A Na,K-ATPase–Fodrin–Actin Membrane Cytoskeleton Complex is Required for Endothelial Fenestra Biogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture and Fenestra Induction
2.3. Immunocytochemistry and Confocal Imaging
2.4. SiRNA Transfection and Correlative Light–Electron Microscopy
2.5. Western Blot Analysis and Immunoprecipitation
2.6. Electron Microscopy
2.7. Animal Studies
2.8. Statistical Analysis
3. Results
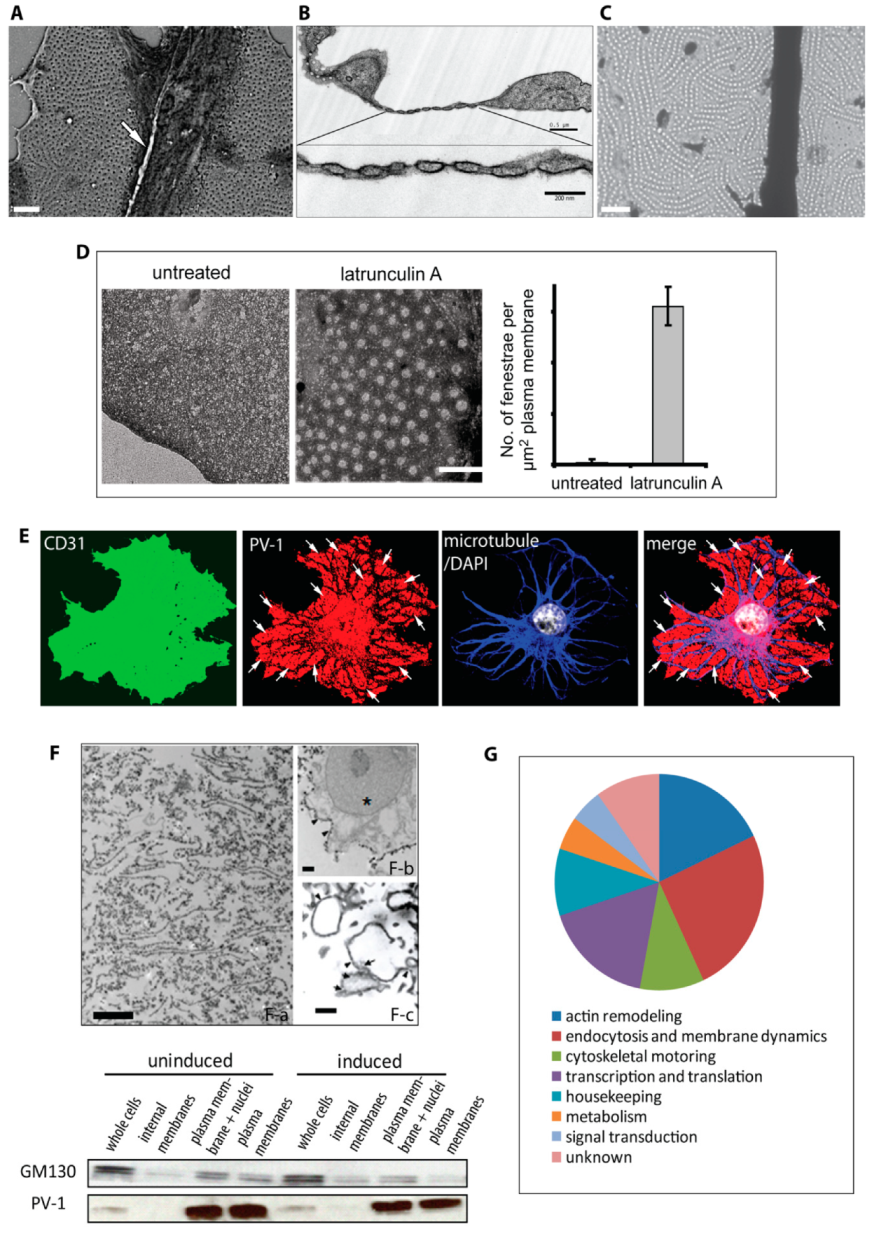
3.1. Actin-Binding Proteins are Enriched in Fenestrated Endothelial Plasma Membranes
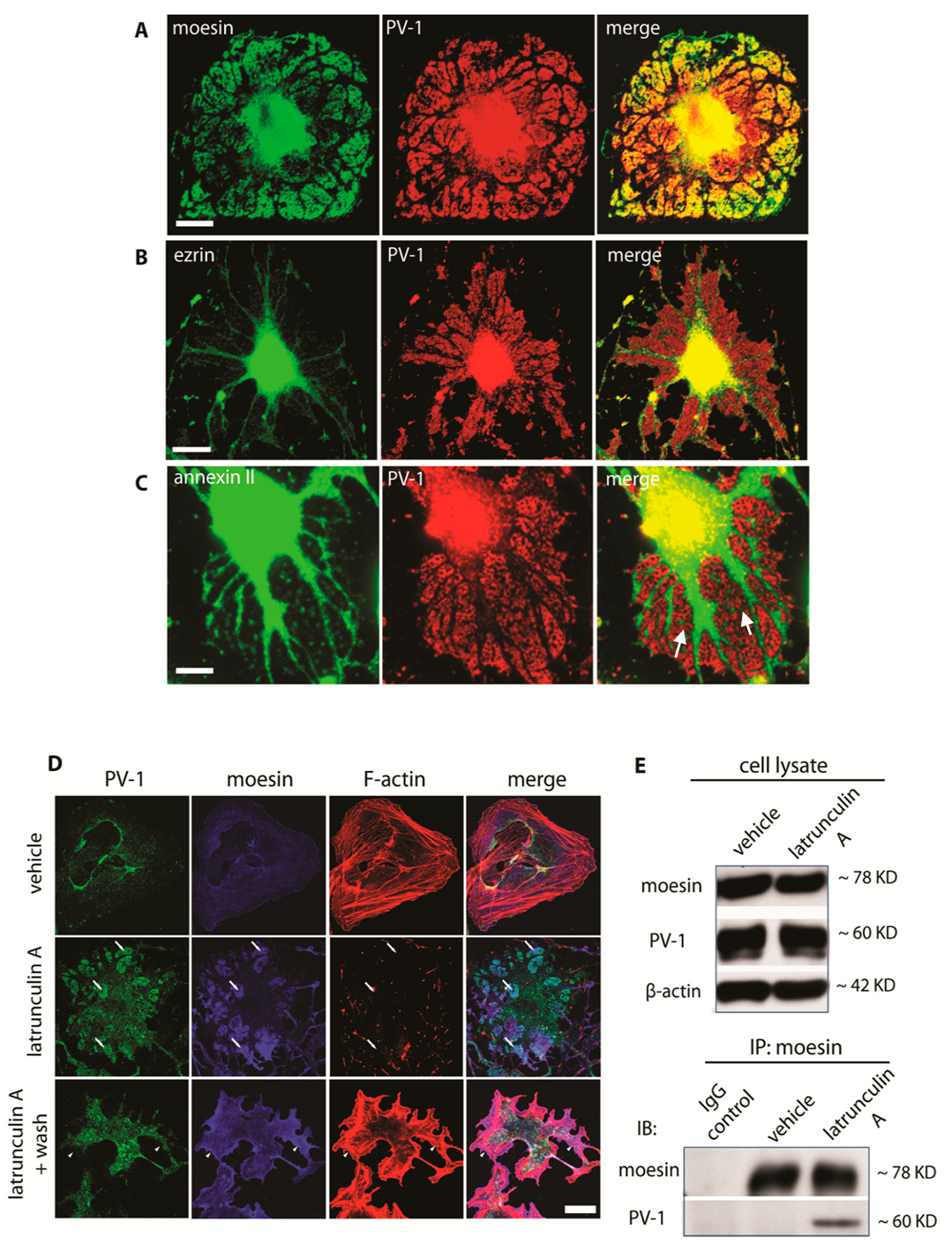
3.2. Moesin and Annexin II Are Present in Fenestral Sieve Plates
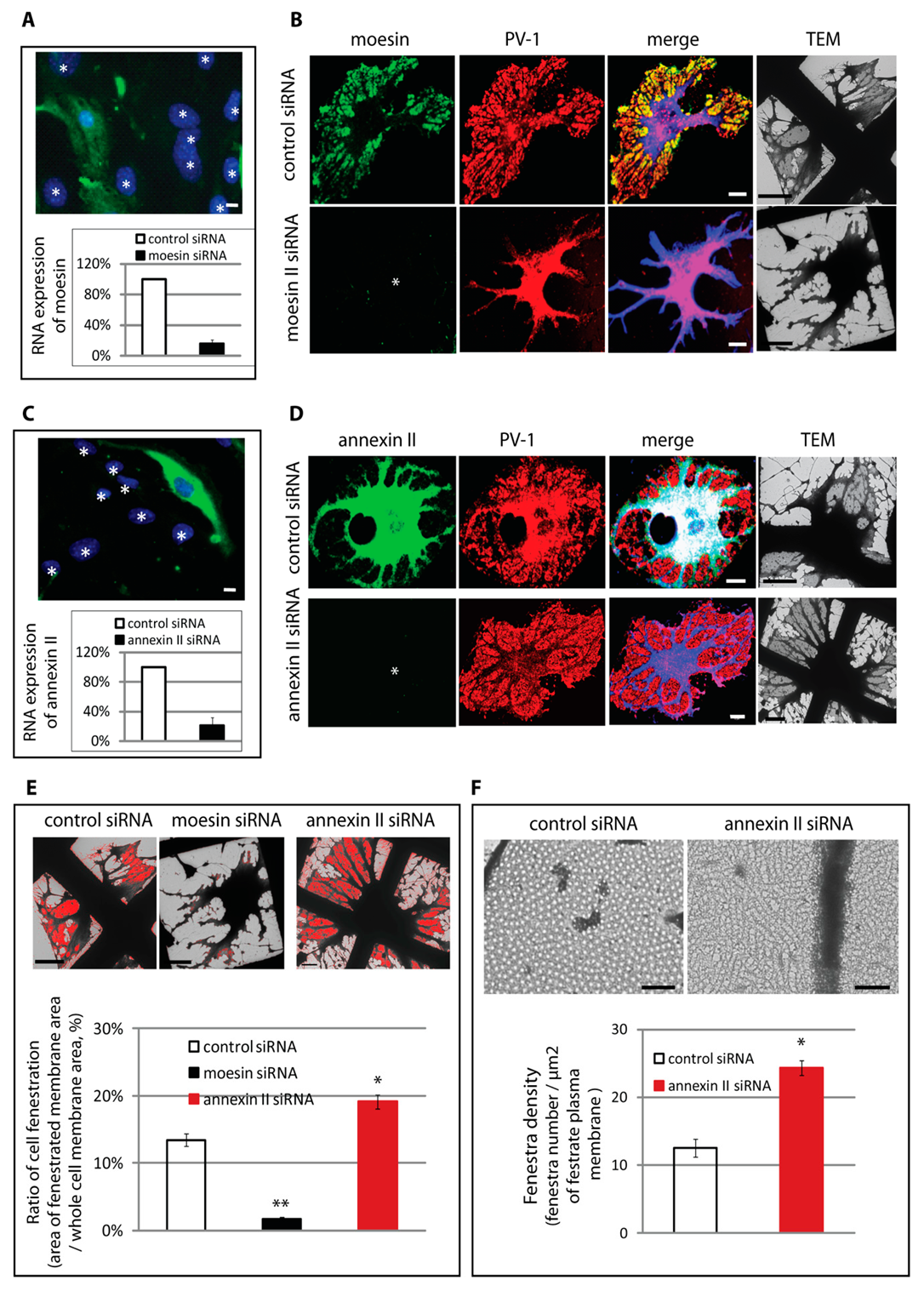
3.3. Differential Regulation of Fenestra Biogenesis by Moesin and Annexin II
3.4. An Actin/Fodrin-Based Membrane Cytoskeleton Is Required for Fenestra Biogenesis
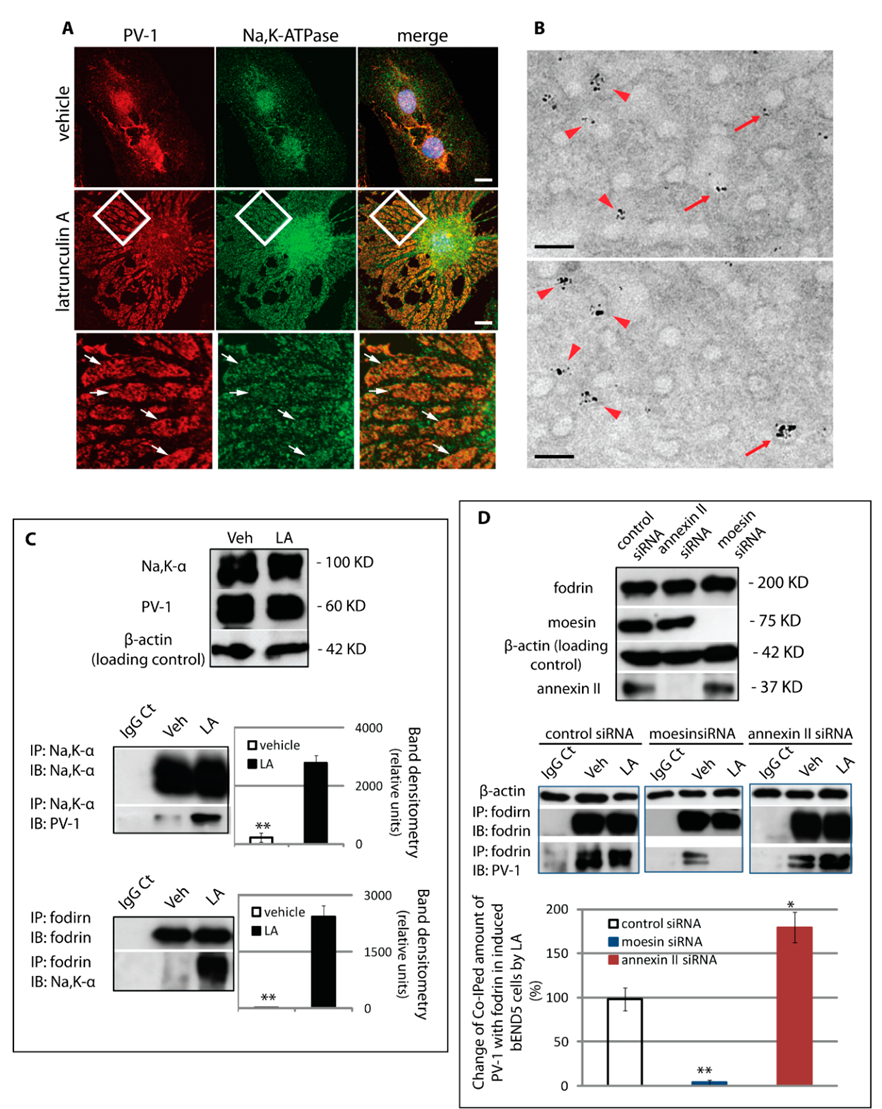
3.5. Assembly of a Fenestra/Na,K-ATPase/Membrane Cytoskeleton Complex Is Regulated by Moesin and Annexin II
3.6. The Na,K-ATPase Is a Negative Regulator of Fenestra Formation In Vitro and In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bennett, H.S.; Luft, J.H.; Hampton, J.C. Morphological classifications of vertebrate blood capillaries. Am. J. Physiol. 1959, 196, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risau, W.; Wolburg, H. Development of the blood-brain barrier. Trends Neurosci. 1990, 13, 174–178. [Google Scholar] [CrossRef]
- Levick, J.R.; Smaje, L.H. An analysis of the permeability of a fenestra. Microvasc. Res. 1987, 33, 233–256. [Google Scholar] [CrossRef]
- Ryan, U.S.; Ryan, J.W.; Smith, D.S.; Winkler, H. Fenestrated endothelium of the adrenal gland: Freeze-fracture studies. Tissue Cell 1975, 7, 181–190. [Google Scholar] [CrossRef]
- Wisse, E.; De Zanger, R.B.; Charels, K.; Van Der Smissen, P.; McCuskey, R.S. The liver sieve: Considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 1985, 5, 683–692. [Google Scholar] [CrossRef]
