Cerebral MRI and Clinical Findings in Children with PTEN Hamartoma Tumor Syndrome: Can Cerebral MRI Scan Help to Establish an Earlier Diagnosis of PHTS in Children?
Abstract
1. Introduction
2. Methods
3. Results
3.1. Macrocephaly
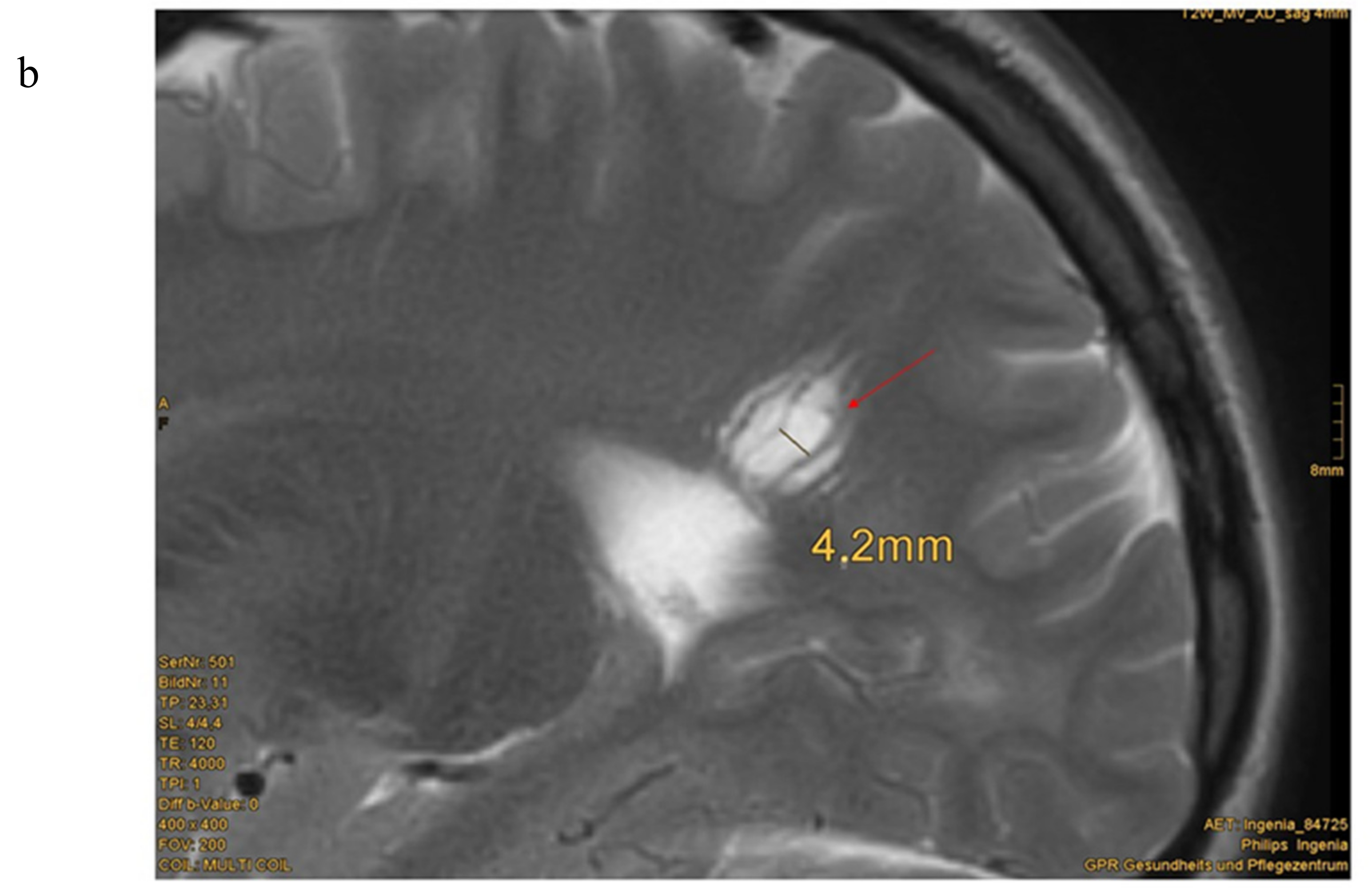
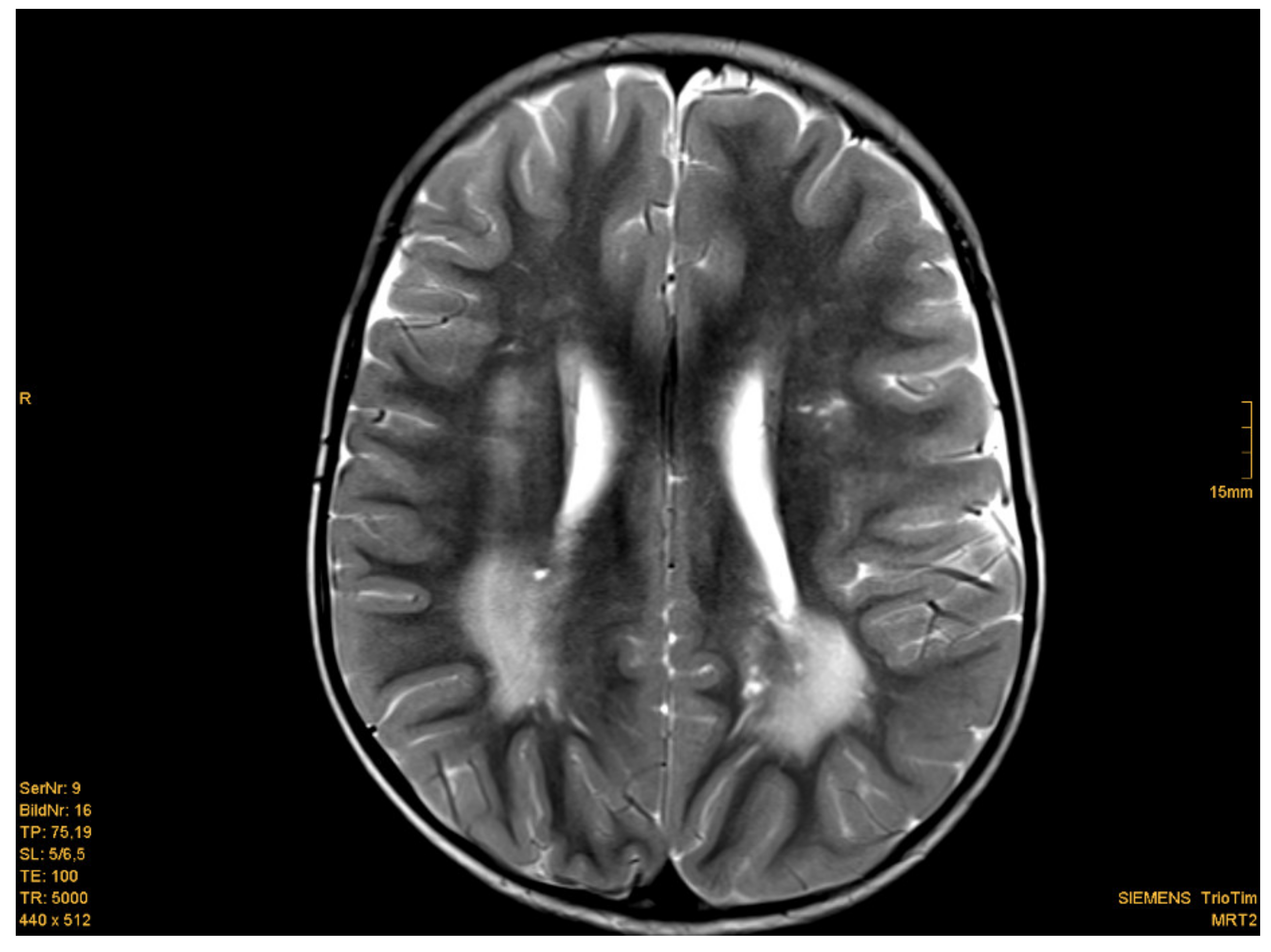
3.2. MRI
3.3. Psychomotor Development
3.4. Additional Phenotypical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, M.H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Plamper, M.; Schreiner, F.; Gohlke, B.; Kionke, J.; Korsch, E.; Kirkpatrick, J.; Born, M.; Aretz, S.; Woelfle, J. Thyroid disease in children and adolescents with PTEN hamartoma tumor syndrome (PHTS). Eur. J. Pediatr. 2018, 177, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Marqusee, E.; Webb, S.; Nose, V.; Fishman, S.J.; Shamberger, R.C.; Frates, M.C.; Huang, S.A. Thyroid nodules and cancer in children with PTEN hamartoma tumor syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Smpokou, P.; Fox, V.L.; Tan, W.H. PTEN hamartoma tumour syndrome: Early tumour development in children. Arch. Dis. Child. 2015, 100, 34–37. [Google Scholar] [CrossRef]
- Marsh, D.J.; Kum, J.B.; Lunetta, K.L.; Bennett, M.J.; Gorlin, R.J.; Ahmed, S.F.; Bodurtha, J.; Crowe, C.; Curtis, M.A.; Dasouki, M.; et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum. Mol. Genet. 1999, 8, 1461–1472. [Google Scholar] [CrossRef]
- Parisi, M.A.; Dinulos, M.B.; Leppig, K.A.; Sybert, V.P.; Eng, C.; Hudgins, L. The spectrum and evolution of phenotypic findings in PTEN mutation positive cases of Bannayan-Riley-Ruvalcaba syndrome. J. Med. Genet. 2001, 38, 52–58. [Google Scholar] [CrossRef]
- Tan, W.H.; Baris, H.N.; Burrows, P.E.; Robson, C.D.; Alomari, A.L.; Mulliken, J.B.; Fishman, S.J.; Irons, M.B. The spectrum of vascular anomalies in patients with PTEN mutations: Implications for diagnosis and management. J. Med. Genet. 2007, 44, 594–602. [Google Scholar] [CrossRef]
