J.M.B.-S.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. Embo J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Yang, Y.; Klionsky, D.J. Autophagy and disease: Unanswered questions. Cell Death Differ. 2020, 27, 858–871. [Google Scholar] [CrossRef]
- Kametaka, S.; Okano, T.; Ohsumi, M.; Ohsumi, Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 22284–22291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Kawamata, T.; Kamada, Y.; Kabeya, Y.; Sekito, T.; Ohsumi, Y. Organization of the Pre-autophagosomal Structure Responsible for Autophagosome Formation. Mol. Boil. Cell 2008, 19, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Fimia, G.M.; Stoykova, A.; Romagnoli, A.; Giunta, L.; Di Bartolomeo, S.; Nardacci, R.; Corazzari, M.; Fuoco, C.; Ucar, A.; Schwartz, P.; et al. Ambra1 regulates autophagy and development of the nervous system. Nature 2007, 447, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Suzuki, K.; Akioka, M.; Kondo-Kakuta, C.; Yamamoto, H.; Ohsumi, Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J. Cell Sci. 2013, 126, 2534–2544. [Google Scholar] [CrossRef]
- Tóth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdélyi, P.; Takács-Vellai, K.; Orosz, L.; Kovács, A.L.; Csikós, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef]
- Simonsen, A.; Cumming, R.C.; Brech, A.; Isakson, P.; Schubert, D.R.; Finley, K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 2007, 4, 176–184. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Singh, R. Autophagy and aging. Single Mol. Single Cell Seq. 2015, 847, 73–87. [Google Scholar] [CrossRef]
- Cassidy, L.D.; Young, A.R.J.; Young, C.N.J.; Soilleux, E.J.; Fielder, E.; Weigand, B.M.; Lagnado, A.; Brais, R.; Ktistakis, N.T.; Wiggins, K.A.; et al. Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat. Commun. 2020, 11, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Garcia-Muse, T. Causes of Genome Instability. Annu. Rev. Genet. 2013, 47, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Menck, C.F.; Leandro, G.S. Autophagy Roles in the Modulation of DNA Repair Pathways. Int. J. Mol. Sci. 2017, 18, 2351. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, E.; Cuervo, A.M. Chaperone-Mediated Autophagy. Proc. Am. Thorac. Soc. 2010, 7, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A. Influence of Normal Aging on Brain Autophagy: A Complex Scenario. Front. Aging Neurosci. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Dice, J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The Chaperone-Mediated Autophagy Receptor Organizes in Dynamic Protein Complexes at the Lysosomal Membrane. Mol. Cell. Boil. 2008, 28, 5747–5763. [Google Scholar] [CrossRef]
- Arias, E.; Koga, H.; Diaz, A.; Mocholí, E.; Patel, B.; Cuervo, A.M. Lysosomal mTORC2/PHLPP1/Akt Regulate Chaperone-Mediated Autophagy. Mol. Cell 2015, 59, 270–284. [Google Scholar] [CrossRef]
- Alfaro, I.E.; Albornoz, A.; Molina, A.; Moreno, J.; Cordero, K.; Criollo, A.; Budini, M. Chaperone Mediated Autophagy in the Crosstalk of Neurodegenerative Diseases and Metabolic Disorders. Front. Endocrinol. 2019, 9, 778. [Google Scholar] [CrossRef]
- Arias, E.; Cuervo, A.M. Pros and Cons of Chaperone-Mediated Autophagy in Cancer Biology. Trends Endocrinol. Metab. 2019, 31, 53–66. [Google Scholar] [CrossRef]