- Engelhardt, B.; Wolburg-Buchholz, K.; Wolburg, H. Involvement of the choroid plexus in central nervous system inflammation. Microsc. Res. Tech. 2001, 52, 112–129. [Google Scholar] [CrossRef]
- Caruso, R.A.; Speciale, G.; Inferrera, A.; Rigoli, L.; Inferrera, C. Ultrastructural observations on the microvasculature in advanced gastric carcinomas. Histol. Histopathol. 2001, 16, 785–792. [Google Scholar] [CrossRef]
- Kohno, R.; Hata, Y.; Mochizuki, Y.; Arita, R.; Kawahara, S.; Kita, T.; Miyazaki, M.; Hisatomi, T.; Ikeda, Y.; Aiello, L.P.; et al. Histopathology of neovascular tissue from eyes with proliferative diabetic retinopathy after intravitreal bevacizumab injection. Am. J. Ophthalmol. 2010, 150, 223.e221–229.e221. [Google Scholar] [CrossRef]
- Rhodin, J.A. The diaphragm of capillary endothelial fenestrations. J. Ultrastruct. Res. 1962, 6, 171–185. [Google Scholar] [CrossRef]
- Palade, G.E.; Simionescu, M.; Simionescu, N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol. Scand. Suppl. 1979, 463, 11–32. [Google Scholar]
- Stan, R.V.; Kubitza, M.; Palade, G.E. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl. Acad. Sci. USA 1999, 96, 13203–13207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidou, S.; Deinhardt, K.; Miotla, J.; Bradley, J.; Cheung, E.; Samuelsson, S.; Ng, Y.S.; Shima, D.T. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16770–16775. [Google Scholar] [CrossRef] [Green Version]
- Seemann, J.; Jokitalo, E.J.; Warren, G. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell 2000, 11, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Mastronarde, D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005, 152, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Mastronarde, D.N. Dual-axis tomography: An approach with alignment methods that preserve resolution. J. Struct. Biol. 1997, 120, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Roberts, W.G.; Palade, G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995, 108, 2369–2379. [Google Scholar]
