Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies
Abstract
:1. Introduction
2. Identification and Plasticity of LCSCs
2.1. Concept of Cancer Stem Cells (CSCs)
2.2. Correlation between Hepatocellular Carcinoma and Cancer Stem Cells
2.3. EpCAM
2.4. CD133
2.5. CD44
2.6. CD13
2.7. CD90
2.8. CD24
2.9. OV-6
2.10. Side Population Cells
2.11. CD47
3. Interactions of LCSCs Influencing HCC and Therapeutic Strategies
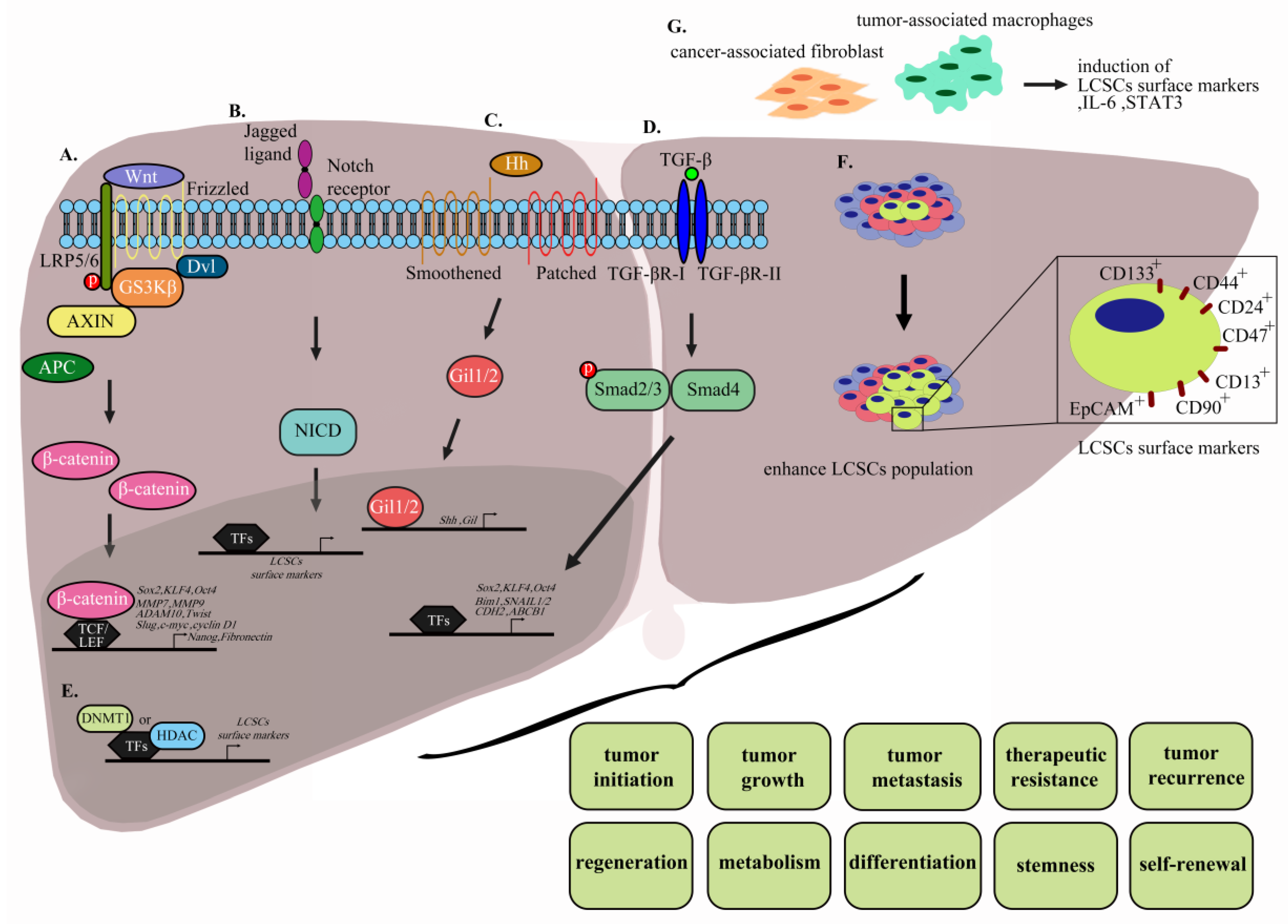
3.1. The Wnt/β-Catenin Pathway
3.2. Notch Signaling
3.3. Hedgehog Signaling Pathway
3.4. TGF-β Signaling Pathway
3.5. Targeting of LCSC Surface Markers
3.6. Epigenetic Changes
3.7. MicroRNAs and Long Non-Coding RNAs
3.8. The LCSC Microenvironment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamashita, T.; Wang, X.W. Cancer stem cells in the development of liver cancer. J. Clin. Investig. 2013, 123, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E. Review article: Steatosis, the metabolic syndrome and cancer. Aliment Pharmacol. Ther. 2005, 22 (Suppl. 2), 40–43. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Newell, P.; Chiang, D.Y.; Friedman, S.L.; Llovet, J.M. Genomics and signaling pathways in hepatocellular carcinoma. Semin. Liver Dis 2007, 27, 55–76. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Pan, J.; Lian, Z. Early molecular and genetic determinants of primary liver malignancy. Surg. Clin. North Am. 2004, 84, 339–354. [Google Scholar] [CrossRef]
- Wang, X.W.; Hussain, S.P.; Huo, T.I.; Wu, C.G.; Forgues, M.; Hofseth, L.J.; Brechot, C.; Harris, C.C. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology 2002, 181–182, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Thorgeirsson, S.S.; Grisham, J.W. Molecular pathogenesis of human hepatocellular carcinoma. Nat. Genet 2002, 31, 339–346. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lu, L.F.; Li, C.J.; Sun, N.K.; Guo, J.Y.; Huang, Y.H.; Yeh, C.T.; Chao, C.C. Hepatitis B Virus X Protein Induces RHAMM-Dependent Motility in Hepatocellular Carcinoma Cells via PI3K-Akt-Oct-1 Signaling. Mol. Cancer Res. 2020, 18, 375–389. [Google Scholar] [CrossRef]
- Whittaker, S.; Marais, R.; Zhu, A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010, 29, 4989–5005. [Google Scholar] [CrossRef]
- Vu, N.B.; Nguyen, T.T.; Tran, L.C.; Do, C.D.; Nguyen, B.H.; Phan, N.K.; Pham, P.V. Doxorubicin and 5-fluorouracil resistant hepatic cancer cells demonstrate stem-like properties. Cytotechnology 2013, 65, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Cacerescortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A Cell Initiating Human Acute Myeloid-Leukemia after Transplantation into Scid Mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.F.B.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.W.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.J.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Maugeri-Sacca, M.; Vigneri, P.; De Maria, R. Cancer stem cells and chemosensitivity. Clin. Cancer Res. 2011, 17, 4942–4947. [Google Scholar] [CrossRef] [Green Version]
- Calcagno, A.M.; Salcido, C.D.; Gillet, J.P.; Wu, C.P.; Fostel, J.M.; Mumau, M.D.; Gottesman, M.M.; Varticovski, L.; Ambudkar, S.V. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J. Natl. Cancer Inst. 2010, 102, 1637–1652. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Wang, X.W. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin. Oncol. 2012, 39, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Lasky, J.L., 3rd; Wu, H. Cancer stem cells. Pediatrics Res. 2006, 59, 59R–64R. [Google Scholar] [CrossRef]
- Zhu, Z.; Hao, X.; Yan, M.; Yao, M.; Ge, C.; Gu, J.; Li, J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int. J. Cancer 2010, 126, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chan, K.W.; Lee, T.K.; Tang, K.H.; Wo, J.Y.; Zheng, B.J.; Guan, X.Y. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol. Cancer Res. 2008, 6, 1146–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Li, H.; Li, J.; Zhu, Z.; Yin, S.; Hao, X.; Yao, M.; Zheng, S.; Gu, J. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis 2008, 29, 2289–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, N.; Utsunomiya, T.; Inoue, H.; Tanaka, F.; Mimori, K.; Barnard, G.F.; Mori, M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 2006, 24, 506–513. [Google Scholar] [CrossRef]
- Xin, H.W.; Ambe, C.M.; Hari, D.M.; Wiegand, G.W.; Miller, T.C.; Chen, J.Q.; Anderson, A.J.; Ray, S.; Mullinax, J.E.; Koizumi, T.; et al. Label-retaining liver cancer cells are relatively resistant to sorafenib. Gut 2013, 62, 1777–1786. [Google Scholar] [CrossRef]
- Piao, L.S.; Hur, W.; Kim, T.K.; Hong, S.W.; Kim, S.W.; Choi, J.E.; Sung, P.S.; Song, M.J.; Lee, B.C.; Hwang, D.; et al. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012, 315, 129–137. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.J.; Chan, K.W.; Guan, X.Y. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008, 27, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.T.; Yang, Z.F.; Ho, D.W.; Ng, M.N.; Yu, W.C.; Wong, J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: A prospective study. Ann. Surg. 2011, 254, 569–576. [Google Scholar] [CrossRef]
- Philip, P.A.; Mooney, M.; Jaffe, D.; Eckhardt, G.; Moore, M.; Meropol, N.; Emens, L.; O’Reilly, E.; Korc, M.; Ellis, L.; et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J. Clin. Oncol. 2009, 27, 5660–5669. [Google Scholar] [CrossRef]
- Yamashita, T.; Budhu, A.; Forgues, M.; Wang, X.W. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007, 67, 10831–10839. [Google Scholar] [CrossRef] [Green Version]