- Tan, M.H.; Mester, J.; Peterson, C.; Yang, Y.; Chen, J.L.; Rybicki, L.A.; Milas, K.; Pederson, H.; Remzi, B.; Orloff, M.S.; et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011, 88, 42–56. [Google Scholar] [CrossRef]
- Kato, K.; Mizuno, S.; Inaba, M.; Fukumura, S.; Kurahashi, N.; Maruyama, K.; Leda, D.; Ohashi, K.; Hori, I.; Negishi, Y.; et al. Distinctive facies, macrocephaly, and developmental delay are signs of PTEN mutation in childhood. Brain Dev. 2018, 40, 678–684. [Google Scholar] [CrossRef]
- Busa, T.; Milh, M.; Degardin, N.; Girard, N.; Sigaudy, S.; Longy, M.; Olshchwang, S.; Sobol, H.; Chabrol, B.; Philip, N. Clicical presentations of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur. J. Paediatr. Neurol. 2015, 19, 188–192. [Google Scholar] [CrossRef]
- Plamper, M.; Gohlke, B.; Schreiner, F.; Woelfle, J. Phenotype-Driven Diagnostic of PTEN Hamartoma Tumor Syndrome: Macrocephaly, But Neither Height nor Weight Development, Is the Important Trait in Children. Cancers 2019, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.; Viseux, V.; Avril, M.F.; Richard, M.A.; Gondry-Jouet, C.; Deramond, H.; Desfossez-Tribout, C.; Courtade, S.; Delaunay, M.; Piette, F.; et al. Cancerology Group of the French Society of Dermatology. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine (Baltimore) 2005, 84, 129–136. [Google Scholar] [CrossRef]
- Balci, T.B.; Davila, J.; Lewis, D.; Boafo, A.; Sell, E.; Richer, J.; Nikkel, S.M.; Armour, C.M.; Tomiak, E.; Lines, M.A.; et al. Broad spectrum of neuropsychiatric phenotypes associated with white matter disease in PTEN hamartoma tumor syndrome. Am. J. Med. Genet. Part B 2018, 177, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, R.; Weindling, S.M.; Porter, A.B.; Hu, L.S.; Wood, C.P.; Hoxworth, J.M. Neuroimaging abnormalities in patients with Cowden syndrome: Retrospective single-center study. Neurol. Clin. Pract. 2018, 8, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shiohama, T.; Levman, J.; Vasung, L.; Takahashi, E. Brain morphological analysis in PTEN hamartoma tumor syndrome. Am. J. Med. Genet. A 2020, 182, 1117–1129. [Google Scholar] [CrossRef]
