Ongoing Research on the Role of Gintonin in the Management of Neurodegenerative Disorders
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
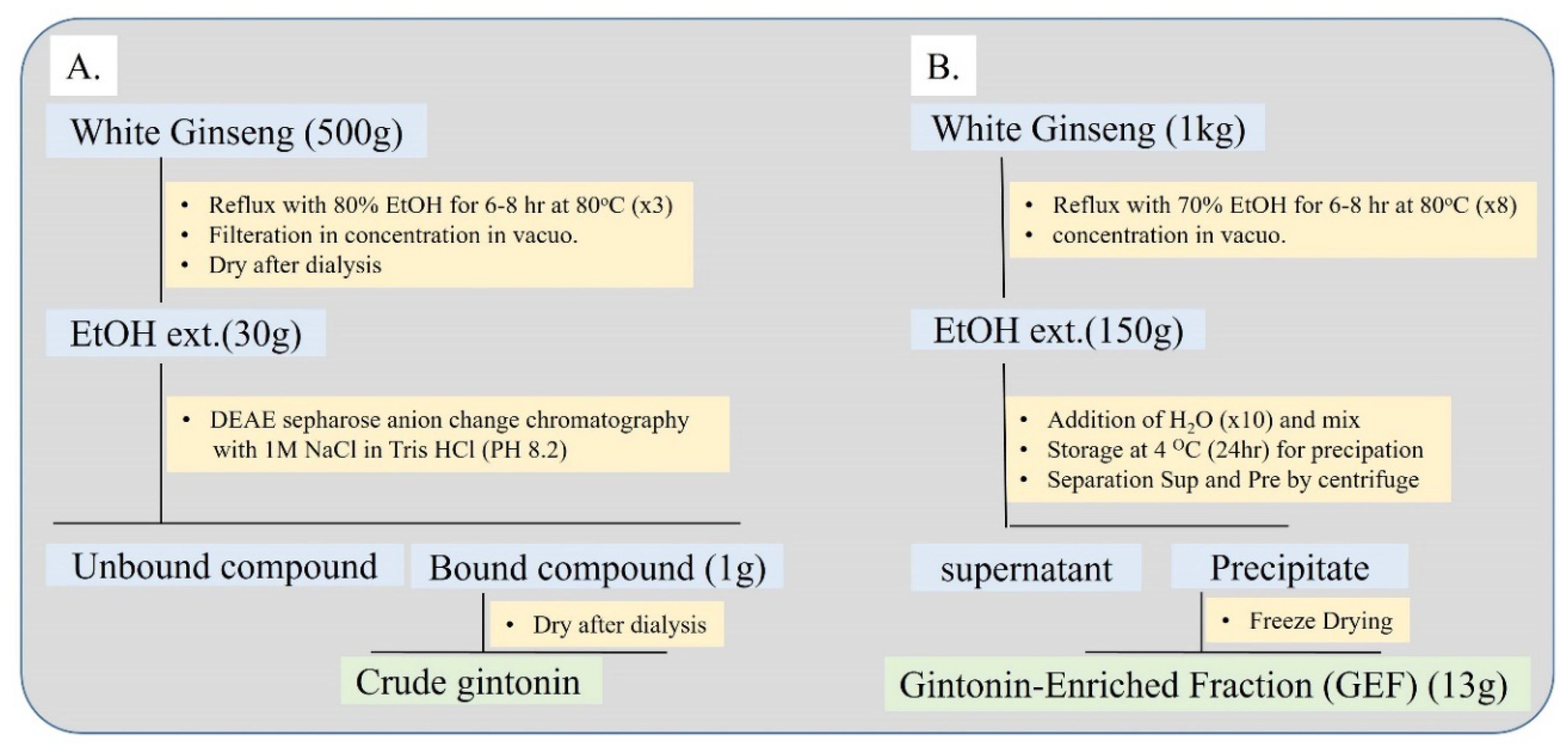
3. Isolation of Gintonin
4. Absorption of Gintonin
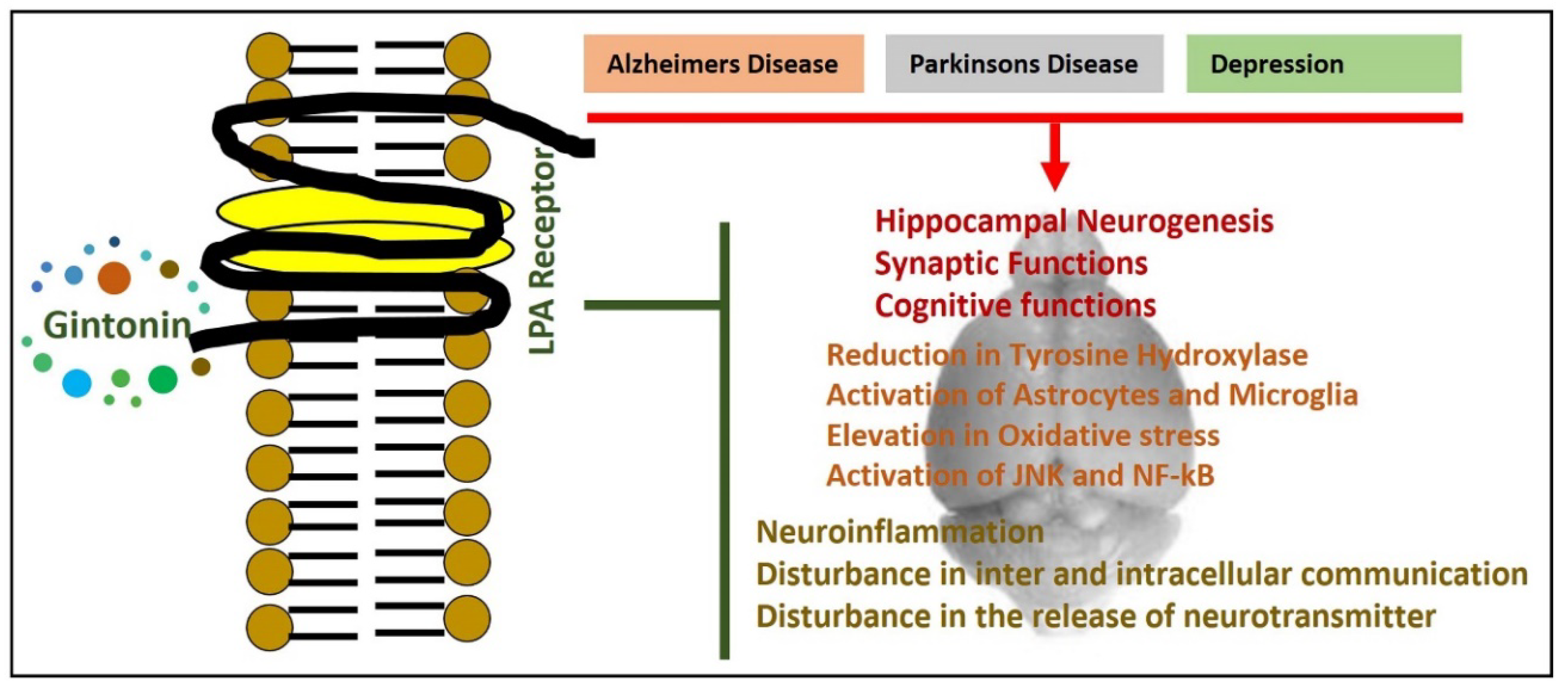
5. Lysophosphatidic Acid Receptor, Gintonin, and Neurological Disorders
6. Effects of Gintonin in Animal Models of Parkinson’s Disease
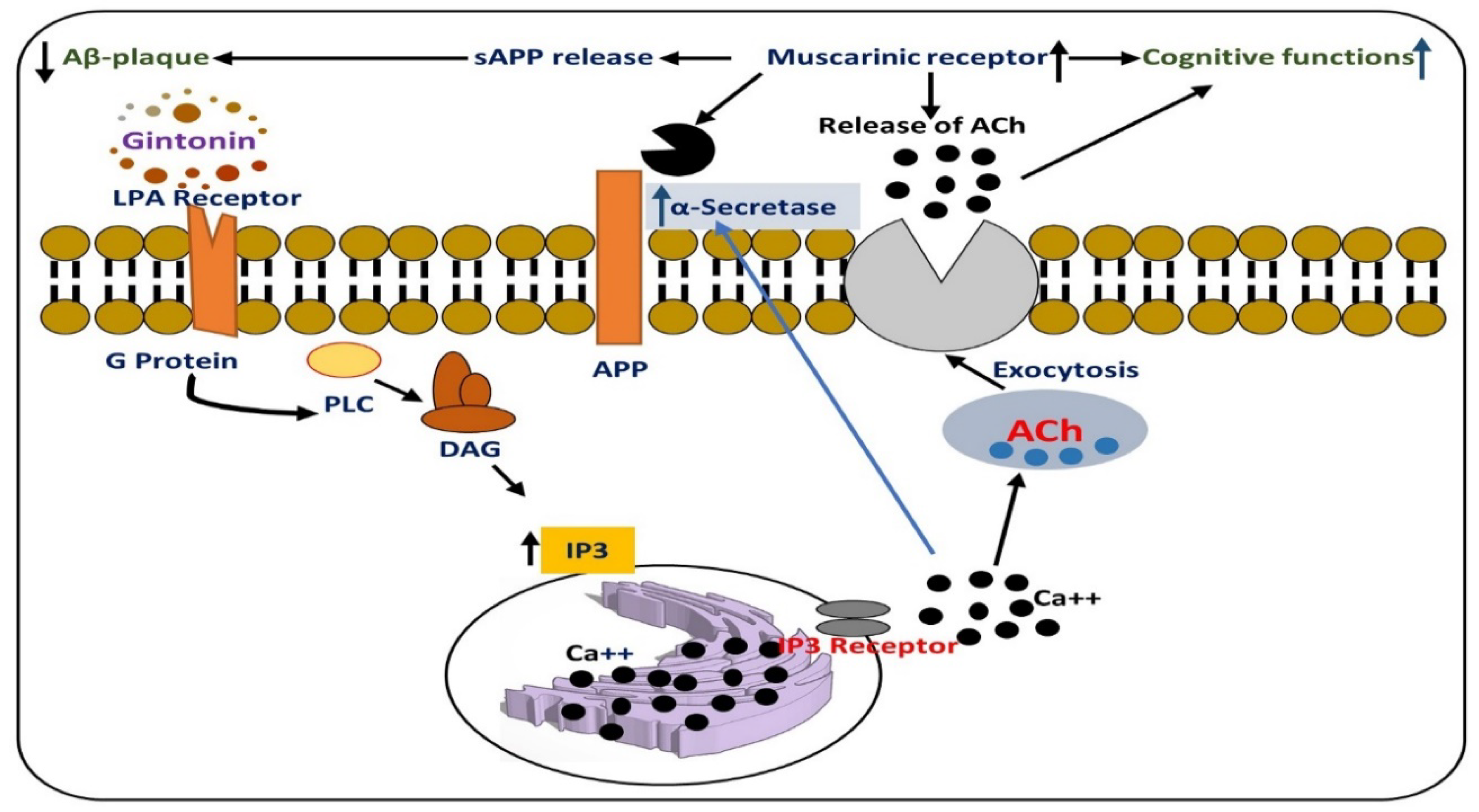
7. Effects of Gintonin Against Alzheimer Disease Pathology
8. Effects of Gintonin Against Huntington’s Disease
9. Effects of Gintonin against Multiple Sclerosis
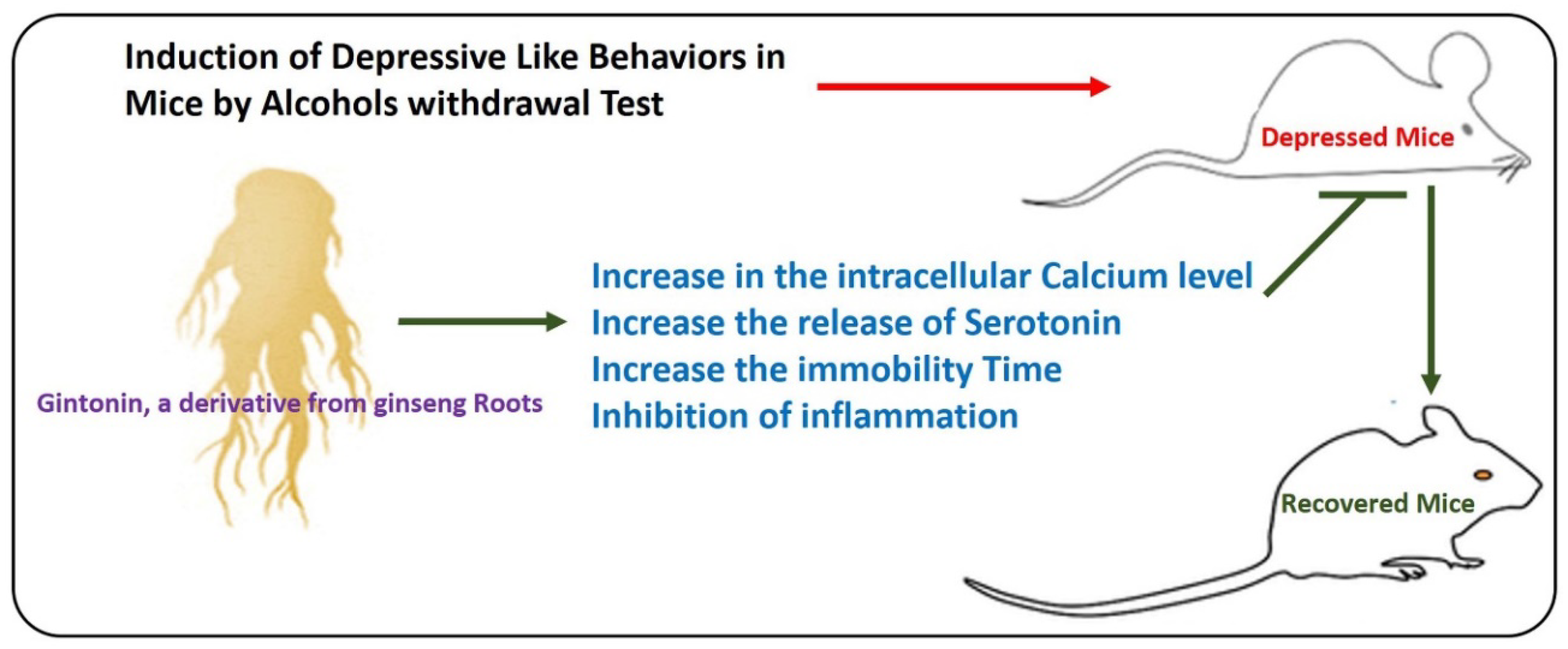
10. Effects of Gintonin on Anxiety and Depressive Behavior in Mice
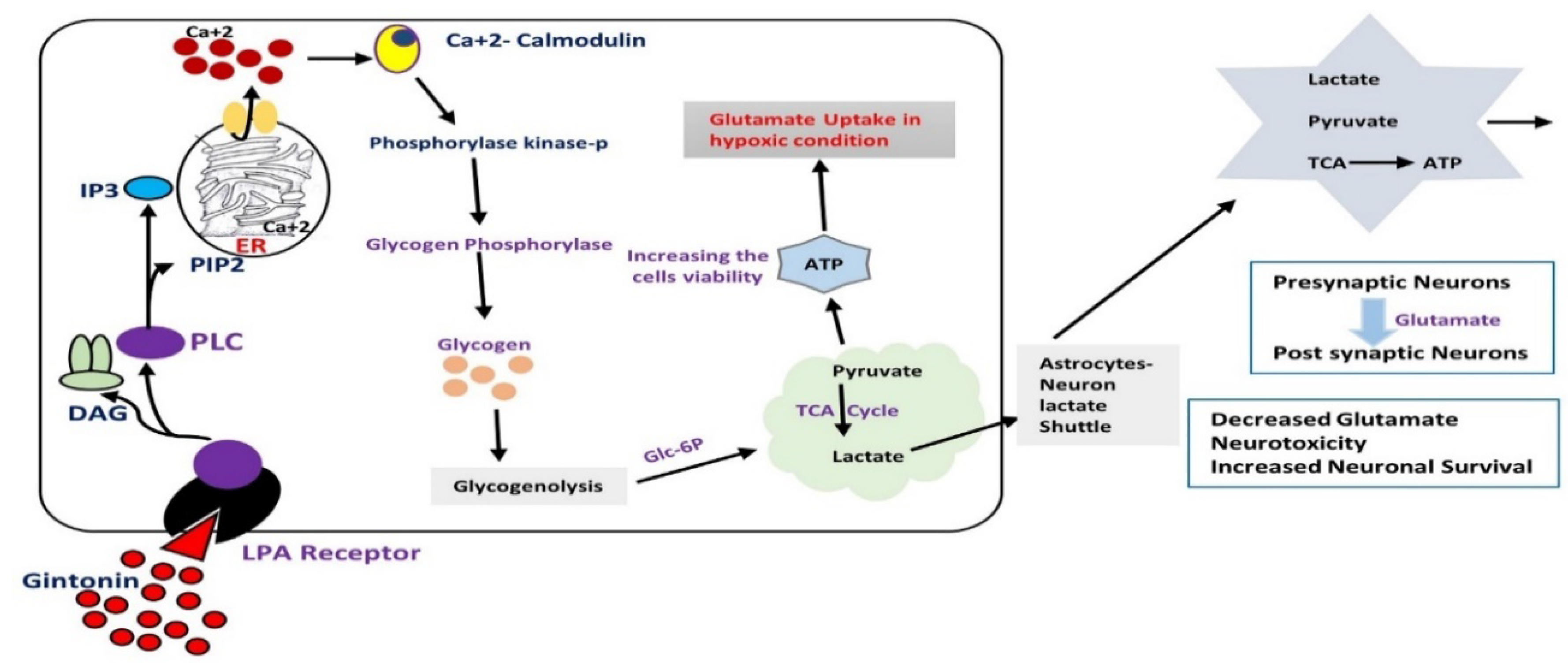
11. Effects of Gintonin against Hypoxic and Re-Oxygenation Stress via the Activation of Astrocytic Glycogenolysis
12. Effects of Gintonin against Synaptic Dysfunctions in Neurodegenerative Conditions
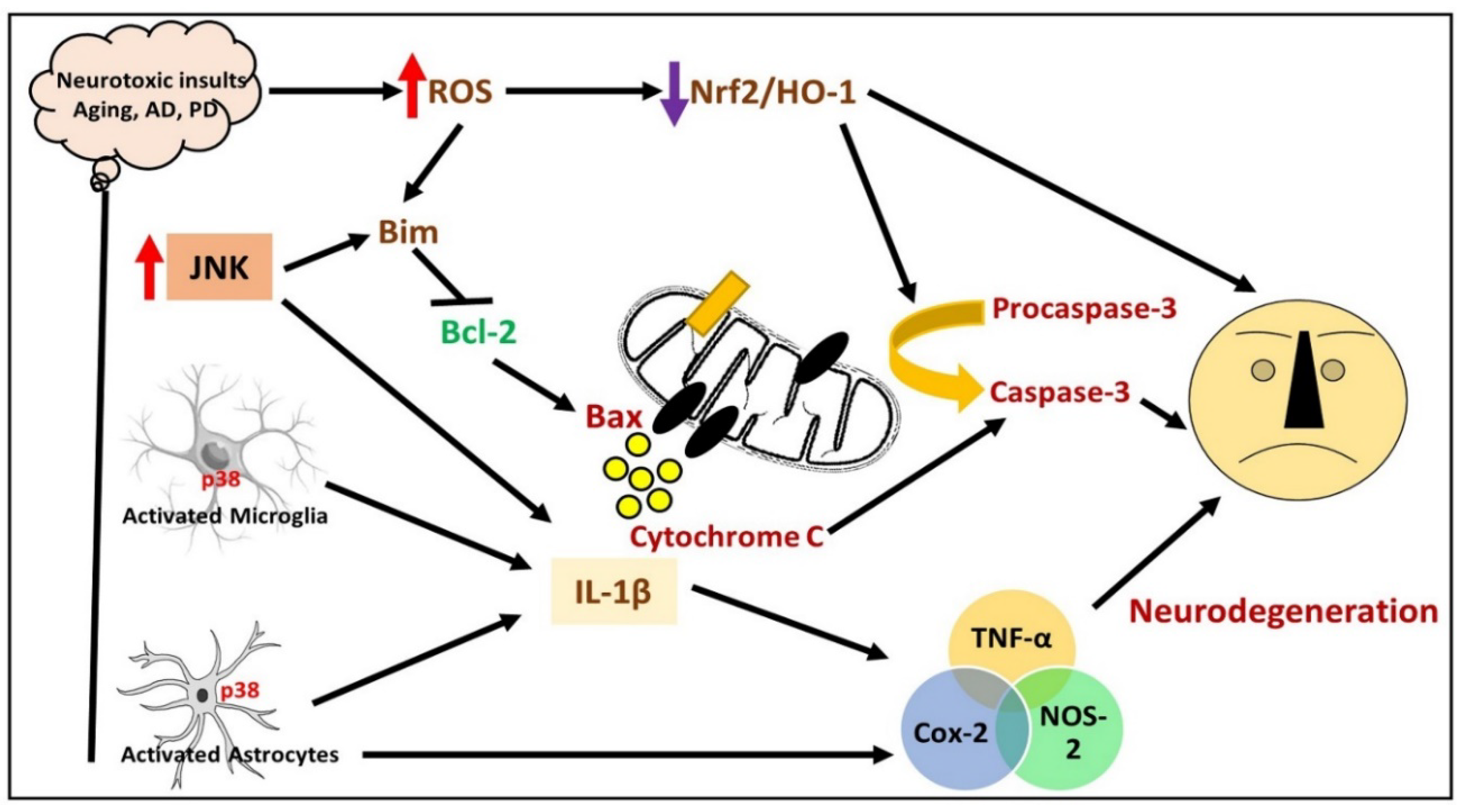
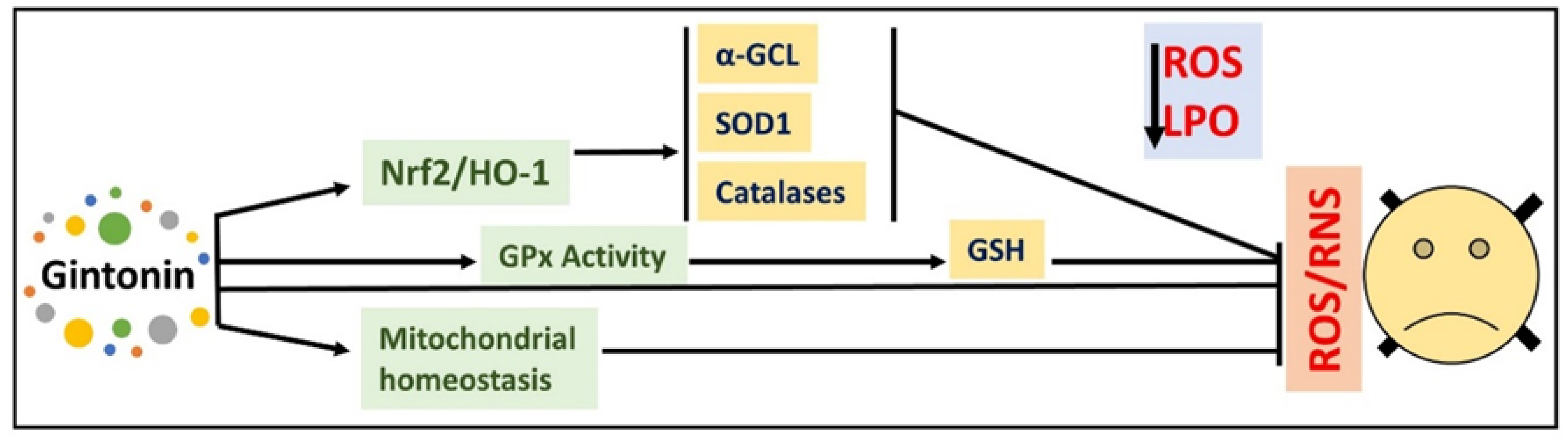
13. Effects of Gintonin against Different Models of Neurodegeneration
14. Conclusions and Future Considerations
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Li, N.; Pu, Y.; Zhang, T.; Wang, B. Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives. Molecules 2019, 24, 2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-kappaB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.G.; Ikram, M.; Jo, M.H.; Yoo, L.; Chung, K.C.; Nah, S.Y.; Hwang, H.; Rhim, H.; Kim, M.O. Gintonin Mitigates MPTP-Induced Loss of Nigrostriatal Dopaminergic Neurons and Accumulation of alpha-Synuclein via the Nrf2/HO-1 Pathway. Mol. Neurobiol 2019, 56, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Mentel, M.; Tielens, A.G.M.; Martin, W.F. Energy metabolism in anaerobic eukaryotes and Earth’s late oxygenation. Free Radic. Biol. Med. 2019, 140, 279–294. [Google Scholar] [CrossRef]
- Li, J.; O, W.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [Green Version]