- Stolz, D.B.; Jacobson, B.S. Examination of transcellular membrane protein polarity of bovine aortic endothelial cells in vitro using the cationic colloidal silica microbead membrane-isolation procedure. J. Cell Sci. 1992, 103, 39–51. [Google Scholar]
- Bretscher, A.; Edwards, K.; Fehon, R.G. ERM proteins and merlin: Integrators at the cell cortex. Nat. Rev. Mol. Cell. Biol. 2002, 3, 586–599. [Google Scholar] [CrossRef]
- Rescher, U.; Gerke, V. Annexins—unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.A.; Demsky, M.; Montague, R.A.; Weymouth, N.; Kiehart, D.P. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 1997, 191, 103–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, F.; Amieva, M.R.; Furthmayr, H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J. Biol. Chem. 1995, 270, 31377–31385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisiadou, L.; Xie, C.; Cho, H.J.; Lin, X.; Gu, X.L.; Long, C.X.; Lobbestael, E.; Baekelandt, V.; Taymans, J.M.; Sun, L.; et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009, 29, 13971–13980. [Google Scholar] [CrossRef] [PubMed]
- Gatmaitan, Z.; Varticovski, L.; Ling, L.; Mikkelsen, R.; Steffan, A.M.; Arias, I.M. Studies on fenestral contraction in rat liver endothelial cells in culture. Am. J. Pathol. 1996, 148, 2027–2041. [Google Scholar]
- Goodman, S.R.; Zagon, I.S.; Kulikowski, R.R. Identification of a spectrin-like protein in nonerythroid cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7570–7574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heltianu, C.; Bogdan, I.; Constantinescu, E.; Simionescu, M. Endothelial cells express a spectrin-like cytoskeletal protein. Circ. Res. 1986, 58, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Boivin, P. [Molecular interactions of membrane proteins and erythrocyte deformability]. Pathol. Biol. (Paris) 1984, 32, 717–735. [Google Scholar]
- Nash, G.B.; Gratzer, W.B. Structural determinants of the rigidity of the red cell membrane. Biorheology 1993, 30, 397–407. [Google Scholar] [CrossRef]
