Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200–CD200R and CX3CL1–CX3CR1 Systems
Abstract
1. Introduction
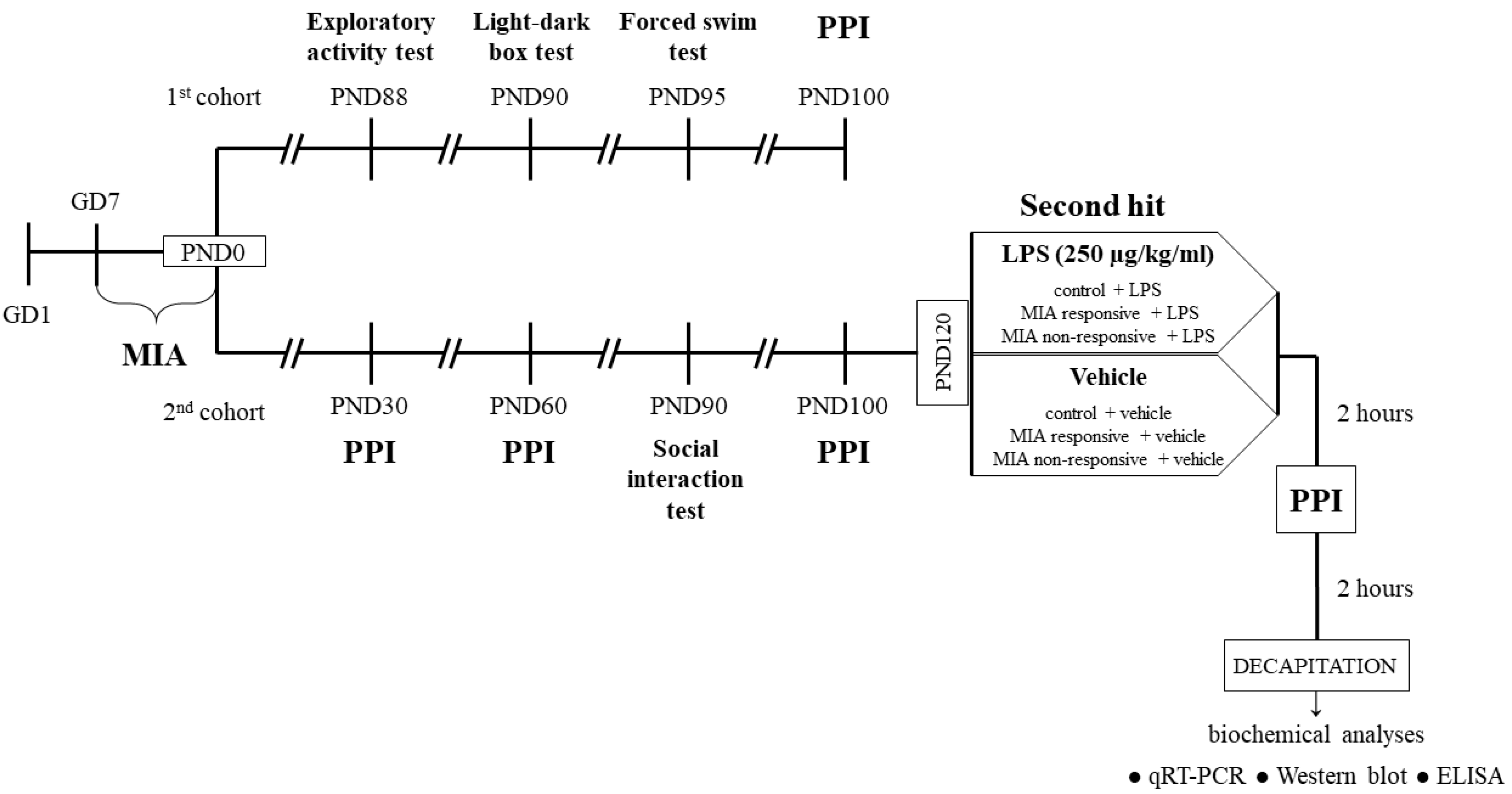
2. Materials and Methods
2.1. Animals
2.2. Drugs and Treatment
2.2.1. Prenatal Administration of LPS
2.2.2. Additional Immune Activation with LPS in Adulthood
2.3. Behavioural Tests
2.3.1. Exploratory Activity Test
2.3.2. Light-Dark Box Test
2.3.3. Forced Swim Test
2.3.4. Social Interaction Test
2.3.5. Prepulse Inhibition Test (PPI)
2.4. Biochemical Analyses
2.4.1. Tissues Collection
2.4.2. Tissues Preparation
2.4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.5. Western Blot
2.5. Statistical Data Analysis
3. Results
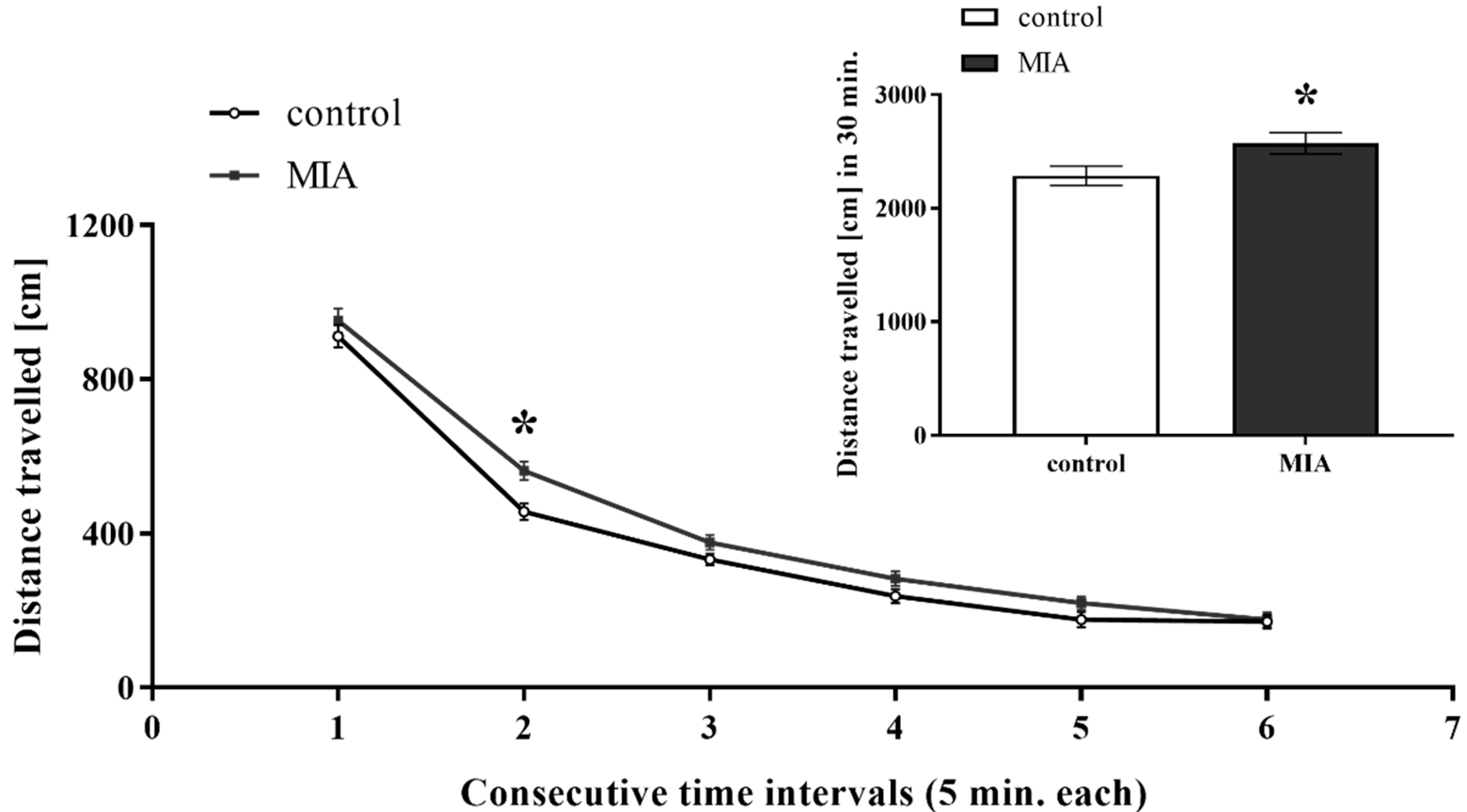
3.1. Exploratory Activity
3.2. Light-Dark Box Test
3.3. Forced Swim Test
3.4. Social Interaction Test
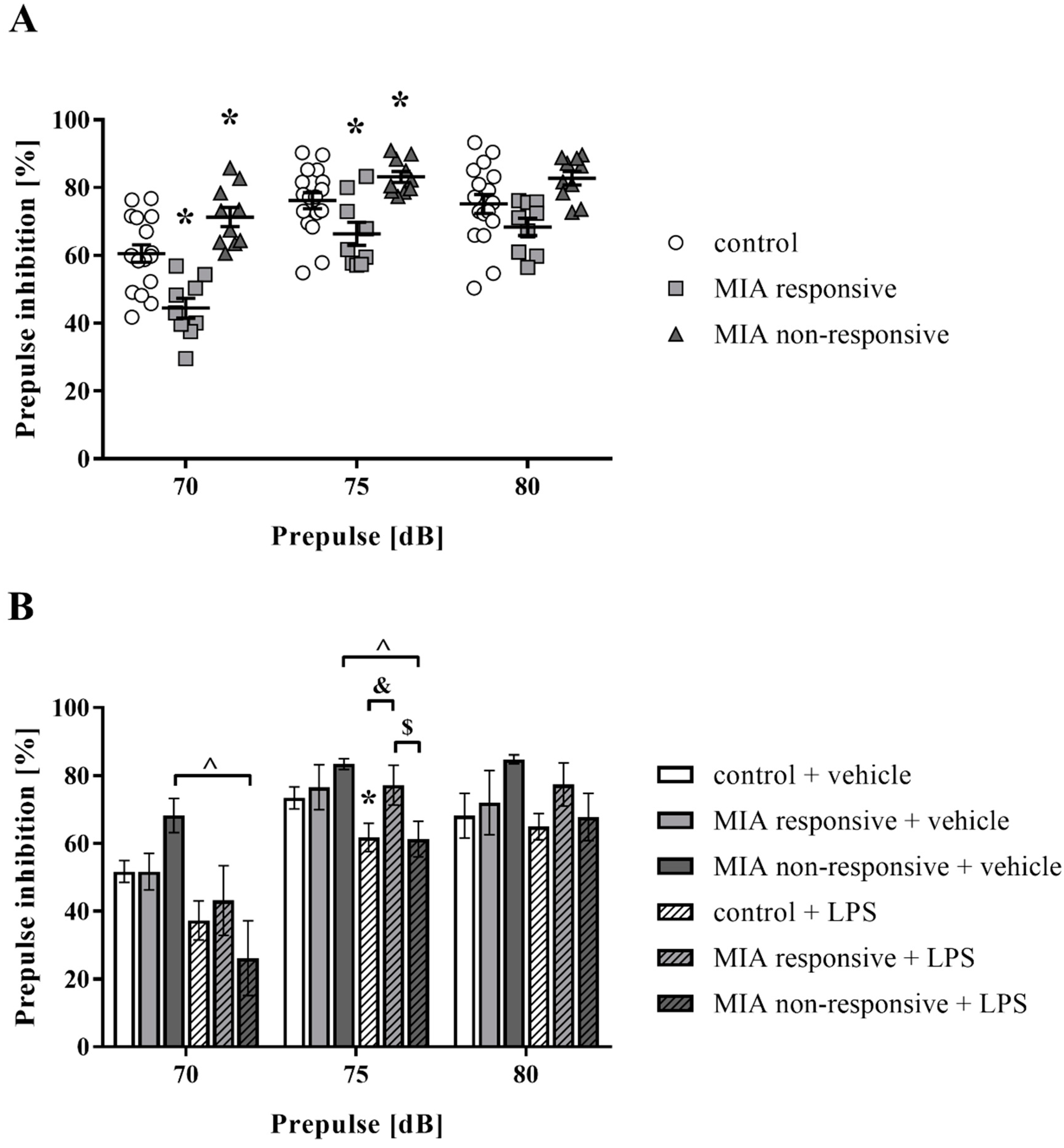
3.5. Prepulse Inhibition of the Acoustic Startle Response
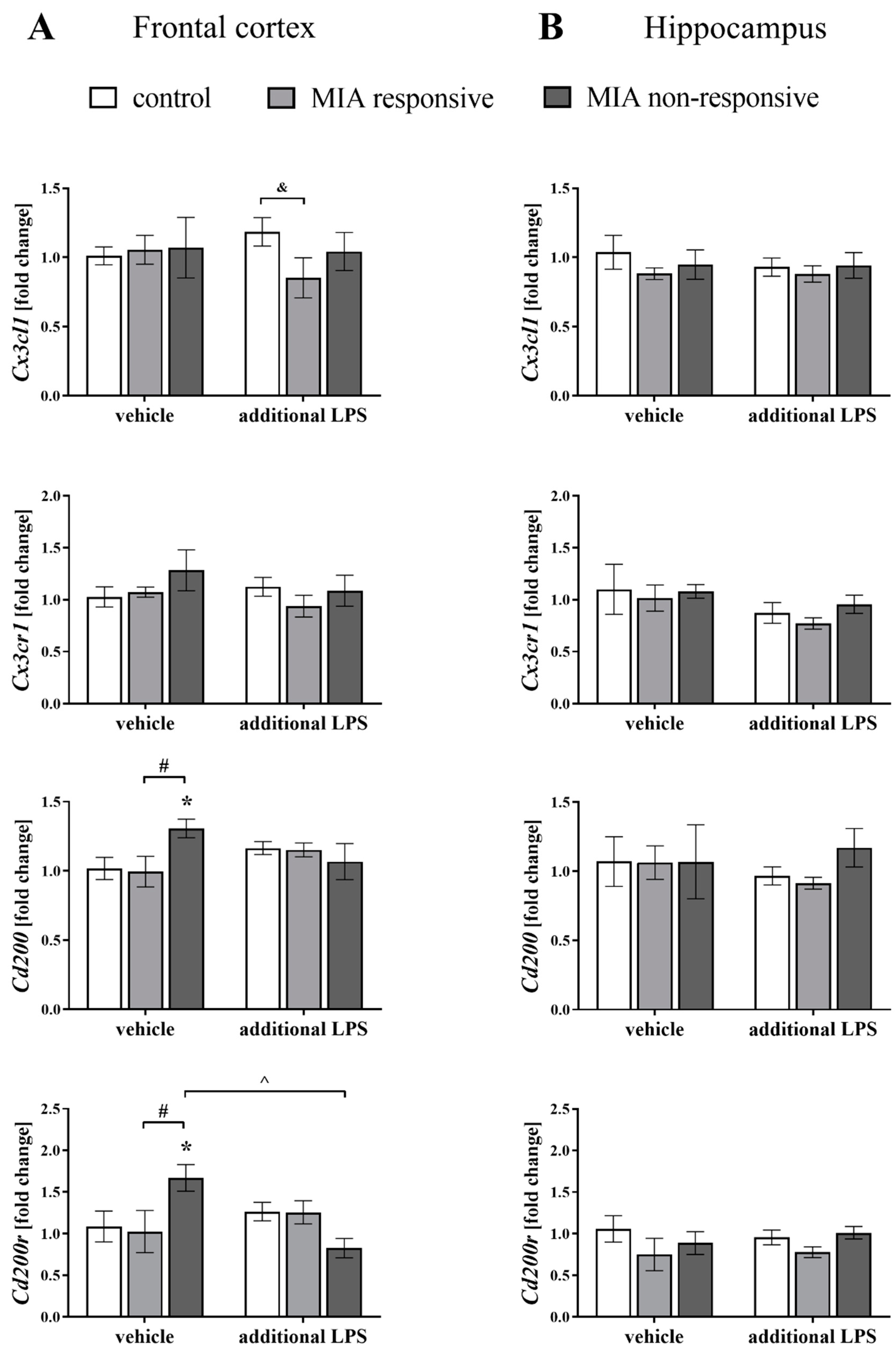
3.6. The mRNA Expression of the Cx3cl1, Cx3cr1, Cd200 and Cd200r in the Frontal Cortices and Hippocampi of Adult Male Offspring
3.7. Levels of the CX3CL1, CX3CR1, CD200 and CD200R Proteins in the Frontal Cortices and Hippocampi of Adult Male Offspring
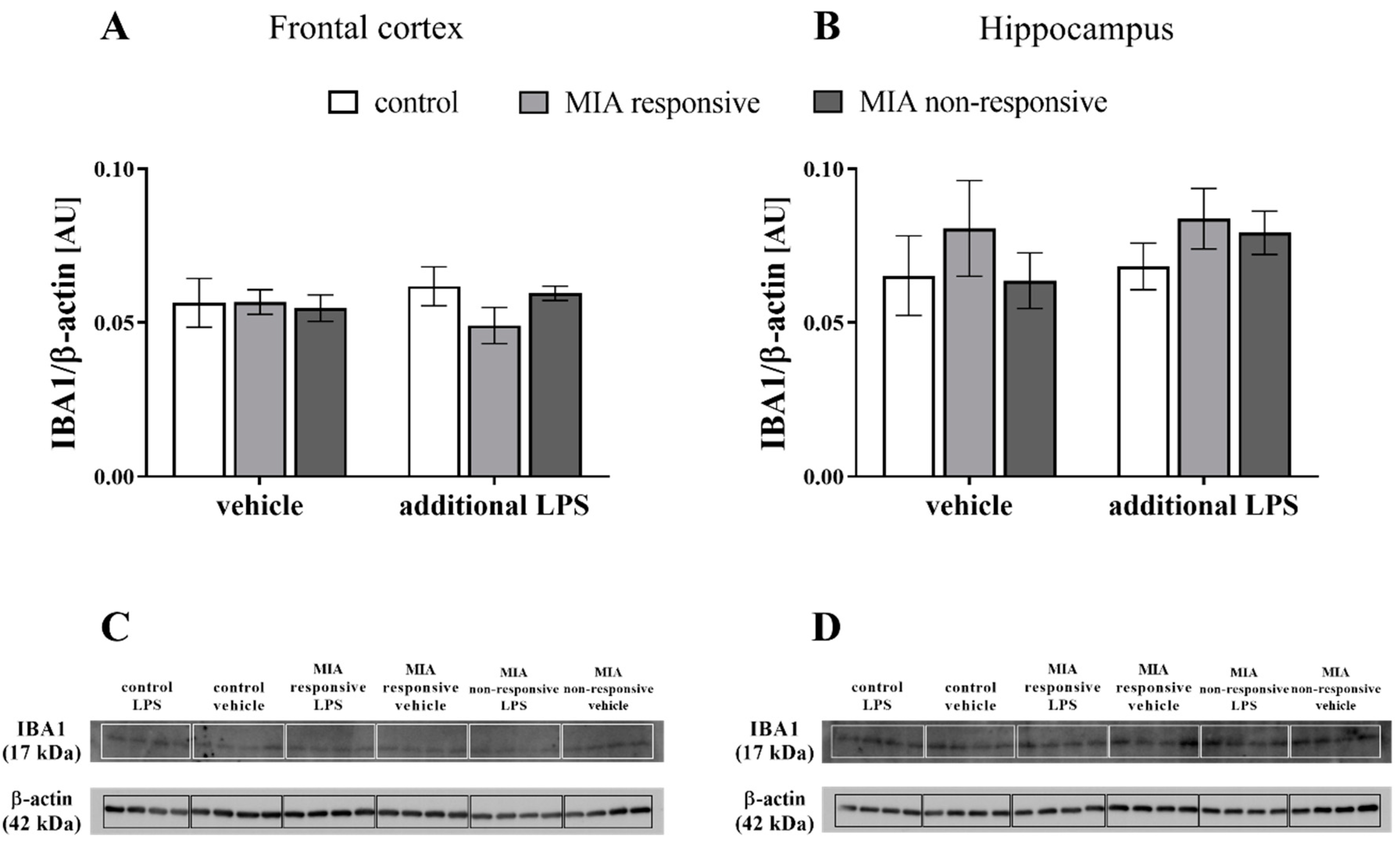