- Dong, S.; Aguirre-Hernandez, C.; Scrivo, A.; Eliscovich, C.; Arias, E.; Bravo-Cordero, J.J.; Cuervo, A.M. Monitoring spatiotemporal changes in chaperone-mediated autophagy in vivo. Nat. Commun. 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.W.-L.; Leung, C.-T.; Liu, H.; Pang, S.Y.-Y.; Lam, C.S.-C.; Xian, J.; Li, L.; Kung, M.H.-W.; Ramsden, D.B.; Ho, S.-L. Age-dependent accumulation of oligomeric SNCA/α-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: Role for therapeutic activation of chaperone-mediated autophagy (CMA). Autophagy 2019, 16, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. Chaperone-Mediated Autophagy. Breast Cancer 2008, 445, 227–244. [Google Scholar] [CrossRef]
- Kiffin, R.; Kaushik, S.; Zeng, M.; Bandyopadhyay, U.; Zhang, C.; Massey, A.C.; Martinez-Vicente, M.; Cuervo, A.M. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J. Cell Sci. 2007, 120, 782–791. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. Age-related Decline in Chaperone-mediated Autophagy. J. Boil. Chem. 2000, 275, 31505–31513. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, J.A.; Cuervo, A.M. Dietary lipids and aging compromise chaperone-mediated autophagy by similar mechanisms. Autophagy 2012, 8, 1152–1154. [Google Scholar] [CrossRef][Green Version]
- Koren, I.; Timms, R.T.; Kula, T.; Xu, Q.; Li, M.Z.; Elledge, S.J. The Eukaryotic Proteome Is Shaped by E3 Ubiquitin Ligases Targeting C-Terminal Degrons. Cell 2018, 173, 1622–1635. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Suryadinata, R.; Roesley, S.N.A.; Yang, G.; Sarcevic, B. Mechanisms of Generating Polyubiquitin Chains of Different Topology. Cells 2014, 3, 674–689. [Google Scholar] [CrossRef]
- Kirisako, T.; Kamei, K.; Murata, S.; Kato, M.; Fukumoto, H.; Kanie, M.; Sano, S.; Tokunaga, F.; Tanaka, K.; Iwai, K. A ubiquitin ligase complex assembles linear polyubiquitin chains. Embo J. 2006, 25, 4877–4887. [Google Scholar] [CrossRef]
- Nijman, S.M.; Luna-Vargas, M.; Velds, A.; Brummelkamp, T.R.; Dirac, A.M.; Sixma, T.K.; Bernards, R. A Genomic and Functional Inventory of Deubiquitinating Enzymes. Cell 2005, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Connell, P.; Ballinger, C.A.; Jiang, J.; Wu, Y.; Thompson, L.J.; Höhfeld, J.; Patterson, C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nature 2000, 3, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xu, X.; Duan, X.; Guo, J.; Wang, Y.; Ren, F.; He, D.-C.; Chang, Z. Hsp70 and Hsp90 oppositely regulate TGF-β signaling through CHIP/Stub1. Biochem. Biophys. Res. Commun. 2014, 446, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Zou, Z.; Xiang, Y.; Chen, S.; Tian, X.-L. Conserved signaling pathways genetically associated with longevity across the species. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1745–1755. [Google Scholar] [CrossRef]
- Tawo, R.; Pokrzywa, W.; Kevei, E.; Akyuz, M.E.; Balaji, V.; Adrian, S.; Höhfeld, J.; Hoppe, T. The Ubiquitin Ligase CHIP Integrates Proteostasis and Aging by Regulation of Insulin Receptor Turnover. Cell 2017, 169, 470–482. [Google Scholar] [CrossRef]
- Scott, M.R.; Rubio, M.D.; Haroutunian, V.; Meador-Woodruff, J.H. Protein Expression of Proteasome Subunits in Elderly Patients with Schizophrenia. Neuropsychopharmacology 2015, 41, 896–905. [Google Scholar] [CrossRef]
- Hwang, J.S.; Chang, I.; Kim, S. Age-Associated Decrease in Proteasome Content and Activities in Human Dermal Fibroblasts: Restoration of Normal Level of Proteasome Subunits Reduces Aging Markers in Fibroblasts From Elderly Persons. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2007, 62, 490–499. [Google Scholar] [CrossRef]
- Tomita, T.; Hirayama, S.; Sakurai, Y.; Ohte, Y.; Yoshihara, H.; Saeki, Y.; Hamazaki, J.; Murata, S. Specific Modification of Aged Proteasomes Revealed by Tag-Exchangeable Knock-In Mice. Mol. Cell. Boil. 2018, 39, e00426-18. [Google Scholar] [CrossRef]