- Byers, T.J.; Branton, D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc. Natl. Acad. Sci. USA 1985, 82, 6153–6157. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.R.; Krebs, K.E.; Whitfield, C.F.; Riederer, B.M.; Zagon, I.S. Spectrin and related molecules. CRC Crit. Rev. Biochem. 1988, 23, 171–234. [Google Scholar] [CrossRef]
- Dunaevsky, A.; Connor, E.A. F-actin is concentrated in nonrelease domains at frog neuromuscular junctions. J. Neurosci. 2000, 20, 6007–6012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinder, J.C.; Ohanian, V.; Gratzer, W.B. Spectrin and protein 4.1 as an actin filament capping complex. FEBS Lett. 1984, 169, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Anong, W.A.; Franco, T.; Chu, H.; Weis, T.L.; Devlin, E.E.; Bodine, D.M.; An, X.; Mohandas, N.; Low, P.S. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 2009, 114, 1904–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, L.L.; Shivdasani, R.A.; Liu, S.C.; Hanspal, M.; John, K.M.; Gonzalez, J.M.; Brugnara, C.; Gwynn, B.; Mohandas, N.; Alper, S.L.; et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 1996, 86, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Nelson, W.J.; Veshnock, P.J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 1986, 103, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. J. Am. Soc. Nephrol. 1998, 9, 2170–2177. [Google Scholar] [CrossRef]
- Krupinski, T.; Beitel, G.J. Unexpected roles of the Na-K-ATPase and other ion transporters in cell junctions and tubulogenesis. Physiology (Bethesda) 2009, 24, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.Y.; Xie, Z.J. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell. 2006, 17, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Haas, M.; Wang, H.; Tian, J.; Xie, Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 2002, 277, 18694–18702. [Google Scholar] [CrossRef] [Green Version]