- Behari, J. The Wnt/beta-catenin signaling pathway in liver biology and disease. Expert. Rev. Gastroenterol. Hepatol. 2010, 4, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Dorado, J.; Baeuerle, P.A.; Heeschen, C. EpCAM/CD3-Bispecific T-cell engaging antibody MT110 eliminates primary human pancreatic cancer stem cells. Clin. Cancer Res. 2012, 18, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, D.X.; Shi, J.; Zhang, Y.; Zhao, J.S.; Long, L.Y.; Chen, T.W.; Zhang, E.B.; Feng, Y.Y.; Bao, W.D.; Deng, Y.Z.; et al. Sorafenib enriches epithelial cell adhesion molecule-positive tumor initiating cells and exacerbates a subtype of hepatocellular carcinoma through TSC2-AKT cascade. Hepatology 2015, 62, 1791–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsugi, A.; Nagaki, M.; Aoki, H.; Motohashi, T.; Kunisada, T.; Moriwaki, H. Characterization of CD133(+) hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem. Bioph. Res. Commun. 2006, 351, 820–824. [Google Scholar] [CrossRef]
- Ma, S.; Chan, K.W.; Hu, L.; Lee, T.K.; Wo, J.Y.; Ng, I.O.; Zheng, B.J.; Guan, X.Y. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007, 132, 2542–2556. [Google Scholar] [CrossRef]
- Rawal, P.; Siddiqui, H.; Hassan, M.; Choudhary, M.C.; Tripathi, D.M.; Nain, V.; Trehanpati, N.; Kaur, S. Endothelial Cell-Derived TGF-beta Promotes Epithelial-Mesenchymal Transition via CD133 in HBx-Infected Hepatoma Cells. Front. Oncol. 2019, 9, 308. [Google Scholar] [CrossRef]
- Underhill, C. CD44: The hyaluronan receptor. J. Cell Sci. 1992, 103 Pt 2, 293–298. [Google Scholar]
- Bourguignon, L.Y. CD44-mediated oncogenic signaling and cytoskeleton activation during mammary tumor progression. J. Mammary Gland Biol. Neoplasia 2001, 6, 287–297. [Google Scholar] [CrossRef]
- Bourguignon, L.Y. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008, 18, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.Y.; Shiina, M.; Li, J.J. Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv. Cancer Res. 2014, 123, 255–275. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ruan, B.; Liu, W.; Wang, J.; Yang, X.; Zhang, Z.; Li, X.; Duan, J.; Zhang, F.; Ding, R.; et al. Knockdown of CD44 inhibits the invasion and metastasis of hepatocellular carcinoma both in vitro and in vivo by reversing epithelial-mesenchymal transition. Oncotarget 2015, 6, 7828–7837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, N.R.; Cha, J.H.; Jang, J.W.; Bae, S.H.; Jang, B.; Kim, J.H.; Hur, W.; Choi, J.Y.; Yoon, S.K. Synergistic effects of CD44 and TGF-beta1 through AKT/GSK-3beta/beta-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem. Biophys. Res. Commun. 2016, 477, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, N.; Ishii, H.; Mimori, K.; Tanaka, F.; Ohkuma, M.; Kim, H.M.; Akita, H.; Takiuchi, D.; Hatano, H.; Nagano, H.; et al. CD13 is a therapeutic target in human liver cancer stem cells. J. Clin. Investig. 2010, 120, 3326–3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhang, L.; Chen, J.; Li, C.; Sun, H.; Wang, J.; Xiao, H. Activation of Tyrosine Metabolism in CD13+ Cancer Stem Cells Drives Relapse in Hepatocellular Carcinoma. Cancer Res. Treat. 2019. [Google Scholar] [CrossRef]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.; Lam, C.T.; Poon, R.T.; Fan, S.T. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Zhang, X.; Deng, T.; Gao, J. Positive correlation of Oct4 and ABCG2 to chemotherapeutic resistance in CD90(+)CD133(+) liver cancer stem cells. Cell Reprogram 2013, 15, 143–150. [Google Scholar] [CrossRef]
- Sukowati, C.H.; Anfuso, B.; Torre, G.; Francalanci, P.; Croce, L.S.; Tiribelli, C. The expression of CD90/Thy-1 in hepatocellular carcinoma: An in vivo and in vitro study. PLoS ONE 2013, 8, e76830. [Google Scholar] [CrossRef]
- Xia, W.; Lo, C.M.; Poon, R.Y.C.; Cheung, T.T.; Chan, A.C.Y.; Chen, L.; Yang, S.; Tsao, G.S.W.; Wang, X.Q. Smad inhibitor induces CSC differentiation for effective chemosensitization in cyclin D1- and TGF-beta/Smad-regulated liver cancer stem cell-like cells. Oncotarget 2017, 8, 38811–38824. [Google Scholar] [CrossRef]
- Yang, X.R.; Xu, Y.; Yu, B.; Zhou, J.; Li, J.C.; Qiu, S.J.; Shi, Y.H.; Wang, X.Y.; Dai, Z.; Shi, G.M.; et al. CD24 Is a Novel Predictor for Poor Prognosis of Hepatocellular Carcinoma after Surgery. Clin. Cancer Res. 2009, 15, 5518–5527. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.K.W.; Castilho, A.; Cheung, V.C.H.; Tang, K.H.; Ma, S.; Irene, O.L.N. CD24(+) Liver Tumor-Initiating Cells Drive Self-Renewal and Tumor Initiation through STAT3-Mediated NANOG Regulation. Cell Stem Cell 2011, 9, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Yao, Y.; Xu, G.L.; Zhou, C.; Zhang, Y.; Sun, J.; Jiang, R.Q.; Shao, Q.; Chen, Y. CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Li, Y.W.; Tsung, A.; Huang, H.; Du, Q.; Yang, M.Q.; Deng, M.H.; Xiong, S.; Wang, X.J.; Zhang, L.Y.; et al. iNOS promotes CD24(+)CD133(+) liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E10127–E10136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Yan, H.X.; Chen, L.; Liu, Q.; He, Y.Q.; Yu, L.X.; Zhang, S.H.; Huang, D.D.; Tang, L.; Kong, X.N.; et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008, 68, 4287–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, M.D.; Cheng, P.; Huang, H.; Dong, W.; Zhang, W.W.; Li, P.P.; Lin, C.; Pan, Z.Y.; Wu, M.C.; et al. Hepatitis B virus X protein promotes the stem-like properties of OV6(+) cancer cells in hepatocellular carcinoma. Cell Death Dis. 2017, 8, e2560. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.M.; Xu, Y.; Fan, J.; Zhou, J.; Yang, X.R.; Qiu, S.J.; Liao, Y.; Wu, W.Z.; Ji, Y.; Ke, A.W.; et al. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J. Cancer Res. Clin. Oncol. 2008, 134, 1155–1163. [Google Scholar] [CrossRef]
- Lo, J.; Lau, E.Y.; Ching, R.H.; Cheng, B.Y.; Ma, M.K.; Ng, I.O.; Lee, T.K. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015, 62, 534–545. [Google Scholar] [CrossRef]