- Vernace, V.A.; Arnaud, L.; Schmidt-Glenewinkel, T.; Figueiredo-Pereira, M.E. Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J. 2007, 21, 2672–2682. [Google Scholar] [CrossRef]
- Thibaudeau, T.A.; Anderson, R.T.; Smith, D. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat. Commun. 2018, 9, 1097. [Google Scholar] [CrossRef]
- Andersson, V.; Hanzén, S.; Liu, B.; Molin, M.; Nystrom, T. Enhancing protein disaggregation restores proteasome activity in aged cells. Aging 2013, 5, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Husom, A.D.; A Peters, E.; A Kolling, E.; A Fugere, N.; Thompson, L.V.; A Ferrington, D. Altered proteasome function and subunit composition in aged muscle. Arch. Biochem. Biophys. 2004, 421, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Pacelli, F.; Costelli, P.; Tortorelli, A.; Rosa, F.; Doglietto, G.B. Proteasome activities in the rectus abdominis muscle of young and older individuals. Biogerontology 2008, 9, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Viteri, G.; Carrard, G.; Birlouez-Aragon, I.; Silva, E.; Friguet, B. Age-dependent protein modifications and declining proteasome activity in the human lens. Arch. Biochem. Biophys. 2004, 427, 197–203. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ovaa, H.; Ponnappan, U. Lower expression of catalytic and structural subunits of the proteasome contributes to decreased proteolysis in peripheral blood T lymphocytes during aging. Int. J. Biochem. Cell Boil. 2007, 39, 799–809. [Google Scholar] [CrossRef]
- Petropoulos, I.; Conconi, M.; Wang, X.; Hoenel, B.; Brégégère, F.; Milner, Y.; Friguet, B. Increase of oxidatively modified protein is associated with a decrease of proteasome activity and content in aging epidermal cells. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2000, 55, B220–B227. [Google Scholar] [CrossRef]
- Kozieł, R.; Greussing, R.; Maier, A.B.; Declercq, L.; Jansen-Duerr, P. Functional Interplay between Mitochondrial and Proteasome Activity in Skin Aging. J. Investig. Dermatol. 2011, 131, 594–603. [Google Scholar] [CrossRef]
- Bulteau, A.-L.; Szweda, L.I.; Friguet, B. Age-Dependent Declines in Proteasome Activity in the Heart. Arch. Biochem. Biophys. 2002, 397, 298–304. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Huang, F.; Markesbery, W. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience 2000, 98, 149–156. [Google Scholar] [CrossRef]
- Breusing, N.; Arndt, J.; Voss, P.; Bresgen, N.; Wiswedel, I.; Gardemann, A.; Siems, W.; Grune, T. Inverse correlation of protein oxidation and proteasome activity in liver and lung. Mech. Ageing Dev. 2009, 130, 748–753. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Ebenezer, P.; Liu, Y.; Fernandez-Kim, S.O.; Bruce-Keller, A.J. Aging and dietary restriction alter proteasome biogenesis and composition in the brain and liver. Mech. Ageing Dev. 2009, 130, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kapphahn, R.J.; Bigelow, E.J.; Ferrington, D.A. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp. Eye Res. 2007, 84, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.-Y.; Medhurst, A.; Jackson, M.; Rose, S.; Jenner, P. Proteasomal activity in brain differs between species and brain regions and changes with age. Mech. Ageing Dev. 2005, 126, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Husom, A.D.; Thompson, L.V. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005, 19, 1–24. [Google Scholar] [CrossRef]