- Barwe, S.P.; Jordan, M.C.; Skay, A.; Inge, L.; Rajasekaran, S.A.; Wolle, D.; Johnson, C.L.; Neco, P.; Fang, K.; Rozengurt, N.; et al. Dysfunction of ouabain-induced cardiac contractility in mice with heart-specific ablation of Na,K-ATPase beta1-subunit. J. Mol. Cell. Cardiol. 2009, 47, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Done, S.C.; Leibiger, I.B.; Efendiev, R.; Katz, A.I.; Leibiger, B.; Berggren, P.O.; Pedemonte, C.H.; Bertorello, A.M. Tyrosine 537 within the Na+,K+-ATPase alpha-subunit is essential for AP-2 binding and clathrin-dependent endocytosis. J. Biol. Chem. 2002, 277, 17108–17111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Xie, Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 2009, 457, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Charlemagne, D. Molecular and cellular level of action of digitalis. Herz 1993, 18, 79–85. [Google Scholar]
- Esser, S.; Wolburg, K.; Wolburg, H.; Breier, G.; Kurzchalia, T.; Risau, W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 1998, 140, 947–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, A.; Amano, M.; Fukata, Y.; Kaibuchi, K. Translocation of Na(+),K(+)-ATPase is induced by Rho small GTPase in renal epithelial cells. Biochem. Biophys. Res. Commun. 2002, 297, 1231–1237. [Google Scholar] [CrossRef]
- Peruchetti, D.B.; Pinheiro, A.A.; Landgraf, S.S.; Wengert, M.; Takiya, C.M.; Guggino, W.B.; Caruso-Neves, C. (Na+ + K+)-ATPase is a target for phosphoinositide 3-kinase/protein kinase B and protein kinase C pathways triggered by albumin. J. Biol. Chem. 2011, 286, 45041–45047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudowski, G.A.; Efendiev, R.; Pedemonte, C.H.; Katz, A.I.; Berggren, P.O.; Bertorello, A.M. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc. Natl. Acad. Sci. USA 2000, 97, 6556–6561. [Google Scholar] [CrossRef] [Green Version]
- Roberts, W.G.; Palade, G.E. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997, 57, 765–772. [Google Scholar]