- Farooq, A.; Walker, L.J.; Bowling, J.; Audisio, R.A. Cowden syndrome. Cancer Treat. Rev. 2010, 36, 577–583. [Google Scholar] [CrossRef]
- Nelen, M.R.; Kremer, H.; Konings, I.B.; Schoute, F.; van Essen, A.J.; Koch, R.; Woods, C.G.; Fryns, J.P.; Hamel, B.; Hoefsloot, L.H.; et al. Novel PTEN mutations in patients with Cowden disease: Absence of clear genotype-phenotype correlations. Eur. J. Hum. Genet. 1999, 7, 267–273. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Axilbund, J.E.; Buys, S.S.; Crawford, B.; Friedman, S.; Garber, J.E.; Horton, C.; Kaklamani, V.; Klein, C.; et al. Genetic/Familial High-Risk Assessment: Breast and Ovarien, Version 1. J. Natl. Compr. Cancer Netw. 2014, 12, 1326–1338. [Google Scholar] [CrossRef]
- Lachlan, K.L.; Lucassen, A.M.; Bunyan, D.; Temple, I.K. Cowden syndrome and Bannayan Riley Ruvalcaba syndrome represent one condition with variable expression and age-related penetrance: Results of a clinical study of PTEN mutation carriers. J. Med. Genet. 2007, 44, 579–585. [Google Scholar] [CrossRef]
- Pavone, P.; Pratico, A.D.; Rizzo, R.; Corsello, G.; Ruggieri, M.; Parano, E.; Falsaperla, R. A clinical review on megalencephaly: A large brain as a possible sign of cerebral impairment. Medicine 2017, 96, e6814. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.M.; Riviere, J.B.; Dobyns, W.B. Megalencephaly syndromes and activation mutatons in the PI3K-AKT pathway: MPPH and MCAP. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.M.; Pduri, A. Megalencephaly and hemimegalencephaly: Breaktroughs in molecular etiology. Am. J. Med. Genet. C Semin. Med. Genet. 2014, 166, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, G.R.; Ammache, Z.; Bell, W.; Yuh, W.T. Unusually prominent perivascular spaces. Neurology 2011, 56, 1242. [Google Scholar] [CrossRef] [PubMed]