- Kabashi, E.; Agar, J.N.; Taylor, D.M.; Minotti, S.; Durham, H.D. Focal dysfunction of the proteasome: A pathogenic factor in a mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2004, 89, 1325–1335. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Ebenezer, P.; Fernandez-Kim, S.O.; Bruce-Keller, A.J.; Szweda, L.I.; Bruce-Keller, A.J. Proteasome alterations during adipose differentiation and aging: Links to impaired adipocyte differentiation and development of oxidative stress. Free Radic. Boil. Med. 2011, 51, 1727–1735. [Google Scholar] [CrossRef][Green Version]
- Chondrogianni, N.; Stratford, F.L.L.; Trougakos, I.P.; Friguet, B.; Gonos, E.S.; Rivett, A.J. Central Role of the Proteasome in Senescence and Survival of Human Fibroblasts. J. Boil. Chem. 2003, 278, 28026–28037. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Tzavelas, C.; Pemberton, A.J.; Nezis, I.; Gonos, E.S.; Rivett, A.J. Overexpression of Proteasome 5 Assembled Subunit Increases the Amount of Proteasome and Confers Ameliorated Response to Oxidative Stress and Higher Survival Rates. J. Boil. Chem. 2005, 280, 11840–11850. [Google Scholar] [CrossRef]
- Motosugi, R.; Murata, S. Dynamic Regulation of Proteasome Expression. Front. Mol. Biosci. 2019, 6, 30. [Google Scholar] [CrossRef]
- Njomen, E.; Tepe, J.J. Proteasome Activation as a New Therapeutic Approach to Target Proteotoxic Disorders. J. Med. Chem. 2019, 62, 6469–6481. [Google Scholar] [CrossRef]
- Ding, Q.; Reinacker, K.; Dimayuga, E.; Nukala, V.; Drake, J.; Butterfield, D.A.; Dunn, J.C.; Martin, S.; Bruce-Keller, A.J.; Bruce-Keller, A.J. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 2003, 546, 228–232. [Google Scholar] [CrossRef]
- Beedholm, R.; Clark, B.F.; Rattan, S. Mild heat stress stimulates 20S proteasome and its 11S activator in human fibroblasts undergoing aging in vitro. Cell Stress Chaperones 2004, 9, 49–57. [Google Scholar] [CrossRef]
- Taylor, D.M.; Kabashi, E.; Agar, J.N.; Minotti, S.; Durham, H.D. Proteasome activity or expression is not altered by activation of the heat shock transcription factor Hsf1 in cultured fibroblasts or myoblasts. Cell Stress Chaperones 2005, 10, 230–241. [Google Scholar] [CrossRef] [PubMed]
- LeComte, S.; Desmots, F.; Le Masson, F.; Le Goff, P.; Michel, D.; Christians, E.S.; Le Dréan, Y. Roles of heat shock factor 1 and 2 in response to proteasome inhibition: Consequence on p53 stability. Oncogene 2010, 29, 4216–4224. [Google Scholar] [CrossRef]
- Bozaykut, P.; Sozen, E.; Kaga, E.; Ece, A.; Ozaltin, E.; Bergquist, J.; Ozer, N.K.; Yilmaz, B.K. HSP70 Inhibition Leads to the Activation of Proteasomal System under Mild Hyperthermia Conditions in Young and Senescent Fibroblasts. Oxidative Med. Cell. Longev. 2020, 2020, 9369524. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Catalgol, B.; Licht, A.; Ermak, G.; Pickering, A.M.; Ngo, J.K.; Davies, K.E. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic. Boil. Med. 2011, 51, 1355–1364. [Google Scholar] [CrossRef]
- Imai, J.; Maruya, M.; Yashiroda, H.; Yahara, I.; Tanaka, K. The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. Embo J. 2003, 22, 3557–3567. [Google Scholar] [CrossRef]
- Barna, J.; Csermely, P.; Vellai, T. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 2018, 75, 2897–2916. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsujimura, A.; Taguchi, K.; Tanaka, M. HSF1 stress response pathway regulates autophagy receptor SQSTM1/p62-associated proteostasis. Autophagy 2016, 13, 133–148. [Google Scholar] [CrossRef]