- De Matteis, M.A.; Morrow, J.S. The role of ankyrin and spectrin in membrane transport and domain formation. Curr. Opin. Cell Biol. 1998, 10, 542–549. [Google Scholar] [CrossRef]
- Drenckhahn, D.; Jons, T.; Kollert-Jons, A.; Koob, R.; Kraemer, D.; Wagner, S. Cytoskeleton and epithelial polarity. Ren. Physiol. Biochem. 1993, 16, 6–14. [Google Scholar] [CrossRef]
- Salcedo-Sicilia, L.; Granell, S.; Jovic, M.; Sicart, A.; Mato, E.; Johannes, L.; Balla, T.; Egea, G. betaIII spectrin regulates the structural integrity and the secretory protein transport of the Golgi complex. J. Biol. Chem. 2013, 288, 2157–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.C.; Derick, L.H.; Duquette, M.A.; Palek, J. Separation of the lipid bilayer from the membrane skeleton during discocyte-echinocyte transformation of human erythrocyte ghosts. Eur. J. Cell Biol. 1989, 49, 358–365. [Google Scholar] [PubMed]
- Nagai, T.; Yokomori, H.; Yoshimura, K.; Fujimaki, K.; Nomura, M.; Hibi, T.; Oda, M. Actin filaments around endothelial fenestrae in rat hepatic sinusoidal endothelial cells. Med. Electron Microsc. 2004, 37, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Wang, H.; Chen, Y.; Liu, L.; Gunning, W.T.; Quintas, L.E.; Xie, Z.J. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J. Cell Biol. 2008, 182, 1153–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liang, M.; Liu, L.; Malhotra, D.; Xie, Z.; Shapiro, J.I. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005, 67, 1844–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, P.F.; Grupp, I.L.; Grupp, G.; Woo, A.L.; Askew, G.R.; Croyle, M.L.; Walsh, R.A.; Lingrel, J.B. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol. Cell 1999, 3, 555–563. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. Drugs 2007, 7, 173–189. [Google Scholar] [CrossRef]
- Nesher, M.; Shpolansky, U.; Rosen, H.; Lichtstein, D. The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sci. 2007, 80, 2093–2107. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Ma, A.Q.; Zhang, L.; Yang, D.Y. Intra-cellular electrolyte changes and levels of endogenous digoxin-like substance within the plasma in patients with congestive heart failure. Int. J. Cardiol. 1990, 27, 47–53. [Google Scholar] [CrossRef]