- Heier, L.A.; Bauer, C.J.; Schwartz, L.; Zimmerman, R.D.; Morgello, S.; Deck, M.D. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am. J. Neuroradiol. 1989, 10, 929–936. [Google Scholar] [PubMed]
- Rudie, J.D.; Rauschecker, A.M.; Nabavizadeh, S.A.; Mohan, S. Neuroimaging of Dilated Perivascular spaces: From Benign and Pathologic Causes to Mimics. J. Neuroimaging 2018, 28, 139–149. [Google Scholar] [CrossRef] [PubMed]
- MacLullich, A.M.; Wardlaw, J.M.; Ferguson, K.J.; Starr, J.M.; Seckl, J.R.; Deary, I.J. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1519–1523. [Google Scholar] [CrossRef]
- Vanderver, A.; Tonduti, D.; Kahn, I.; Schmidt, J.; Medne, L.; Vento, J.; Van Der Knaap, M.S. Characteristic brain magnetic resonance imaging pattern in patients with macrocephaly and PTEN mutations. Am. J. Med. Genet. Part A 2014, 164, 627–633. [Google Scholar] [CrossRef]
- Busch, R.M.; Strivasta, S.; Hogue, O.; Frazier, T.W.; Klaas, P.; Hardan, A.; Martinez-Agosto, J.A.; Sahin, M.; Eng, C. Developmental Synaptopathies Consortium, Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Transl. Psychiatry 2019, 9, 253. [Google Scholar] [CrossRef]
- Busch, R.M.; Chapin, J.S.; Mester, J.; Fergueson, L.; Haut, J.S.; Frazier, T.W.; Eng, C. The Cognitive Characteristics of PTEN Hamartoma Tumor Syndromes. Genet. Med. 2013, 15, 548–553. [Google Scholar] [CrossRef][Green Version]
- Frazier, T.W.; Embacher, R.; Tilot, A.K.; Koenig, K.; Mester, J.; Eng, C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol. Psychiatry 2015, 20, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- AWMG Guidelins: S1-Leitlinie. Diagnostik und Management von Patientin mit PTEN Hamartom Tumor Syndrom (PHTS) im Kinder- und Jugendalter. Registernr.: 174-025. Available online: https://www.awmf.org/leitlinien/detail/ll/174-025.html (accessed on 30 October 2019).