- Aparicio, R.; Hansen, M.; Walker, D.W.; Kumsta, C. The selective autophagy receptor SQSTM1/p62 improves lifespan and proteostasis in an evolutionarily conserved manner. Autophagy 2020, 16, 772–774. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Lee, R.; Tan, E.P.; Yang, Y.; Loureiro, R.; Choy, E.H.; Lim, S.H.Y.; Saez, I.; Springhorn, A.; et al. The autophagy receptor p62/SQST-1 promotes proteostasis and longevity in C. elegans by inducing autophagy. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.-I.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Nezis, I.; Stenmark, H. p62 at the Interface of Autophagy, Oxidative Stress Signaling, and Cancer. Antioxid. Redox Signal. 2012, 17, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Rodríguez-Enríquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Sabatini, D.M. Redox Regulation of the Nutrient-sensitive Raptor-mTOR Pathway and Complex. J. Boil. Chem. 2005, 280, 39505–39509. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-H.; Cao, J.; Tang, Z.; Dai, S.; He, Y.; Sampson, S.B.; Benjamin, I.J.; Dai, C. HSF1 critically attunes proteotoxic stress sensing by mTORC1 to combat stress and promote growth. Nature 2016, 18, 527–539. [Google Scholar] [CrossRef]
- Dai, S.; Tang, Z.; Cao, J.; Zhou, W.; Li, H.; Sampson, S.; Dai, C. Suppression of the HSF 1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. Embo J. 2014, 34, 275–293. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y. AMPK and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 85–108. [Google Scholar] [CrossRef]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD + elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef]
- Piskovatska, V.; Stefanyshyn, N.; Storey, K.B.; Vaiserman, A.; Lushchak, O. Metformin as a geroprotector: Experimental and clinical evidence. Biogerontology 2018, 20, 33–48. [Google Scholar] [CrossRef]
- Joshi, V.; Amanullah, A.; Upadhyay, A.; Mishra, R.; Kumar, A.; Mishra, A. A Decade of Boon or Burden: What Has the CHIP Ever Done for Cellular Protein Quality Control Mechanism Implicated in Neurodegeneration and Aging? Front. Mol. Neurosci. 2016, 9, 5115. [Google Scholar] [CrossRef] [PubMed]
- McDonough, H.; Patterson, C. CHIP: A link between the chaperone and proteasome systems. Cell Stress Chaperones 2003, 8, 303–308. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, C.; Wu, Y.; McDonough, H.; Whaley, R.A.; Godfrey, V.; Li, H.; Madamanchi, N.; Xu, W.; Neckers, L.; et al. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. Embo J. 2003, 22, 5446–5458. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Chen, S.; Lu, J.; Wang, X.; Liu, Q.; Zhang, Y.; Long, Y.; Hu, Z.; Xu, G. The E3 ubiquitin ligase STUB1 attenuates cell senescence by promoting the ubiquitination and degradation of the core circadian regulator BMAL1. J. Boil. Chem. 2020, 295, 4696–4708. [Google Scholar] [CrossRef]
- Sha, Y.; Rao, L.; Settembre, C.; Ballabio, A.; Eissa, N.T. STUB 1 regulates TFEB -induced autophagy-lysosome pathway. Embo J. 2017, 36, 2544–2552. [Google Scholar] [CrossRef]
- Guo, D.; Ying, Z.; Wang, H.; Chen, N.; Gao, F.; Ren, H.-G.; Wang, G. Regulation of autophagic flux by CHIP. Neurosci. Bull. 2015, 31, 469–479. [Google Scholar] [CrossRef]
- Ravi, S.; Parry, T.L.; Willis, M.S.; Lockyer, P.; Patterson, C.; Bain, J.R.; Stevens, R.D.; Ilkayeva, O.R.; Newgard, C.B.; Schisler, J. Adverse Effects of Fenofibrate in Mice Deficient in the Protein Quality Control Regulator, CHIP. J. Cardiovasc. Dev. Dis. 2018, 5, 43. [Google Scholar] [CrossRef]