- Marin, D.P.; Bolin, A.P.; Campoio, T.R.; Guerra, B.A.; Otton, R. Oxidative stress and antioxidant status response of handball athletes: Implications for sport training monitoring. Int. Immunopharmacol. 2013, 17, 462–470. [Google Scholar] [CrossRef]
- Scott, T.L.; Rangaswamy, S.; Wicker, C.A.; Izumi, T. Repair of oxidative DNA damage and cancer: Recent progress in DNA base excision repair. Antioxid. Redox Signal. 2014, 20, 708–726. [Google Scholar] [CrossRef] [Green Version]
- Haas, R.H. Mitochondrial Dysfunction in Aging and Diseases of Aging. Biology (Basel) 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef] [Green Version]
- Nah, S.Y. Gintonin: A novel ginseng-derived ligand that targets G protein- coupled lysophosphatidic acid receptors. Curr. Drug Targets 2012, 13, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 2011, 6, 193–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyo, M.K.; Choi, S.H.; Shin, T.J.; Hwang, S.H.; Lee, B.H.; Kang, J.; Kim, H.J.; Lee, S.H.; Nah, S.Y. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J. Ginseng Res. 2011, 35, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.H.; Choi, S.H.; Kim, H.J.; Park, S.D.; Rhim, H.; Kim, H.C.; Hwang, S.H.; Nah, S.Y. Gintonin absorption in intestinal model systems. J. Ginseng Res. 2018, 42, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J.J.N. Lysophosphatidic acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aikawa, S.; Hashimoto, T.; Kano, K.; Aoki, J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem. 2015, 157, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Matas-Rico, E.; García-Diaz, B.; Llebrez-Zayas, P.; López-Barroso, D.; Santín, L.; Pedraza, C.; Smith-Fernández, A.; Fernández-Llebrez, P.; Tellez, T.; Redondo, M.J.M.; et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell. Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Castilla-Ortega, E.; Sánchez-López, J.; Hoyo-Becerra, C.; Matas-Rico, E.; Zambrana-Infantes, E.; Chun, J.; De Fonseca, F.R.; Pedraza, C.; Estivill-Torrús, G.; Santin, L.J.; et al. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol. Learn. Mem. 2010, 94, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Castilla-Ortega, E.; Pedraza, C.; Chun, J.; de Fonseca, F.R.; Estivill-Torrús, G.; Santín, L.J. Hippocampal c-Fos activation in normal and LPA1-null mice after two object recognition tasks with different memory demands. Behav. Brain Res. 2012, 232, 400–405. [Google Scholar] [CrossRef]
- Das, A.K.; Hajra, A.K. Quantification, characterization and fatty acid composition of lysophosphatidic acid in different rat tissues. Lipids 1989, 24, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jang, M.; Oh, S.; Nah, S.Y.; Cho, I.H. Multi-Target Protective Effects of Gintonin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Mediated Model of Parkinson’s Disease via Lysophosphatidic Acid Receptors. Front. Pharmacol. 2018, 9, 515. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease. Redox Biol. 2019, 22, 101133. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aprahamian, I.; Stella, F.; Forlenza, O.V. New treatment strategies for Alzheimer’s disease: Is there a hope? Indian J. Med. Res. 2013, 138, 449–460. [Google Scholar]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, E.J.; Lee, B.H.; Choi, S.H.; Jung, S.W.; Cho, I.H.; Hwang, S.H.; Kim, J.Y.; Han, J.S.; Chung, C.; et al. Oral Administration of Gintonin Attenuates Cholinergic Impairments by Scopolamine, Amyloid-beta Protein, and Mouse Model of Alzheimer’s Disease. Mol. Cells 2015, 38, 796–805. [Google Scholar] [CrossRef]
- Damiano, M.; Galvan, L.; Déglon, N.; Brouillet, E. Mitochondria in Huntington’s disease. Biochim. Biophys. Acta 2010, 1802, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Gil-Mohapel, J.; Brocardo, P.S.; Christie, B.R. The role of oxidative stress in Huntington’s disease: Are antioxidants good therapeutic candidates? Curr. Drug Targets 2014, 15, 454–468. [Google Scholar] [CrossRef]