- Wolff, J.R.; Schieweck, C.; Emmenegger, H.; Meier-Ruge, W. Cerebrovascular ultrastructural alterations after intra-arterial infusions of ouabain, scilla-glycosides, heparin and histamine. Acta Neuropathol. 1975, 31, 45–58. [Google Scholar] [CrossRef]
- Levick, J.R. An analysis of the interaction between interstitial plasma protein, interstitial flow, and fenestral filtration and its application to synovium. Microvasc. Res. 1994, 47, 90–125. [Google Scholar] [CrossRef] [PubMed]
- Stan, R.V.; Tse, D.; Deharvengt, S.J.; Smits, N.C.; Xu, Y.; Luciano, M.R.; McGarry, C.L.; Buitendijk, M.; Nemani, K.V.; Elgueta, R.; et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev. Cell 2012, 23, 1203–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, M.; Ioannidou, S.; Munro, P.; Rämö, O.; Vihinen, H.; Jokitalo, E.; Shima, D.T. A Na,K-ATPase–Fodrin–Actin Membrane Cytoskeleton Complex is Required for Endothelial Fenestra Biogenesis. Cells 2020, 9, 1387. https://doi.org/10.3390/cells9061387
Ju M, Ioannidou S, Munro P, Rämö O, Vihinen H, Jokitalo E, Shima DT. A Na,K-ATPase–Fodrin–Actin Membrane Cytoskeleton Complex is Required for Endothelial Fenestra Biogenesis. Cells. 2020; 9(6):1387. https://doi.org/10.3390/cells9061387
Chicago/Turabian StyleJu, Meihua, Sofia Ioannidou, Peter Munro, Olli Rämö, Helena Vihinen, Eija Jokitalo, and David T. Shima. 2020. "A Na,K-ATPase–Fodrin–Actin Membrane Cytoskeleton Complex is Required for Endothelial Fenestra Biogenesis" Cells 9, no. 6: 1387. https://doi.org/10.3390/cells9061387
APA StyleJu, M., Ioannidou, S., Munro, P., Rämö, O., Vihinen, H., Jokitalo, E., & Shima, D. T. (2020). A Na,K-ATPase–Fodrin–Actin Membrane Cytoskeleton Complex is Required for Endothelial Fenestra Biogenesis. Cells, 9(6), 1387. https://doi.org/10.3390/cells9061387