| Pat.No | Sex | Mutation/Deletion in PTEN Gene (Localisation) | Age at DIAGNOSIS (Years) | Age at cMRI (Years) | Results of cMRI Scan | IQ > 85 | Delay in Motor Develop-ment | Muscle Hypotonia | Confirmed Autism | More Detailed Description of Neurological Features and Academic Performance | Age at Start of Walking Independently (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | c.389G > A; Arg130 Gln (exon 5) | 3.8 | n.d. | n.d. | + | + | - | - | moderate delay in motor development, normal intelligence, secondary school −>university | 22 |
| 2 | male | c.389G > A; Arg130 Gln (exon 5) | 4 | 0.25 2.5 | enlarged perivascular spaces | - | + | + | + | muscle hypotonia, autism, developmental delay in motor and language development, no expressive speech | 26 |
| 3 | male | c.540C > A; p.Y180X (exon 6) | 5.3 | 6 | enlarged perivascular spaces | + | + | - | - | delay of fine motor skills, normal intelligence, secondary school | 18 |
| 4 | male | c.737C > T.p.Pro246Leu (exon 7) | 1.3 | normal MRI scan (reported) | + | - | - | - | None, normal intelligence, secondary school | 17 | |
| 5 | male | c.209 + 5G > A (Intron 3) | 2 | 1.75 | white matter abnormalities, (periventricular posterior white matter), enlarged perivascular spaces | + | + | - | - | delay of gross and fine motor skills, normal intelligence, impulsivity, secondary school | 18 |
| 6 | male | c.445C > T; Gln149X (exon 5) | 3 | 2.0 | white matter abnormalities (posterior horn up to parietal white matter; smaller frontal and periventricular lesions), enlarged perivascular spaces | + | - | - | - | None, normal intelligence, elementary school | 18 |
| 7 | male | c.509G > A; pSer170Asn (exon 6) | 4 | n.d. | n.d. | + | + | - | - | moderat delay in fine motor skills, normal intelligence, elementary school | 14.5 |
| 8 | male | heterozygous deletion (exon 1–2) | 8 | 4.5 4.75 5.3 | Ventriculo-peritoneal shunt, enlarged perivascular spaces | - | + | - | (-) | social behaviour problems, impulsivity, developmental delay, pseudotumor cerebri, Difficulties in regular school | ? |
| 9 | male | partial deletion (exon 6) | 1.5 | 1 2.5 4.5 | Periventricular, occipital, parietal and and smaller frontal white matter abnormalities; enlarged perivascular spaces | + | + | + | - | muscle hypotonia, moderate delay in motor development, normal intelligence, secondary school diagnosis of PHTS because of MC and EPVS | 20 |
| 10 | male | c.697C > T;pArg233*(exon 7) | 11 | n.d. | n.d. | + | - | - | - | None, normal intelligence, secondary school | 18 |
| 11 | male | c.959T > G (p.Leu320*) | 7.5 | 0.3 7.8 8.3 9.3 | Cavernoma right side cerebellum, enlarged perivascular spaces; slight parieto-occipital white matter abnormalities | + IQ 91 | - | + | - | muscle hypotonia, difficulties in logical reasoning, impulsivity normal intelligence: HAWIK IV with 8 years: IQ 91 Special needs school | 14 |
| 12 | male | c.987dup T (p.Lys330*) (exon 8) | 6.5 | n.d. | n.d. | + IQ 84 | + | + | - | muscle hypotonia WPPSI-III, 2009–HAWIVA-III with 6 years: IQ 84, elementary school | 24 |