- Jang, M.; Choi, J.H.; Chang, Y.; Lee, S.J.; Nah, S.Y.; Cho, I.H. Gintonin, a ginseng-derived ingredient, as a novel therapeutic strategy for Huntington’s disease: Activation of the Nrf2 pathway through lysophosphatidic acid receptors. Brain Behav. Immun. 2019, 80, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, R.A.; Maghazachi, A.A. Multiple sclerosis and the role of immune cells. World J. Exp. Med. 2014, 4, 27–37. [Google Scholar] [CrossRef]
- Volpe, E.; Battistini, L.; Borsellino, G. Advances in T Helper 17 Cell Biology: Pathogenic Role and Potential Therapy in Multiple Sclerosis. Mediators Inflamm. 2015, 2015, 475158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; De Girolamo, G.; De Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Berk, M.; Hyphantis, T.N.; McIntyre, R.S. The integrative management of treatment-resistant depression: A comprehensive review and perspectives. Psychother. Psychosom. 2014, 83, 70–88. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S.; Alves, G.S.; Fountoulakis, K.N.; Carvalho, A.F. Beyond monoamines-novel targets for treatment-resistant depression: A comprehensive review. Curr. Neuropharmacol. 2015, 13, 636–655. [Google Scholar] [CrossRef] [Green Version]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef]
- Köhler, C.; Freitas, T.; Maes, M.d.; De Andrade, N.; Liu, C.; Fernandes, B.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psuchiatry 2013, 70, 31–41. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.; et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: Systematic review and meta-analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, M.; Ota, K.T.; Duman, R.S. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013, 31, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondelli, V.; Vernon, A.C.; Turkheimer, F.; Dazzan, P.; Pariante, C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017, 4, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, P.P.; Bertrand, R.L. Serotonin release and uptake in the gastrointestinal tract. Auton. Neurosci. 