- Roberts, D.D.; Kaur, S.; Soto-Pantoja, D.R. Therapeutic targeting of the thrombospondin-1 receptor CD47 to treat liver cancer. J. Cell. Commun. Signal 2015, 9, 101–102. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zheng, D.X.; Yu, X.J.; Sun, H.W.; Xu, Y.T.; Zhang, Y.J.; Xu, J. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology 2019, 8, e1652540. [Google Scholar] [CrossRef]
- Oikawa, T.; Kamiya, A.; Zeniya, M.; Chikada, H.; Hyuck, A.D.; Yamazaki, Y.; Wauthier, E.; Tajiri, H.; Miller, L.D.; Wang, X.W.; et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013, 57, 1469–1483. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Li, W.; Shen, H.G.; Ren, R.; Xu, Z.H.; Wan, D.W.; Han, Y.; Kuang, Y.T.; Zhi, Q.M. SALL4 expression is associated with poor outcome in hepatocellular carcinoma. Int. J. Clin. Exp. Med. 2017, 10, 4267–4277. [Google Scholar]
- Yamashita, T.; Honda, M.; Nakamoto, Y.; Baba, M.; Nio, K.; Hara, Y.; Zeng, S.S.; Hayashi, T.; Kondo, M.; Takatori, H.; et al. Discrete nature of EpCAM+ and CD90+ cancer stem cells in human hepatocellular carcinoma. Hepatology 2013, 57, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, W.; Wang, J.H.; Liu, R. Evidence of CD90+CXCR4+cells as circulating tumor stem cells in hepatocellular carcinoma. Tumor. Biol. 2015, 36, 5353–5360. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Baeuerle, P.A.; Gires, O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009, 69, 5627–5629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelzer, E.; Reid, L.M. EpCAM expression in normal, non-pathological tissues. Front Biosci. 2008, 13, 3096–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Trzpis, M.; McLaughlin, P.M.; de Leij, L.M.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Alibolandi, M.; Ramezani, M.; Sadeghi, F.; Abnous, K.; Hadizadeh, F. Epithelial cell adhesion molecule aptamer conjugated PEG-PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro. Int. J. Pharm. 2015, 479, 241–251. [Google Scholar] [CrossRef]
- Sun, Y.F.; Xu, Y.; Yang, X.R.; Guo, W.; Zhang, X.; Qiu, S.J.; Shi, R.Y.; Hu, B.; Zhou, J.; Fan, J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 2013, 57, 1458–1468. [Google Scholar] [CrossRef]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Qin, L.X.; Wauthier, E.; et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef] [Green Version]
- Lacoste, B.; Raymond, V.A.; Cassim, S.; Lapierre, P.; Bilodeau, M. Highly tumorigenic hepatocellular carcinoma cell line with cancer stem cell-like properties. PLoS ONE 2017, 12, e0171215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nio, K.; Yamashita, T.; Okada, H.; Kondo, M.; Hayashi, T.; Hara, Y.; Nomura, Y.; Zeng, S.S.; Yoshida, M.; Hayashi, T.; et al. Defeating EpCAM(+) liver cancer stem cells by targeting chromatin remodeling enzyme CHD4 in human hepatocellular carcinoma. J. Hepatol. 2015, 63, 1164–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, C.J.; Li, C.J.; Wu, M.Y.; Chu, P.Y. Overexpression of epithelial cell adhesion molecule as a predictor of poor outcome in patients with hepatocellular carcinoma. Exp. Ther. Med. 2018, 16, 4810–4816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Lin, P.Y.; Ming, Y.Z.; Huang, W.C.; Chen, R.F.; Chen, P.M.; Chu, P.Y. The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer 2017, 17, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Guler, G.; Guven, U.; Oktem, G. Characterization of CD133(+)/CD44(+) human prostate cancer stem cells with ATR-FTIR spectroscopy. Analyst 2019, 144, 2138–2149. [Google Scholar] [CrossRef]
- Gzil, A.; Zarebska, I.; Bursiewicz, W.; Antosik, P.; Grzanka, D.; Szylberg, L. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol. Biol. Rep. 2019, 46, 6629–6645. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Fu, J.; Wang, W.; Hofman, F.M.; Chen, T.C.; Chen, L. Distribution of cancer stem cells in two human brain gliomas. Oncol. Lett. 2019, 17, 2123–2130. [Google Scholar] [CrossRef]
- Yin, S.; Li, J.; Hu, C.; Chen, X.; Yao, M.; Yan, M.; Jiang, G.; Ge, C.; Xie, H.; Wan, D.; et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int. J. Cancer 2007, 120, 1444–1450. [Google Scholar] [CrossRef]
- Song, W.; Li, H.; Tao, K.; Li, R.; Song, Z.; Zhao, Q.; Zhang, F.; Dou, K. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int. J. Clin. Pract. 2008, 62, 1212–1218. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Kamimura, H.; Takamura, M.; Yamagiwa, S.; Matsuda, Y.; Sato, Y.; Nomoto, M.; Ichida, T.; Aoyagi, Y. Clinicopathological analysis of CD133 and NCAM human hepatic stem/progenitor cells in damaged livers and hepatocellular carcinomas. Hepatol. Res. 2009, 39, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Zen, Y.; Fujii, T.; Sato, Y.; Ohta, T.; Aoyagi, Y.; Nakanuma, Y. Characterization of CD133+ parenchymal cells in the liver: Histology and culture. World J. Gastroenterol. 2009, 15, 4896–4906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, G.; Fu, X.; Xu, S.; Wang, T.; Zhang, Q.; Yang, Y. Aquaporin 3 maintains the stemness of CD133+ hepatocellular carcinoma cells by activating STAT3. Cell Death Dis. 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Hao, M.; Ouyang, Y.; Zheng, J.; Chen, D. CD133(+) cancer stem cells promoted by VEGF accelerate the recurrence of hepatocellular carcinoma. Sci. Rep. 2017, 7, 41499. [Google Scholar] [CrossRef]
- Qiu, M.T.; Hu, J.W.; Yin, R.; Xu, L. Long noncoding RNA: An emerging paradigm of cancer research. Tumour. Biol. 2013, 34, 613–620. [Google Scholar] [CrossRef]
- Ma, S.; Tang, K.H.; Chan, Y.P.; Lee, T.K.; Kwan, P.S.; Castilho, A.; Ng, I.; Man, K.; Wong, N.; To, K.F.; et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010, 7, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Zhao, E.; Kryczek, I.; Vatan, L.; Sadovskaya, A.; Ludema, G.; Simeone, D.M.; Zou, W.; Welling, T.H. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014, 147, 1393–1404. [Google Scholar] [CrossRef] [Green Version]