3.8. The IBA1 Levels in the Frontal Cortices and Hippocampi of Adult Male Offspring
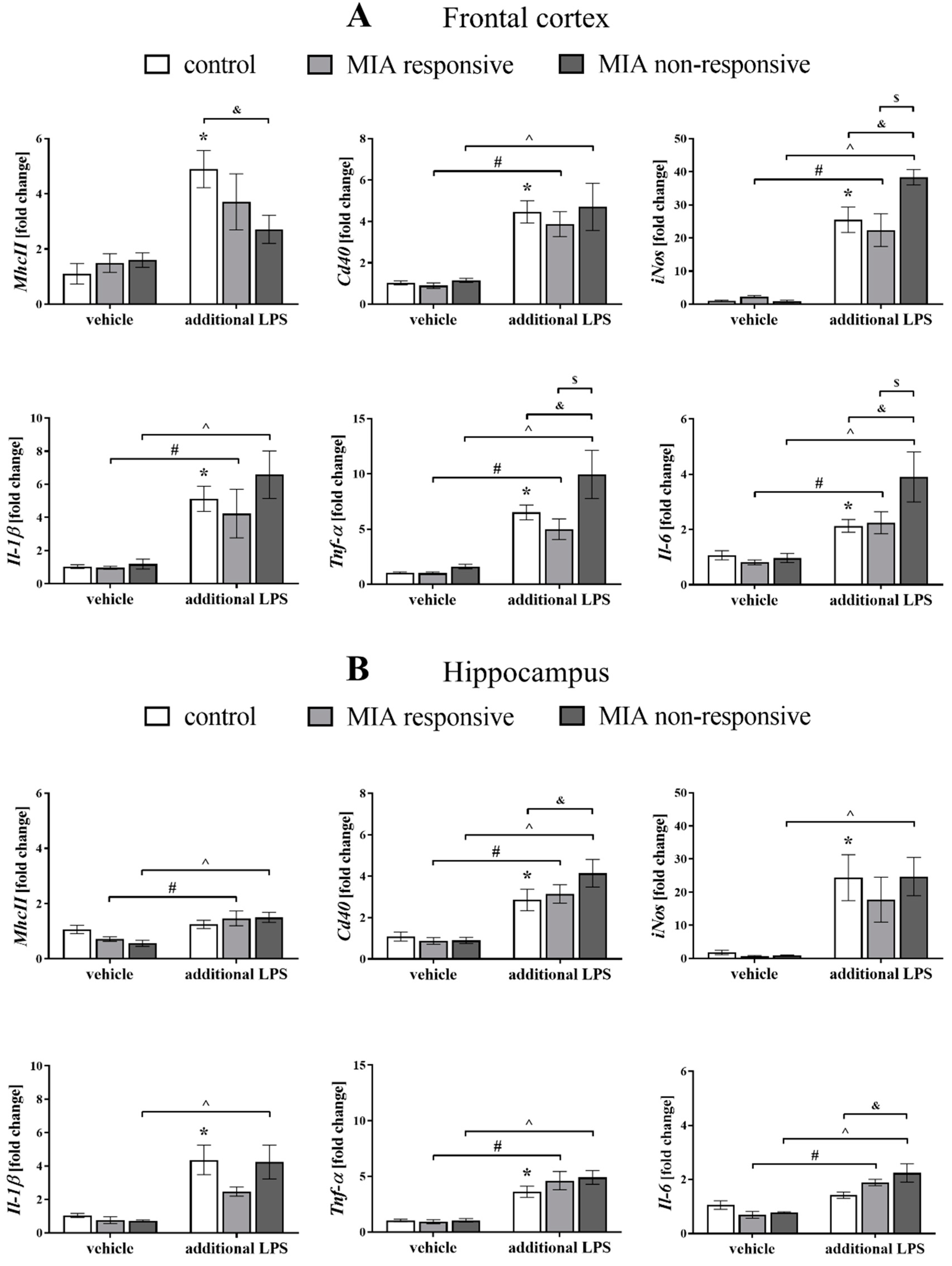
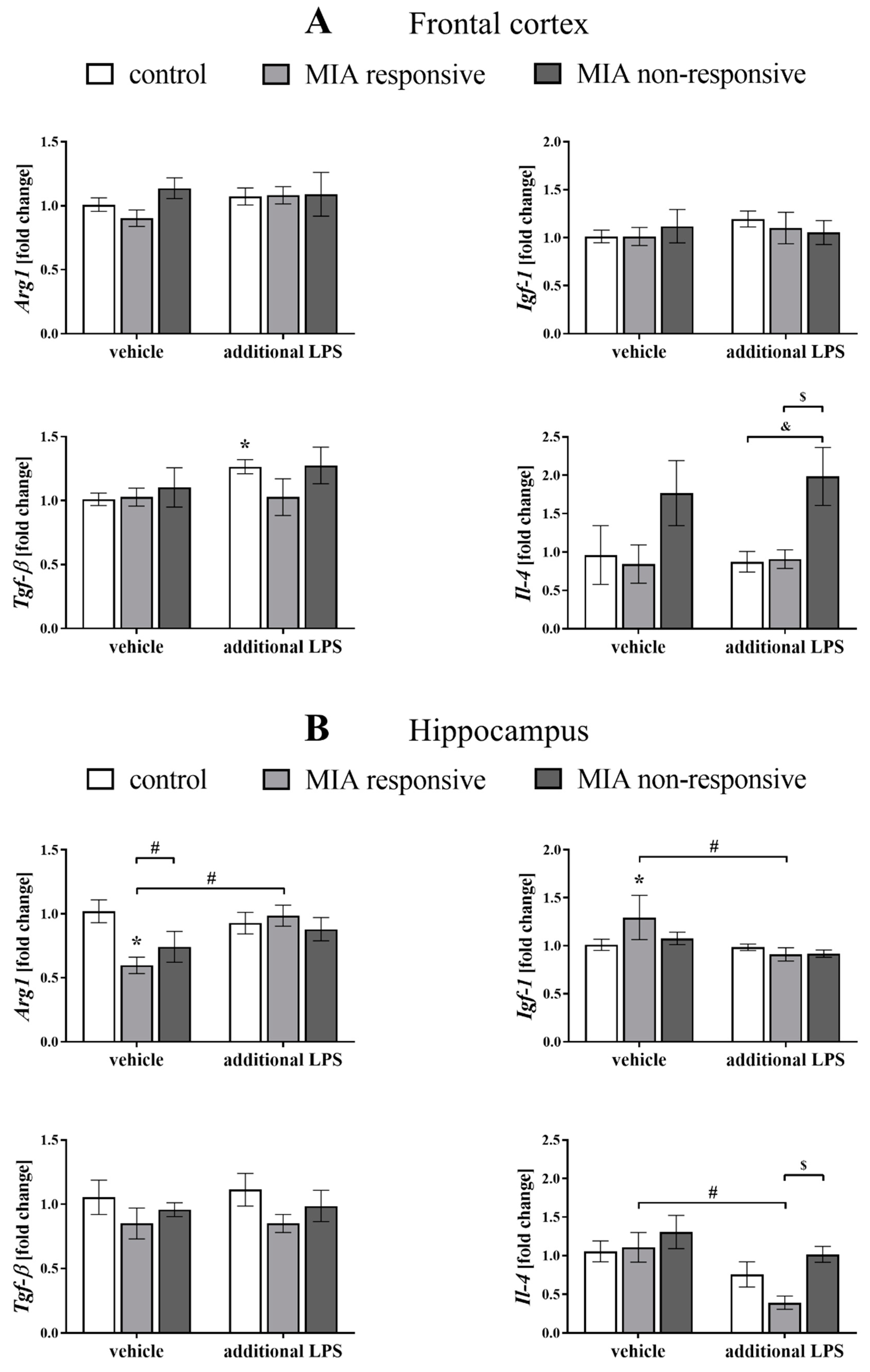
3.9. The mRNA Expression of the Microglial Markers in the Frontal Cortices and Hippocampi of Adult Male Offspring
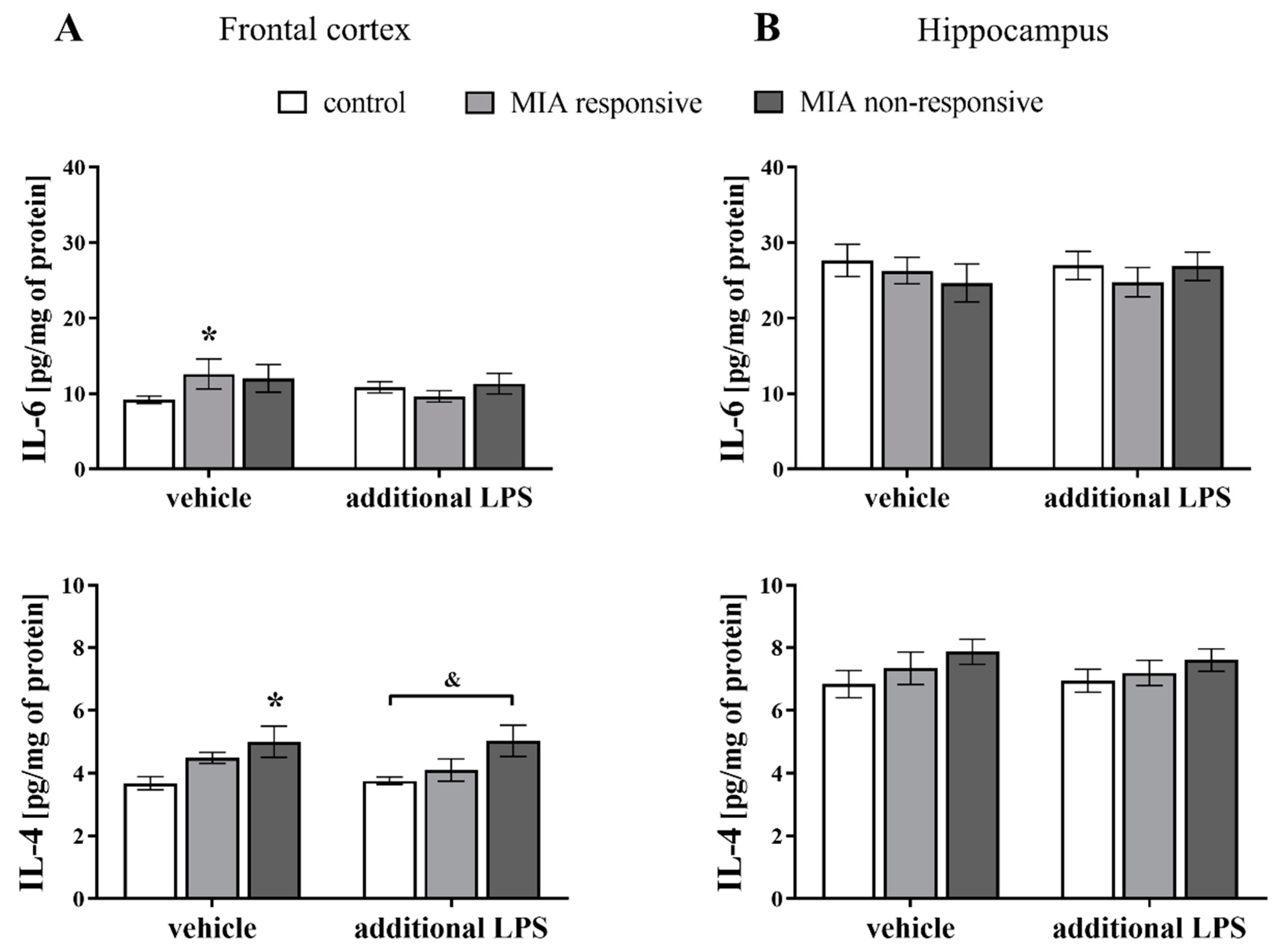
3.10. Levels of the IL-6 and IL-4 Proteins in the Frontal Cortices and Hippocampi of Adult Male Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Al-Haddad, B.J.S.; Oler, E.; Armistead, B.; Elsayed, N.A.; Weinberger, D.R.; Bernier, R.; Burd, I.; Kapur, R.; Jacobsson, B.; Wang, C.; et al. The fetal origins of mental illness. Am. J. Obstet. Gynecol. 2019, 221, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.; Murphy, S.K.; Ellman, L.M. Prenatal Maternal Stress and the Cascade of Risk to Schizophrenia Spectrum Disorders in Offspring. Curr. Psychiatry Rep. 2019, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X.; Niu, W.; Ma, G.; Sun, Q.; Bi, Y.; Guo, Z.; Ren, D.; Hu, J.; Yuan, F.; et al. Metabolomic profiling on rat brain of prenatal malnutrition: Implicated for oxidative stress and schizophrenia. Metab. Brain Dis. 2019, 34, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Schwarz, J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012, 33, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Graciarena, M.; Depino, A.M.; Pitossi, F.J. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFβ1 downregulation. Brain. Behav. Immun. 2010, 24, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Nyffeler, M.; Engler, A.; Urwyler, A.; Schedlowski, M.; Knuesel, I.; Yee, B.K.; Feldon, J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006, 26, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Q.; Wang, J.; Tang, M.; Huang, S.; Peng, K.; Han, Y.; Zhang, J.; Liu, G.; Fang, Q.; et al. Maternal immune activation-induced PPARγ-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring. Neurobiol. Dis. 2019, 125, 1–13. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Drexhage, R.C.; Padmos, R.C.; de Wit, H.; Versnel, M.A.; Hooijkaas, H.; van der Lely, A.J.; van Beveren, N.; DeRijk, R.H.; Cohen, D. Patients with schizophrenia show raised serum levels of the pro-inflammatory chemokine CCL2: Association with the metabolic syndrome in patients? Schizophr. Res. 2008, 102, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schwarz, M.J. Immune System and Schizophrenia. Curr. Immunol. Rev. 2010, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- van Berckel, B.N.; Bossong, M.G.; Boellaard, R.; Kloet, R.; Schuitemaker, A.; Caspers, E.; Luurtsema, G.; Windhorst, A.D.; Cahn, W.; Lammertsma, A.A.; et al. Microglia Activation in Recent-Onset Schizophrenia: A Quantitative (R)-[11C]PK11195 Positron Emission Tomography Study. Biol. Psychiatry 2008, 64, 820–822. [Google Scholar] [CrossRef]
- Steiner, J.; Bielau, H.; Brisch, R.; Danos, P.; Ullrich, O.; Mawrin, C.; Bernstein, H.G.; Bogerts, B. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008, 42, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.A.; Kipnis, J. Central Nervous System: (Immunological) Ivory Tower or Not. Neuropsychopharmacology 2017, 42, 28–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Savage, J.C.; Carrier, M.; Tremblay, M.È. Morphology of Microglia Across Contexts of Health and Disease. Methods Mol. Biol. 2019, 2034, 13–26. [Google Scholar] [CrossRef]
- Hirbec, H.; Rassendren, F.; Audinat, E. Microglia Reactivity: Heterogeneous Pathological Phenotypes. Methods Mol. Biol. 2019, 2034, 41–55. [Google Scholar] [CrossRef]
- Böttcher, C.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; Knop, A.; Paza, E.; Fidzinski, P.; Kraus, L.; Snijders, G.J.L.; Kahn, R.S.; et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019, 22, 78–90. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Hu, X.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol. Stress 2016, 4, 62–70. [Google Scholar] [CrossRef]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.V.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007, 7, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Monte, A.S.; Mello, B.S.F.; Borella, V.C.M.; da Silva Araujo, T.; da Silva, F.E.R.; de Sousa, F.C.F.; de Oliveira, A.C.P.; Gama, C.S.; Seeman, M.V.; Vasconcelos, S.M.M.; et al. Two-hit model of schizophrenia induced by neonatal immune activation and peripubertal stress in rats: Study of sex differences and brain oxidative alterations. Behav. Brain Res. 2017, 331, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef]
- Chamera, K.; Trojan, E.; Szuster-Głuszczak, M.; Basta-Kaim, A. The Potential Role of Dysfunctions in Neuron–Microglia Communication in the Pathogenesis of Brain Disorders. Curr. Neuropharmacol. 2020, 18, 408–430. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Cardona, A.E.; Sasse, M.E.; Liu, L.; Cardona, S.M.; Mizutani, M.; Savarin, C.; Hu, T.; Ransohoff, R.M. Scavenging roles of chemokine receptors: Chemokine receptor deficiency is associated with increased levels of ligand In circulation and tissues. Blood 2008, 112, 256–263. [Google Scholar] [CrossRef]