2010, 153, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racke, K.; Schwörer, H. Characterization of the role of calcium and sodium channels in the stimulus secretion coupling of 5-hydroxytryptamine release from porcine enterochromaffin cells. Naunyn Schmiedebergs Arch. Pharmacol. 1993, 347, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McLean, P.G.; Borman, R.A.; Lee, K. 5-HT in the enteric nervous system: Gut function and neuropharmacology. Trends Neurosci. 2007, 30, 9–13. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Horn, C.C. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton. Neurosci. 2006, 125, 100–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilla-Ortega, E.; Pavón, F.J.; Sánchez-Marín, L.; Estivill-Torrús, G.; Pedraza, C.; Blanco, E.; Suárez, J.; Santín, L.; de Fonseca, F.R.; Serrano, A. Both genetic deletion and pharmacological blockade of lysophosphatidic acid LPA1 receptor results in increased alcohol consumption. Neuropharmacology 2016, 103, 92–103. [Google Scholar] [CrossRef]
- Almeida-Montes, L.G.; Valles-Sanchez, V.; Moreno-Aguilar, J.; Chavez-Balderas, R.A.; García-Marín, J.A.; Sotres, J.C.; Hheinze-Martin, G. Neuroscience. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J. Psychiatry Neurosci. 2000, 25, 371. [Google Scholar]
- Ronaldson, P.T.; Davis, T.P. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol. Rev. 2013, 65, 291–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobysheva, N.V.; Tonshin, A.A.; Selin, A.A.; Yaguzhinsky, L.S.; Nartsissov, Y.R. Diversity of neurodegenerative processes in the model of brain cortex tissue ischemia. Neurochem. Int. 2009, 54, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yuan, W.; Cao, M.; Chen, R.; Wu, X.; Yan, J. Cyclophilin A Protects Cardiomyocytes against Hypoxia/Reoxygenation-Induced Apoptosis via the AKT/Nox2 Pathway. Oxid. Med. Cell Longev. 