- Asai, R.; Tsuchiya, H.; Amisaki, M.; Makimoto, K.; Takenaga, A.; Sakabe, T.; Hoi, S.; Koyama, S.; Shiota, G. CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 2019, 8, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Okabe, H.; Ishimoto, T.; Hayashi, H.; Nakagawa, S.; Kuroki, H.; Watanabe, M.; Beppu, T.; Tamada, M.; Nagano, O.; et al. CD44s regulates the TGF-beta-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012, 72, 3414–3423. [Google Scholar] [CrossRef] [Green Version]
- Mima, K.; Hayashi, H.; Imai, K.; Kuroki, H.; Nakagawa, S.; Okabe, H.; Chikamoto, A.; Watanabe, M.; Beppu, T.; Baba, H. High CD44s expression is associated with the EMT expression profile and intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J. Hepatobiliary Pancreat. Sci. 2013, 20, 429–434. [Google Scholar] [CrossRef]
- Dhar, D.; Antonucci, L.; Nakagawa, H.; Kim, J.Y.; Glitzner, E.; Caruso, S.; Shalapour, S.; Yang, L.; Valasek, M.A.; Lee, S.; et al. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell 2018, 33, 1061–1077.e1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovuu, L.O.; Imura, S.; Utsunomiya, T.; Morine, Y.; Ikemoto, T.; Arakawa, Y.; Mori, H.; Hanaoka, J.; Kanamoto, M.; Sugimoto, K.; et al. Role of CD44 expression in non-tumor tissue on intrahepatic recurrence of hepatocellular carcinoma. Int. J. Clin. Oncol. 2013, 18, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Shipp, M.A.; Look, A.T. Hematopoietic Differentiation Antigens That Are Membrane-Associated Enzymes - Cutting Is the Key. Blood 1993, 82, 1052–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.M.; Haraguchi, N.; Ishii, H.; Ohkuma, M.; Okano, M.; Mimori, K.; Eguchi, H.; Yamamoto, H.; Nagano, H.; Sekimoto, M.; et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann. Surg Oncol. 2012, 19 (Suppl. 3), S539–S548. [Google Scholar] [CrossRef]
- Yamanaka, C.; Wada, H.; Eguchi, H.; Hatano, H.; Gotoh, K.; Noda, T.; Yamada, D.; Asaoka, T.; Kawamoto, K.; Nagano, H.; et al. Clinical significance of CD13 and epithelial mesenchymal transition (EMT) markers in hepatocellular carcinoma. Jpn. J. Clin. Oncol. 2018, 48, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Barboni, E.; Gormley, A.M.; Rivero, F.B.P.; Vidal, M.; Morris, R.J. Activation of Lymphocytes-T by Cross-Linking of Glycophospholipid-Anchored Thy-1 Mobilizes Separate Pools of Intracellular 2nd Messengers to Those Induced by the Antigen Receptor Cd3 Complex. Immunology 1991, 72, 457–463. [Google Scholar] [PubMed]
- Morris, R.J.; Tiveron, M.C.; Xue, G.P. The Relation of the Expression and Function of the Neuronal Glycoprotein Thy-I to Axonal Growth. Biochem. Soc. T 1992, 20, 401–405. [Google Scholar] [CrossRef]
- Jeng, C.J.; McCarroll, S.A.; Martin, T.F.J.; Floor, E.; Adams, J.; Krantz, D.; Butz, S.; Edwards, R.; Schweitzer, E.S. Thy-1 is a component common to multiple populations of synaptic vesicles. J. Cell Biol. 1998, 140, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Leyton, L.; Schneider, P.; Labra, C.V.; Ruegg, C.; Hetz, C.A.; Quest, A.F.; Bron, C. Thy-1 binds to integrin beta(3) on astrocytes and triggers formation of focal contact sites. Curr. Biol. 2001, 11, 1028–1038. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.F.; Ngai, P.; Ho, D.W.; Yu, W.C.; Ng, M.N.; Lau, C.K.; Li, M.L.; Tam, K.H.; Lam, C.T.; Poon, R.T.; et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology 2008, 47, 919–928. [Google Scholar] [CrossRef]
- Chiba, T.; Kanai, F.; Iwama, A.; Yokosuka, O. Circulating cancer stem cells: A novel prognostic predictor of hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2013, 2, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, L.Q.; Jiang, J.H.; Ou, C.; Zeng, L.X.; Xiang, B.D. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Park, J.W.; Kim, J.S.; Lee, S.K.; Hong, E.K. Stem Cell Markers Predict the Response to Sorafenib in Patients with Hepatocellular Carcinoma. Gut Liver 2019, 13, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.C.; Zhou, J.; Chen, K.F.; Gong, J.; Liu, J.; He, J.Y.; Guan, P.; Li, B.; Qin, Y. The prognostic value of combination of CD90 and OCT4 for hepatocellular carcinoma after curative resection. Neoplasma 2016, 63, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliam, D.T.; Menon, V.; Bretz, N.P.; Pruszak, J. The CD24 surface antigen in neural development and disease. Neurobiol. Dis. 2017, 99, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Hernandez, J.C.; Dean, A.M.; Rao, P.H.; Darlington, G.J. CD24-Positive Cells from Normal Adult Mouse Liver Are Hepatocyte Progenitor Cells. Stem Cells Dev. 2011, 20, 2177–2188. [Google Scholar] [CrossRef]
- Dunsford, H.A.; Sell, S. Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable Morris hepatomas. Cancer Res. 1989, 49, 4887–4893. [Google Scholar]
- Strain, A.J.; Crosby, H.A.; Nijjar, S.; Kelly, D.A.; Hubscher, S.G. Human liver-derived stem cells. Semin. Liver Dis. 2003, 23, 373–384. [Google Scholar] [CrossRef]
- Yang, W.; Wang, C.; Lin, Y.; Liu, Q.; Yu, L.X.; Tang, L.; Yan, H.X.; Fu, J.; Chen, Y.; Zhang, H.L.; et al. OV6(+) tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J. Hepatol. 2012, 57, 613–620. [Google Scholar] [CrossRef]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Falciatori, I.; Borsellino, G.; Haliassos, N.; Boitani, C.; Corallini, S.; Battistini, L.; Bernardi, G.; Stefanini, M.; Vicini, E. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. Faseb. J. 2003, 17, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Kita, K.; Zheng, Y.W.; Yokosuka, O.; Saisho, H.; Iwama, A.; Nakauchi, H.; Taniguchi, H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology 2006, 44, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Fujii, T.; Yoshikawa, S.; Takamura, H.; Tani, T.; Ohta, T.; Nakanuma, Y. Histological and culture studies with respect to ABCG2 expression support the existence of a cancer cell hierarchy in human hepatocellular carcinoma. Am. J. Pathol. 2007, 170, 1750–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [Green Version]