- Zujovic, V.; Benavides, J.; Vigé, X.; Carter, C.; Taupin, V. Fractalkine Modulates TNF-Secretion and Neurotoxicity Induced by Microglial Activation. Glia 2000, 29, 305–315. [Google Scholar] [CrossRef]
- Catalano, M.; Lauro, C.; Cipriani, R.; Chece, G.; Ponzetta, A.; Di Angelantonio, S.; Ragozzino, D.; Limatola, C. CX3CL1 protects neurons against excitotoxicity enhancing GLT-1 activity on astrocytes. J. Neuroimmunol. 2013, 263, 75–82. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Trojan, E.; Wydra, K.; Głombik, K.; Chamera, K.; Kucharczyk, M.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Filip, M.; et al. Beneficial impact of intracerebroventricular fractalkine administration on behavioral and biochemical changes induced by prenatal stress in adult rats: Possible role of NLRP3 inflammasome pathway. Biochem. Pharmacol. 2016, 113, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H. Control of glial immune function by neurons. Glia 2001, 36, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.J.; Tian, L.P.; Pan, J.; Lu, G.Q.; Zhang, Y.J.; Ding, J.Q.; Chen, S. Di CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J. Neuroinflammation 2011, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.; Hoek, R.M.; Forrester, J.V.; Liversidge, J.; Sedgwick, J.D.; Dick, A.D. Constitutive Retinal CD200 Expression Regulates Resident Microglia and Activation State of Inflammatory Cells during Experimental Autoimmune Uveoretinitis. Am. J. Pathol. 2002, 161, 1669–1677. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Budziszewska, B.; Leśkiewicz, M.; Fijał, K.; Regulska, M.; Kubera, M.; Wędzony, K.; Lasoń, W. Hyperactivity of the hypothalamus-pituitary-adrenal axis in lipopolysaccharide-induced neurodevelopmental model of schizophrenia in rats: Effects of antipsychotic drugs. Eur. J. Pharmacol. 2011, 650, 586–595. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Fijał, K.; Budziszewska, B.; Regulska, M.; Leśkiewicz, M.; Kubera, M.; Gołembiowska, K.; Lasoń, W.; Wȩdzony, K. Prenatal lipopolysaccharide treatment enhances MK-801-induced psychotomimetic effects in rats. Pharmacol. Biochem. Behav. 2011, 98, 241–249. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Szczęsny, E.; Leśkiewicz, M.; Głombik, K.; Slusarczyk, J.; Budziszewska, B.; Regulska, M.; Kubera, M.; Nowak, W.; Wędzony, K.; et al. Maternal immune activation leads to age-related behavioral and immunological changes in male rat offspring-the effect of antipsychotic drugs. Pharmacol. Reports 2012, 64, 1400–1410. [Google Scholar] [CrossRef]