2019, 2019, 2717986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.E. Role of Glycogenolysis in Memory and Learning: Regulation by Noradrenaline, Serotonin and ATP. Front. Integr. Neurosci. 2015, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienel, G.A. The metabolic trinity, glucose-glycogen-lactate, links astrocytes and neurons in brain energetics, signaling, memory, and gene expression. Neurosci. Lett. 2017, 637, 18–25. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.L.; Stella, N.; Magistretti, P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Hwang, S.H.; Shin, T.-J.; Choi, S.-H.; Cho, H.-J.; Lee, B.-H.; Pyo, M.K.; Lee, J.-H.; Kang, J.; Kim, H.-J.; Park, C.-W.; et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol. Cells 2012, 33, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Tabuchi, S.; Kume, K.; Aihara, M.; Shimizu, T. Expression of lysophosphatidic acid receptor in rat astrocytes: Mitogenic effect and expression of neurotrophic genes. Neurochem. Res. 2000, 25, 573–582. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990, 27, 457–464. [Google Scholar] [CrossRef]
- Marksteiner, J.; Kaufmann, W.A.; Gurka, P.; Humpel, C. Synaptic proteins in Alzheimer’s disease. J. Mol. Neurosci. 2002, 18, 53–63. [Google Scholar] [CrossRef]
- Brown, D.F.; Risser, R.C.; Bigio, E.H.; Tripp, P.; Stiegler, A.; Welch, E.; Eagan, K.P.; Hladik, C.L.; White, C.L., 3rd. Neocortical synapse density and Braak stage in the Lewy body variant of Alzheimer disease: A comparison with classic Alzheimer disease and normal aging. J. Neuropathol. Exp. Neurol. 1998, 57, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Scheff, S.W.; DeKosky, S.T.; Price, D.A. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol. Aging 1990, 11, 29–37. [Google Scholar] [CrossRef]
- Mota, S.I.; Ferreira, I.L.; Rego, A.C. Dysfunctional synapse in Alzheimer’s disease–A focus on NMDA receptors. Neuropharmacology 2014, 76, 16–26. [Google Scholar] [CrossRef]