- Lo, J.; Lau, E.Y.; So, F.T.; Lu, P.; Chan, V.S.; Cheung, V.C.; Ching, R.H.; Cheng, B.Y.; Ma, M.K.; Ng, I.O.; et al. Anti-CD47 antibody suppresses tumour growth and augments the effect of chemotherapy treatment in hepatocellular carcinoma. Liver Int. 2016, 36, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.K.; Shao, C.; Wang, J.; Wei, Q.; Wang, X.; Collier, Z.; Tang, S.; Liu, H.; Zhang, F.; Huang, J.; et al. Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016, 3, 11–40. [Google Scholar] [CrossRef] [Green Version]
- Kraus, C.; Liehr, T.; Hulsken, J.; Behrens, J.; Birchmeier, W.; Grzeschik, K.H.; Ballhausen, W.G. Localization of the human beta-catenin gene (CTNNB1) to 3p21: A region implicated in tumor development. Genomics 1994, 23, 272–274. [Google Scholar] [CrossRef]
- Noordermeer, J.; Klingensmith, J.; Perrimon, N.; Nusse, R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 1994, 367, 80–83. [Google Scholar] [CrossRef]
- Peifer, M.; Berg, S.; Reynolds, A.B. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 1994, 76, 789–791. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Morin, P.J. beta-catenin signaling and cancer. Bioessays 1999, 21, 1021–1030. [Google Scholar] [CrossRef]
- Gedaly, R.; Galuppo, R.; Daily, M.F.; Shah, M.; Maynard, E.; Chen, C.; Zhang, X.; Esser, K.A.; Cohen, D.A.; Evers, B.M.; et al. Targeting the Wnt/beta-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS ONE 2014, 9, e99272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, J.; von Kries, J.P.; Kuhl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996, 382, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, Y.; Brown, A.M. Wnt signaling in liver cancer. Curr. Drug Targets 2008, 9, 1013–1024. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.Y.; Park, K.K.; Choi, Y.K.; Nam, J.S.; Hong, I.S. CWP232228 targets liver cancer stem cells through Wnt/beta-catenin signaling: A novel therapeutic approach for liver cancer treatment. Oncotarget 2016, 7, 20395–20409. [Google Scholar] [CrossRef] [Green Version]
- Handeli, S.; Simon, J.A. A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities. Mol. Cancer Ther. 2008, 7, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Ishige, N. FH535 suppresses the proliferation and motility of hepatocellular carcinoma cells. Int. J. Oncol. 2016, 48, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Turcios, L.; Vilchez, V.; Acosta, L.F.; Poyil, P.; Butterfield, D.A.; Mitov, M.; Marti, F.; Gedaly, R. Sorafenib and FH535 in combination act synergistically on hepatocellular carcinoma by targeting cell bioenergetics and mitochondrial function. Dig. Liver Dis. 2017, 49, 697–704. [Google Scholar] [CrossRef]
- Galuppo, R.; Maynard, E.; Shah, M.; Daily, M.F.; Chen, C.; Spear, B.T.; Gedaly, R. Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and WNT/beta-catenin pathways. Anticancer Res. 2014, 34, 1709–1713. [Google Scholar]
- Ni, C.X.; Qi, Y.; Zhang, J.; Liu, Y.; Xu, W.H.; Xu, J.; Hu, H.G.; Wu, Q.Y.; Wang, Y.; Zhang, J.P. WM130 preferentially inhibits hepatic cancer stem-like cells by suppressing AKT/GSK3beta/beta-catenin signaling pathway. Oncotarget 2016, 7, 79544–79556. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Liu, Y.; Xu, Y.; Ji, W.; Wu, Q.; Liu, Y.; Gao, Q.; Su, C. Matrine derivative WM130 inhibits hepatocellular carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Cancer Lett. 2015, 368, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, Y.; Bai, Z.H.; Ni, C.X.; Ren, Q.H.; Xu, W.H.; Xu, J.; Hu, H.G.; Qiu, L.; Li, J.Z.; et al. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol. Sin. 2017, 38, 120–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.K.; Lo, R.C. Deregulation of Frizzled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 19, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Juillerat-Jeanneret, L.; Golshayan, D. Notch Antagonists: Potential Modulators of Cancer and Inflammatory Diseases. J. Med. Chem. 2016, 59, 7719–7737. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Wang, P.; Wang, R.H.; Wang, J.L.; Liu, M.; Xiong, S.; Li, Y.W.; Cheng, B. The Notch pathway promotes the cancer stem cell characteristics of CD90(+) cells in hepatocellular carcinoma. Oncotarget 2016, 7, 9526–9538. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Wang, Y.; Du, Y.; He, L.; Huang, G.; Zhang, G.; Yan, X.; Fan, Z. C8orf4 negatively regulates self-renewal of liver cancer stem cells via suppression of NOTCH2 signalling. Nat. Commun. 2015, 6, 7122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Y.; Lu, C.J.; Fang, T.; Wang, Y.C.; Hu, W.H.; Qiao, J.; Liu, B.; Liu, J.; Chen, N.P.; Li, M.Y.; et al. Notch3 functions as a regulator of cell self-renewal by interacting with the beta-catenin pathway in hepatocellular carcinoma. Oncotarget 2015, 6, 3669–3679. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Sun, Q.; Wang, P.; Liu, M.; Xiong, S.; Luo, J.; Huang, H.; Du, Q.; Geller, D.A.; Cheng, B. Notch and Wnt/beta-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget 2016, 7, 5754–5768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.X.; Xu, A.; Zhang, C.C.; Olson, P.; Chen, L.; Lee, T.K.; Cheung, T.T.; Lo, C.M.; Wang, X.Q. Notch Inhibitor PF-03084014 Inhibits Hepatocellular Carcinoma Growth and Metastasis via Suppression of Cancer Stemness due to Reduced Activation of Notch1-Stat3. Mol. Cancer Ther. 2017, 16, 1531–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihrie, R.A.; Shah, J.K.; Harwell, C.C.; Levine, J.H.; Guinto, C.D.; Lezameta, M.; Kriegstein, A.R.; Alvarez-Buylla, A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron 2011, 71, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buller, N.V.; Rosekrans, S.L.; Westerlund, J.; van den Brink, G.R. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiol. (Bethesda) 2012, 27, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solanas, G.; Benitah, S.A. Regenerating the skin: A task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol. 2013, 14, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.; Joyner, A.L. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, A.; Shevde, L.A. Hedgehog signaling: Modulation of cancer properies and tumor mircroenvironment. Mol. Cancer 2016, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Arzumanyan, A.; Sambandam, V.; Clayton, M.M.; Choi, S.S.; Xie, G.; Diehl, A.M.; Yu, D.Y.; Feitelson, M.A. Hedgehog signaling blockade delays hepatocarcinogenesis induced by hepatitis B virus X protein. Cancer Res. 2012, 72, 5912–5920. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Cao, L.; Li, Y.; Lu, H.; Yang, X.; Xue, P. Expression of glioma-associated oncogene 2 (Gli 2) is correlated with poor prognosis in patients with hepatocellular carcinoma undergoing hepatectomy. World J. Surg. Oncol. 2013, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Chun, H.W.; Hong, R. Significance of the hedgehog pathway-associated proteins Gli-1 and Gli-2 and the epithelial-mesenchymal transition-associated proteins Twist and E-cadherin in hepatocellular carcinoma. Oncol. Lett. 2016, 12, 1753–1762. [Google Scholar] [CrossRef]
- Wang, F.; Ma, L.; Zhang, Z.; Liu, X.; Gao, H.; Zhuang, Y.; Yang, P.; Kornmann, M.; Tian, X.; Yang, Y. Hedgehog Signaling Regulates Epithelial-Mesenchymal Transition in Pancreatic Cancer Stem-Like Cells. J. Cancer 2016, 7, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers (Basel) 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incardona, J.P.; Gaffield, W.; Kapur, R.P.; Roelink, H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 1998, 125, 3553–3562. [Google Scholar] [PubMed]
- Sicklick, J.K.; Li, Y.X.; Jayaraman, A.; Kannangai, R.; Qi, Y.; Vivekanandan, P.; Ludlow, J.W.; Owzar, K.; Chen, W.; Torbenson, M.S.; et al. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis 2006, 27, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Hirotsu, M.; Setoguchi, T.; Sasaki, H.; Matsunoshita, Y.; Gao, H.; Nagao, H.; Kunigou, O.; Komiya, S. Smoothened as a new therapeutic target for human osteosarcoma. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef] [Green Version]
- Mimeault, M.; Johansson, S.L.; Henichart, J.P.; Depreux, P.; Batra, S.K. Cytotoxic Effects Induced by Docetaxel, Gefitinib, and Cyclopamine on Side Population and Nonside Population Cell Fractions from Human Invasive Prostate Cancer Cells. Mol. Cancer Ther. 2010, 9, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Robarge, K.D.; Brunton, S.A.; Castanedo, G.M.; Cui, Y.; Dina, M.S.; Goldsmith, R.; Gould, S.E.; Guichert, O.; Gunzner, J.L.; Halladay, J.; et al. GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med. Chem. Lett. 2009, 19, 5576–5581. [Google Scholar] [CrossRef]
- Philips, G.M.; Chan, I.S.; Swiderska, M.; Schroder, V.T.; Guy, C.; Karaca, G.F.; Moylan, C.; Venkatraman, T.; Feuerlein, S.; Syn, W.K.; et al. Hedgehog Signaling Antagonist Promotes Regression of Both Liver Fibrosis and Hepatocellular Carcinoma in a Murine Model of Primary Liver Cancer. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Song, K.; Han, C.; Chen, W.; Wang, Y.; Dash, S.; Lim, K.; Wu, T. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology 2016, 63, 1155–1169. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Rodova, M.; Roy, S.K.; Sharma, J.; Singh, K.P.; Srivastava, R.K.; Shankar, S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013, 330, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, C.; Lu, L.; Magliato, S.; Wu, T. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology 2013, 58, 995–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Liu, J.; Derynck, R. Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS Lett. 2012, 586, 1871–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meindl-Beinker, N.M.; Matsuzaki, K.; Dooley, S. TGF-beta signaling in onset and progression of hepatocellular carcinoma. Dig. Dis. 2012, 30, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Sakaki-Yumoto, M.; Katsuno, Y.; Derynck, R. TGF-beta family signaling in stem cells. Biochim. Biophys. Acta 2013, 1830, 2280–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Q.M.; Jing, Y.Y.; Yu, G.F.; Kou, X.R.; Ye, F.; Gao, L.; Li, R.; Zhao, Q.D.; Yang, Y.; Lu, Z.H.; et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014, 352, 160–168. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Ding, W.; Rountree, C.B. Epigenetic regulation of cancer stem cell marker CD133 by transforming growth factor-beta. Hepatology 2010, 51, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Serova, M.; Tijeras-Raballand, A.; Dos Santos, C.; Albuquerque, M.; Paradis, V.; Neuzillet, C.; Benhadji, K.A.; Raymond, E.; Faivre, S.; de Gramont, A. Effects of TGF-beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget 2015, 6, 21614–21627. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, Q.; Li, Z.; Zhang, R.; Jia, C.; Yang, Z.; Zhao, H.; Ya, S.; Mao, R.; Ailijiang, T.; et al. GP73 promotes epithelial-mesenchymal transition and invasion partly by activating TGF-beta1/Smad2 signaling in hepatocellular carcinoma. Carcinogenesis 2018, 39, 900–910. [Google Scholar] [CrossRef]