- Lizama, B.; Palubinsky, A.; Raveendran, V.A.; Moore, A.M.; Federspiel, J.D.; Codreanu, S.; Liebler, D.C.; McLaughlin, B. Neuronal Preconditioning Requires the Mitophagic Activity of C-terminus of HSC70-Interacting Protein. J. Neurosci. 2018, 38, 6825–6840. [Google Scholar] [CrossRef]
- Takayama, S.; Reed, J.C. Molecular chaperone targeting and regulation by BAG family proteins. Nature 2001, 3, E237–E241. [Google Scholar] [CrossRef]
- Kabbage, M.; Dickman, M. The BAG proteins: A ubiquitous family of chaperone regulators. Cell. Mol. Life Sci. 2008, 65, 1390–1402. [Google Scholar] [CrossRef]
- Stürner, E.; Behl, C. The Role of the Multifunctional BAG3 Protein in Cellular Protein Quality Control and in Disease. Front. Mol. Neurosci. 2017, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Gamerdinger, M.; Hajieva, P.; Kaya, A.M.; Wolfrum, U.; Hartl, F.U.; Behl, C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. Embo J. 2009, 28, 889–901. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.; VanDusseldorp, T.; Ulrich, C.; Lanphere, R.; Dokladny, K.; Mosely, P.; Mermier, C. The effect of aging on the autophagic and heat shock response in human peripheral blood mononuclear cells. Physiol. Int. 2018, 105, 247–256. [Google Scholar] [CrossRef] [PubMed]

| Subject | Normal Conditions | Response to Stress | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSF1 | mRNA | Protein | HSF1 | mRNA | Protein | ||||||||
| The Whole HSR | Y | O | Y | O | Y | O | Y | O | Y | O | Y | O | |
| Mouse brain cells | + | + | + | + | + | + | [26] | ||||||
| Mouse myocardium | ++ | + | ++ | + | ++ | + | [26] | ||||||
| Hsp70 | |||||||||||||
| Rat brain, lung, skin | ++ | + | [29] | ||||||||||
| Rat neuron | ++ | + | [30] | ||||||||||
| Rat hepatocytes | ++ | + | ++ | + | ++ | + | [31] | ||||||
| Rat macrophages | ++ | + | ++ | + | ++ | + | [32] | ||||||
| IMR-90 cells, human fibroblasts | ++ | + | ++ | + | ++ | + | [25] | ||||||
| Human fibroblasts (senescence) | + | ++ | [33] | ||||||||||
| Human dermal fibroblasts | ++ | + | + | + | + | + | ++ | + | [34] | ||||
| Human monocytes, lymphocytes | ++ | + | [35] | ||||||||||
| Rat skeletal muscle | + | ++ | [36] | ||||||||||
| Rat muscle | ++ | + | ++ | + | [37] | ||||||||
| Hsp90 | |||||||||||||
| Human fibroblasts | ++ | + | [33] | ||||||||||
| Rat liver (chaperone activity) | ++ | + | [38] | ||||||||||
| Gerbil brain cells | + | ++ | [39] | ||||||||||
| Hsp27 (small Hsp family) | |||||||||||||
| Human fibroblasts | + | ++ | [33] | ||||||||||
| Rat skeletal muscle | + | ++ | [36] | ||||||||||
| Rat muscle | + | ++ | [40] | ||||||||||
| Hsp20 & Hsp22 (small Hsp family) | |||||||||||||
| Gerbil brain cells | + | ++ | [39] | ||||||||||
| Rat muscle | + | ++ | [41] | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margulis, B.; Tsimokha, A.; Zubova, S.; Guzhova, I. Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis in Aging Cells. Cells 2020, 9, 1308. https://doi.org/10.3390/cells9051308
Margulis B, Tsimokha A, Zubova S, Guzhova I. Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis in Aging Cells. Cells. 2020; 9(5):1308. https://doi.org/10.3390/cells9051308
Chicago/Turabian StyleMargulis, Boris, Anna Tsimokha, Svetlana Zubova, and Irina Guzhova. 2020. "Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis in Aging Cells" Cells 9, no. 5: 1308. https://doi.org/10.3390/cells9051308
APA StyleMargulis, B., Tsimokha, A., Zubova, S., & Guzhova, I. (2020). Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis in Aging Cells. Cells, 9(5), 1308. https://doi.org/10.3390/cells9051308