- Coleman, P.D.; Yao, P.J. Synaptic slaughter in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.E.; Dall’Armi, C.; Voronov, S.V.; McIntire, L.B.J.; Zhang, H.; Moore, A.Z.; Staniszewski, A.; Arancio, O.; Kim, T.-W.; Di Paolo, G. Oligomeric amyloid-β peptide disrupts phosphatidylinositol-4, 5-bisphosphate metabolism. Nat. Neurosci. 2008, 11, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pooler, A.M.; Noble, W.; Hanger, D.P. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology 2014, 76, 1–8. [Google Scholar] [CrossRef]
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32. [Google Scholar] [CrossRef]
- Harrison, S.; Reavill, C.; Brown, G.; Brown, J.; Cluderay, J.; Crook, B.; Davies, C.; Dawson, L.; Grau, E.; Heidbreder, C.; et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell Neurosci. 2003, 24, 1170–1179. [Google Scholar] [CrossRef]
- Dash, P.K.; Orsi, S.A.; Moody, M.; Moore, A.N. A role for hippocampal Rho–ROCK pathway in long-term spatial memory. Biochem. Biophys. Res. Commun. 2004, 322, 893–898. [Google Scholar] [CrossRef]
- Lin, M.E.; Rivera, R.R.; Chun, J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J. Biol. Chem. 2012, 287, 17608–17617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedraza, C.; Sanchez-Lopez, J.; Castilla-Ortega, E.; Rosell-Valle, C.; Zambrana-Infantes, E.; Garcia-Fernandez, M.; Rodriguez de Fonseca, F.; Chun, J.; Santin, L.J.; Estivill-Torrus, G. Fear extinction and acute stress reactivity reveal a role of LPA(1) receptor in regulating emotional-like behaviors. Brain Struct. Funct. 2014, 219, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, S.; Rhee, J.; Kim, H.J.; Han, J.S.; Nah, S.Y.; Chung, C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J. Neurophysiol. 2015, 113, 1493–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine May Abrogate LPS-Induced Oxidative Stress and Neuroinflammation by Regulating Nrf2/TLR4 in Adult Mouse Brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.M.; Hwang, H.; Seo, M.