- Bach, P.; Abel, T.; Hoffmann, C.; Gal, Z.; Braun, G.; Voelker, I.; Ball, C.R.; Johnston, I.C.; Lauer, U.M.; Herold-Mende, C.; et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013, 73, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Tanaka, S.; Matsumura, S.; Murakata, A.; Ban, D.; Ochiai, T.; Irie, T.; Kudo, A.; Nakamura, N.; Tanabe, M.; et al. EpCAM-targeted therapy for human hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 1314–1322. [Google Scholar] [CrossRef]
- Biggers, K.; Scheinfeld, N. VB4-845, a conjugated recombinant antibody and immunotoxin for head and neck cancer and bladder cancer. Curr. Opin. Mol. 2008, 10, 176–186. [Google Scholar]
- Wang, L.; Su, W.; Liu, Z.; Zhou, M.; Chen, S.; Chen, Y.; Lu, D.; Liu, Y.; Fan, Y.; Zheng, Y.; et al. CD44 antibody-targeted liposomal nanoparticles for molecular imaging and therapy of hepatocellular carcinoma. Biomaterials 2012, 33, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sui, Z.G.; Xu, W.; Quan, X.H.; Sun, J.L.; Li, X.; Ji, H.Y.; Jing, F.B. Ubenimex suppresses Pim-3 kinase expression by targeting CD13 to reverse MDR in HCC cells. Oncotarget 2017, 8, 72652–72665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Rosner, M.; Hengstschlager, M. Targeting epigenetic readers in cancer. N. Engl. J. Med. 2012, 367, 1764–1765. [Google Scholar] [CrossRef]
- Waldmann, T.; Schneider, R. Targeting histone modifications--epigenetics in cancer. Curr. Opin. Cell Biol. 2013, 25, 184–189. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.J.; Mukhopadhyay, A.; Kim, K.S.; Xing, J. Statistical mechanics model for the dynamics of collective epigenetic histone modification. Phys. Rev. Lett. 2014, 112, 068101. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, J.U.; Factor, V.M.; Thorgeirsson, S.S. Epigenetic regulation of cancer stem cells in liver cancer: Current concepts and clinical implications. J. Hepatol. 2010, 53, 568–577. [Google Scholar] [CrossRef] [Green Version]
- Raggi, C.; Factor, V.M.; Seo, D.; Holczbauer, A.; Gillen, M.C.; Marquardt, J.U.; Andersen, J.B.; Durkin, M.; Thorgeirsson, S.S. Epigenetic reprogramming modulates malignant properties of human liver cancer. Hepatology 2014, 59, 2251–2262. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.S.; Yamashita, T.; Kondo, M.; Nio, K.; Hayashi, T.; Hara, Y.; Nomura, Y.; Yoshida, M.; Hayashi, T.; Oishi, N.; et al. The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J. Hepatol. 2014, 60, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luo, N.; Luo, Y.; Peng, Z.P.; Zhang, T.; Li, S.L. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb. Int. J. Oncol. 2012, 40, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Z.; Yang, Z.; Shan, J.J.; Liu, L.M.; Liu, C.G.; Shen, J.J.; Chen, X.J.; Xu, Y.M.; Chen, J.; Ma, Q.H.; et al. MicroRNA-449a maintains self-renewal in liver cancer stem-like cells by targeting Tcf3. Oncotarget 2017, 8, 110187–110200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, S.; Ng, K.Y.; Tong, M.; Lau, E.Y.; Lee, T.K.; Chan, K.W.; Yuan, Y.F.; Cheung, T.T.; Cheung, S.T.; Wang, X.Q.; et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/beta-catenin activation in liver cancer stem cells. Hepatology 2016, 64, 2062–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.N.; Zeng, Q.; Wang, H.Y.; Zhang, B.; Li, S.T.; Nan, X.; Cao, N.; Fu, C.J.; Yan, X.L.; Jia, Y.L.; et al. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology 2015, 62, 801–815. [Google Scholar] [CrossRef]
- Xia, H.P.; Ooi, L.L.P.J.; Hui, K.M. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology 2013, 58, 629–641. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wu, M.H.; Huang, Y.H.; Yeh, C.T.; Chi, H.C.; Tsai, C.Y.; Chuang, W.Y.; Yu, C.J.; Chung, I.H.; Chen, C.Y.; et al. Thyroid hormone negatively regulates tumorigenesis through suppression of BC200. Endocr.-Relat. Cancer 2018, 25, 967–979. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Z.; Huang, L.; Wu, Y.H.; Zhai, W.J.; Zhu, P.P.; Gao, Y.F. LncSox4 promotes the self-renewal of liver tumour-initiating cells through Stat3-mediated Sox4 expression. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Du, Y.; Zhu, P.; Huang, G.; Luo, J.; Yan, X.; Ye, B.; Li, C.; Xia, P.; et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell 2015, 16, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.D.; Lin, Z.J.; Xu, J.; Lu, Y.N.; Meng, Q.Y.; Wang, C.; Yang, Y.X.; Xin, X.R.; Li, X.N.; Pu, H.; et al. Long noncoding RNA MEG3 suppresses liver cancer cells growth through inhibiting beta-catenin by activating PKM2 and inactivating PTEN. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Huang, G.; Ye, B.; Liu, B.; Wu, J.; Du, Y.; He, L.; Fan, Z. lnc-beta-Catm elicits EZH2-dependent beta-catenin stabilization and sustains liver CSC self-renewal. Nat. Struct. Mol. Biol. 2016, 23, 631–639. [Google Scholar] [CrossRef]
- Yuan, S.X.; Wang, J.; Yang, F.; Tao, Q.F.; Zhang, J.; Wang, L.L.; Yang, Y.; Liu, H.; Wang, Z.G.; Xu, Q.G.; et al. Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology 2016, 63, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Shen, W.; Xia, M.; Chen, C.; Xiang, D.; Ning, B.; Cui, X.; Li, H.; Li, X.; et al. Long non-coding RNA DILC regulates liver cancer stem cells via IL-6/STAT3 axis. J. Hepatol. 2016, 64, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, P.; Lu, T.; Du, Y.; Wang, Y.; He, L.; Ye, B.; Liu, B.; Yang, L.; Wang, J.; et al. The long non-coding RNA LncHDAC2 drives the self-renewal of liver cancer stem cells via activation of Hedgehog signaling. J. Hepatol. 2019, 70, 918–929. [Google Scholar] [CrossRef]