| 13 | male | c.(492 + 1_493–1)_(1026 + 1_1027–1)del | 0.9 | 0.6 | enlarged perivascular spaces | + | + | + | - | muscle hypotonia, delay in fine motor skills, normal intelligence, kindergarten | 19 |
| 14 | male | heterozygous deletion PTEN and BMPR1A Gene | 0.7 | 0.75 2 | arachnoid cysts left and right of the pineal region, enlarged perivascular spaces, parietal and temporal white matter abnormalities (left sided pronounced), parietal Pacchioni granulation | + | - | + | - | muscle hypotonia | 18 |
| 15 | male | c.800_801delAG (exon 7) | 10 | 4.5 | enlarged perivascular spaces, slight parietal white matter abnormalities | −/+ IQ with 4.5 years: 70; with 5.9 years: IQ 89 | + | - | - | developmental delay in speech, cognition and motor development, HAWIWA III with 4.5 years: 70; HAWIVA III with 5.9 years: IQ 89, special needs school | 14 |
| 16 | male | c.464a > G; p.Tyr155Cys | 12 | 11.75 | enlarged perivascular spaces | + IQ 85 | + | - | - | problems in sense of balance, dyslexia, panic attacks, diagnosis of PHTS because of MC and EPVS | ? |
| 17 | male | p.Arg130Ter*;c.388C > T | 4.5 | n.d. | n.d. | + | + | - | - | delay in motor development, normal intelligence | 30 |
| 18 | male | c.266C > G (p.Pro89Arg) | 9 | 0.75 2.25 8 | subependymal heterotopia at the top of the right lateral ventricle, enlarged perivascular spaces | + IQ 93 | + | + | + | delay in language and motor development. autism, ADHD, muscle hypotonia, obsessive-compulsive disorder, social behaviour problems, HAWIK: IQ 93, special needs school | 30 |
| 19 | female | c.741dupA; p.Pro248Thrfs*5 (exon 7) | 13.5 | n.d. | n.d. | + | - | - | - | None, secondary school −> university | 13 |
| 20 | female | c.302T > C; p.Ile101Thr (exon 5) | 5 | n.d. | n.d. | + IQ 89 | + | + | - | global developmental delay, muscle hypotonia, IQ testing: 89 | 24 |
| 21 | female | c.762dupA; p.Val255Serfs*43 (exon 7) | 5.5 | 6.2 | enlarged perivascular spaces | + IQ 96 | + | ++ | - | severe muscle hypotonia, difficulties in logical reasoning, HAWIK-IV/WISC-IV: IQ 96, special needs school | 24 |
| 22 | female | c.49C > T;p.Gln17* (exon1) | 6.8 | 8.5 | normal MRI scan, but enlarged perivascular spaces (reported) | + IQ 95 | + | + | - | ADHS, orofacial hypotonia, delay in motor development, normal intelligence, IQ with 6 years:95 Problems in elementary school | 18 |
| 23 | female | c.1008C > G;p.Tyr336* (exon 8) | 5.8 | 2.75 3 10.75 | extremely large perivascular spaces, arachnoidal cysts | - | + | - | - | problems in sense of balance, ataxia, global developmental delay, special needs school | 19 |
| 24 | female | c.492delG; p.Gly165Glufs*2 (exon 5) | 2.8 | 1.25 | normal MRI scan enlarged perivascular spaces | + | - | - | - | None, normal intelligence, secondary school | 17 |
| 25 | female | c.1133_1136del.pArg378ilefs*37 (exon 9) | 3.5 | 1 | Chiari malformation type I, enlarged perivascular spaces | ? | + | - | - | delay in cross motor skills, language developmental delay, kindergarten | 29 |