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Rhim, H.; Kim, H.C.; Cho, I.H.; Nah, S.Y. Gintonin Attenuates D-Galactose-Induced Hippocampal Senescence by Improving Long-Term Hippocampal Potentiation, Neurogenesis, and Cognitive Functions. Gerontology 2018, 64, 562–575. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, H.J.; Cho, H.J.; Park, S.D.; Lee, N.E.; Hwang, S.H.; Rhim, H.; Kim, H.C.; Cho, I.H.; Nah, S.Y. Gintonin-mediated release of astrocytic vascular endothelial growth factor protects cortical astrocytes from hypoxia-induced cell damages. J. Ginseng Res. 2019, 43, 305–311. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, S.H.; Lee, N.E.; Cho, H.J.; Rhim, H.; Kim, H.C.; Hwang, S.H.; Nah, S.Y. Effects of Gintonin-Enriched Fraction on Methylmercury-Induced Neurotoxicity and Organ Methylmercury Elimination. Int. J. Environ. Res. Public Health 2020, 17, 838. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Choi, S.H.; Shim, J.Y.; Park, H.J.; Oh, M.J.; Kim, M.; Nah, S.Y. Gintonin Administration is Safe and Potentially Beneficial in Cognitively Impaired Elderly. Alzheimer Dis. Assoc. Disord. 2018, 32, 85–87. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, M.; Ullah, R.; Khan, A.; Kim, M.O. Ongoing Research on the Role of Gintonin in the Management of Neurodegenerative Disorders. Cells 2020, 9, 1464. https://doi.org/10.3390/cells9061464
Ikram M, Ullah R, Khan A, Kim MO. Ongoing Research on the Role of Gintonin in the Management of Neurodegenerative Disorders. Cells. 2020; 9(6):1464. https://doi.org/10.3390/cells9061464
Chicago/Turabian StyleIkram, Muhammad, Rahat Ullah, Amjad Khan, and Myeong Ok Kim. 2020. "Ongoing Research on the Role of Gintonin in the Management of Neurodegenerative Disorders" Cells 9, no. 6: 1464. https://doi.org/10.3390/cells9061464
APA StyleIkram, M., Ullah, R., Khan, A., & Kim, M. O. (2020). Ongoing Research on the Role of Gintonin in the Management of Neurodegenerative Disorders. Cells, 9(6), 1464. https://doi.org/10.3390/cells9061464