- Postovit, L.M.; Margaryan, N.V.; Seftor, E.A.; Kirschmann, D.A.; Lipavsky, A.; Wheaton, W.W.; Abbott, D.E.; Seftor, R.E.; Hendrix, M.J. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc. Natl. Acad. Sci. USA 2008, 105, 4329–4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Kasai, T.; Li, Y.; Sugii, Y.; Jin, G.; Okada, M.; Vaidyanath, A.; Mizutani, A.; Satoh, A.; Kudoh, T.; et al. A model of cancer stem cells derived from mouse induced pluripotent stem cells. PLoS ONE 2012, 7, e33544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhang, W.; Jia, Y.; Yu, Q.; Grau, G.E.; Peng, L.; Ran, Y.; Yang, Z.; Deng, H.; Lou, J. Single-cell clones of liver cancer stem cells have the potential of differentiating into different types of tumor cells. Cell Death Dis. 2013, 4, e857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukowati, C.H.; Anfuso, B.; Croce, L.S.; Tiribelli, C. The role of multipotent cancer associated fibroblasts in hepatocarcinogenesis. BMC Cancer 2015, 15, 188. [Google Scholar] [CrossRef] [Green Version]
- Lau, E.Y.; Lo, J.; Cheng, B.Y.; Ma, M.K.; Lee, J.M.; Ng, J.K.; Chai, S.; Lin, C.H.; Tsang, S.Y.; Ma, S.; et al. Cancer-Associated Fibroblasts Regulate Tumor-Initiating Cell Plasticity in Hepatocellular Carcinoma through c-Met/FRA1/HEY1 Signaling. Cell Rep. 2016, 15, 1175–1189. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, S.; Tanaka, S.; Mogushi, K.; Adikrisna, R.; Aihara, A.; Ban, D.; Ochiai, T.; Irie, T.; Kudo, A.; Nakamura, N.; et al. Visualization of stem cell features in human hepatocellular carcinoma reveals in vivo significance of tumor-host interaction and clinical course. Hepatology 2013, 58, 218–228. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Wu, Z.; Liu, S.; Sun, L.; Zhong, Y.; Zhang, X.; Kong, X.; Qian, P.; Zhang, H.; et al. Artemin is hypoxia responsive and promotes oncogenicity and increased tumor initiating capacity in hepatocellular carcinoma. Oncotarget 2016, 7, 3267–3282. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; McConville, C.; Kannappan, V.; Tawari, P.E.; Brown, J.; Ding, J.; Armesilla, A.L.; Irache, J.M.; Mei, Q.B.; et al. Poly lactic-co-glycolic acid controlled delivery of disulfiram to target liver cancer stem-like cells. Nanomedicine 2017, 13, 641–657. [Google Scholar] [CrossRef] [PubMed]
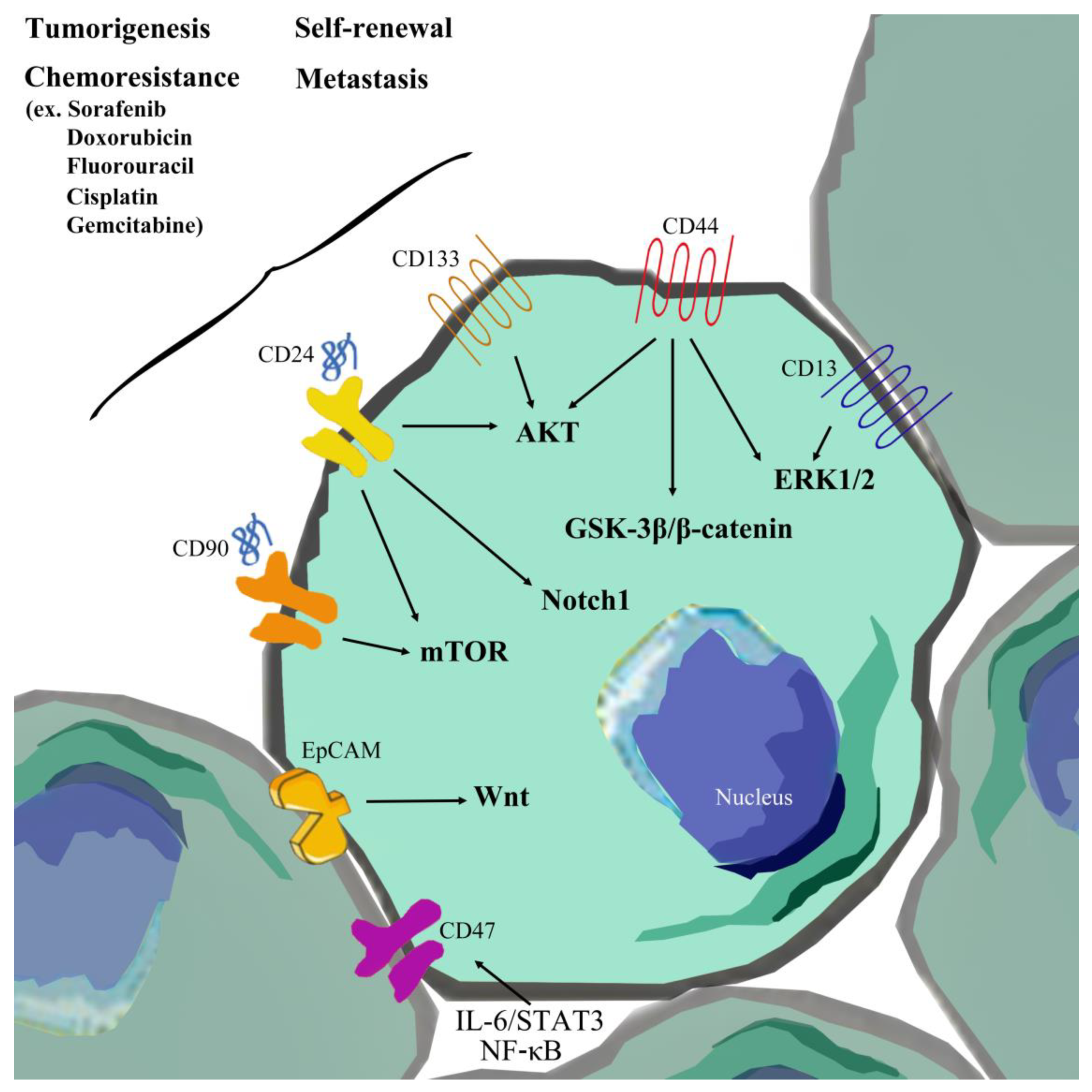
| LCSCs | Phenotypes of LCSCs (Source) | Signaling Involving LCSCs | Resistance to Clinical Drug | Ref. |
|---|---|---|---|---|
| EpCAM | cell–cell adhesion, metabolism, cell signaling, differentiation, metastasis, regeneration, organogenesis, tumorigenesis, chemoresistance and self-renewal (Hep3B, HepG2, Huh7, Huh1, and Dt81Hepa1-6 cells) | Activation of the Wnt signaling pathway | Sorafenib | [30,31,32,33] |
| CD133 | tumorigenic, cell cycle progression, differentiation, chemoresistance, and self-renewal (Huh7, SMMC7721, PLC8024,PLC8024, HepG2, and HCCLM3 cells) | Activation of AKT/PKB, | Doxorubicin, Fluorouracil (5-FU) and Sorafenib | [27,34,35,36] |
| CD44 | proliferation, survival, migration/invasion, and chemoresistance, and self-renewal (primary HCC, HepG2, Hep3B, Huh7, SUN-368, SUN-354, SMMC-7721, and MHCC97-H cells) | Activation of AKT/GSK-3β/β-catenin, and ERK/Snail pathways | Doxorubicin | [21,37,38,39,40,41,42] |
| CD13 | chemoresistance, tumorigenesis and self-renewal (Huh7, PLC, and HepG2 cells) | Activation of ERK1/2 signaling pathway | Sorafenib, Doxorubicin, and Fluorouracil (5-FU) | [43,44] |
| CD90 | tumorigenesis, metastasis, self-renewal and chemoresistance (MHCC97L, PLC, HepG2, Hep3B, primary HCC, and JHH-6 cells) | Activation of mTOR signaling pathway | Doxorubicin | [45,46,47,48] |
| CD24 | metastasis, differentiation, self-renewal and chemoresistance (MHCC97H, HCCLM3, PLC/PRF/5, Huh7, and Hep3B cells) | Autophagy activation, activation of AKT/mTOR signaling pathway, and Notch1 signaling pathway | Cisplatin, Sorafenib | [49,50,51,52] |
| OV-6 | self-renewal, tumorigenicity, and chemoresistance (SMMC7721, and HuH7 cells) | Activation of Wnt/β-catenin signaling | Cisplatin | [53,54] |
| Side population | differentiation, chemoresistance, and metastasis (Huh7, PLC/PRF/5, HCCLM3, MHCC97-H, MHCC97-L, and Hep3B cells) | Activation of AKT signaling pathway | Doxorubicin, Fluorouracil (5-FU), and Gemcitabine | [23,24,55] |
| CD47 | self-renewal, tumor initiating, tumorigenicity, and chemoresistance (MHCC97L, PLC, and Huh7 cells) | Activation of IL-6/STAT3 signaling pathway, and NF-κB | Doxorubicin, Sorafenib | [56,57,58] |
| SALL4 | proliferation, differentiation, and chemoresistance (Huh7, PLC/PRF/5, and patients of HCC) | Interaction with NuRD, regulation of PTEN, and PI3K/AKT signaling pathway | Fluorouracil (5-FU) | [59,60] |
| CD13+CD133+ | tumor initiation, chemoresistance, and anti-apoptosis (Huh7 and PLC cells) | Reduction of ROS-induced DNA damage and inhibition of apoptosis | Doxorubicin, Fluorouracil (5-FU) | [43] |
| CD13+CD90+ | tumor initiation, chemoresistance, and anti-apoptosis (Huh7 and PLC cells) | Reduction of ROS-induced DNA damage and inhibition of apoptosis | Doxorubicin, Fluorouracil (5-FU) | [43] |
| EpCAM+ CD90+ | metastasis, tumorigenesis (patients of HCC and primary HCC) | activation of the TGF-β pathway | [61] | |
| CD90+CXCR4+and CD133+CD90+ | tumor development, tumor spheres, and metastasis (primary HCC) | [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies. Cells 2020, 9, 1331. https://doi.org/10.3390/cells9061331
Liu Y-C, Yeh C-T, Lin K-H. Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies. Cells. 2020; 9(6):1331. https://doi.org/10.3390/cells9061331
Chicago/Turabian StyleLiu, Yu-Chin, Chau-Ting Yeh, and Kwang-Huei Lin. 2020. "Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies" Cells 9, no. 6: 1331. https://doi.org/10.3390/cells9061331
APA StyleLiu, Y.-C., Yeh, C.-T., & Lin, K.-H. (2020). Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies. Cells, 9(6), 1331. https://doi.org/10.3390/cells9061331