| 26 | female | c.389G > A; p.(Arg130 Gln) (exon 5) | 2.3 | 0.8 | Supraventricular white matter abnormalities, left-sided; enlarged perivascular spaces | ? | + | + | - | muscle hypotonia, delay in language and motor development, kindergarten | 28 |
| 27 | female | c.406T > C(p.Cys136Arg) | 3 | n.d. | n.d. | ? | + | + | - | autism, muscle hypotonia, delay in language development, kindergarten | 20 |
| (a) | |||
| Major Criteria | Minor Criteria | ||
| Macrocephaly | Autism spectrum disorder | ||
| Positive family history | Mental retardation (i.e., IQ of 75 and below) | ||
| Facial trichilemmomas (>/= 3) | Esophageal acanthosis | ||
| Oral papilloma | Lipoma | ||
| Macular pigmentation of glans penis | Renal cell carcinoma | ||
| Multiple GI hamartomas or ganglioneuroma | Testicular lipomatosis | ||
| Thyroid carcinoma/adenoma | Other thyroid lesions (e.g., adenoma, multinodular goiter) | ||
| Breast cancer | Vascular anomalies | ||
| Endometrial cancer | Enlarged perivascular spaces in cMRI | ||
| White matter abnormalities | |||
| (b) | |||
| Analysis of PTEN Gene, If | Macrocephaly Plus | No Macrocephaly/Positive Family History | Positive Family History (Positive PTEN Gene Mutation) |
| At least one of the following criteria: | 2 major criteria | Genetical testing without any other criteria, if a parent is positive for a PTEN gene mutation | |
| autism spectrum disorder or developmental delay | 1 major criteria +2 minor criteria | ||
| dermatologic features, including lipomas, trichilemmomas, oral papillomas, penile freckling | 3 minor criteria | ||
| vascular pathologies | |||
| multiple GI hamartomas or ganglioneuroma | |||
| thyroid lesions (especially adenoma and carcinoma) | |||
| enlarged perivascular spaces in cMRI | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plamper, M.; Born, M.; Gohlke, B.; Schreiner, F.; Schulte, S.; Splittstößer, V.; Woelfle, J. Cerebral MRI and Clinical Findings in Children with PTEN Hamartoma Tumor Syndrome: Can Cerebral MRI Scan Help to Establish an Earlier Diagnosis of PHTS in Children? Cells 2020, 9, 1668. https://doi.org/10.3390/cells9071668
Plamper M, Born M, Gohlke B, Schreiner F, Schulte S, Splittstößer V, Woelfle J. Cerebral MRI and Clinical Findings in Children with PTEN Hamartoma Tumor Syndrome: Can Cerebral MRI Scan Help to Establish an Earlier Diagnosis of PHTS in Children? Cells. 2020; 9(7):1668. https://doi.org/10.3390/cells9071668
Chicago/Turabian StylePlamper, Michaela, Mark Born, Bettina Gohlke, Felix Schreiner, Sandra Schulte, Vera Splittstößer, and Joachim Woelfle. 2020. "Cerebral MRI and Clinical Findings in Children with PTEN Hamartoma Tumor Syndrome: Can Cerebral MRI Scan Help to Establish an Earlier Diagnosis of PHTS in Children?" Cells 9, no. 7: 1668. https://doi.org/10.3390/cells9071668
APA StylePlamper, M., Born, M., Gohlke, B., Schreiner, F., Schulte, S., Splittstößer, V., & Woelfle, J. (2020). Cerebral MRI and Clinical Findings in Children with PTEN Hamartoma Tumor Syndrome: Can Cerebral MRI Scan Help to Establish an Earlier Diagnosis of PHTS in Children? Cells, 9(7), 1668. https://doi.org/10.3390/cells9071668