- Quiñones, M.M.; Maldonado, L.; Velazquez, B.; Porter, J.T. Candesartan Ameliorates Impaired Fear Extinction Induced by Innate Immune Activation. Brain. Behav. Immun. 2016, 52, 169–177. [Google Scholar] [CrossRef]
- Kupferschmid, B.J.; Therrien, B.A. Spatial Learning Responses to Lipopolysaccharide in Adult and Aged Rats. Biol. Res. Nurs. 2018, 20, 32–39. [Google Scholar] [CrossRef]
- Basta-Kaim, A.; Fijał, K.; Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Leśkiewicz, M.; Regulska, M.; Kubera, M.; Lasoń, W.; et al. Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 2015, 287, 78–92. [Google Scholar] [CrossRef]
- Chocyk, A.; Bobula, B.; Dudys, D.; Przyborowska, A.; Majcher-Maślanka, I.; Hess, G.; Wedzony, K. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur. J. Neurosci. 2013, 38, 2089–2107. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Johnson, J.; Lucki, I. Acute and Chronic Antidepressant Drug Treatment in the Rat Forced Swimming Test Model of Depression. Exp. Clin. Psychopharmacol. 1997, 5, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Szczesny, E.; Glombik, K.; Stachowicz, K.; Slusarczyk, J.; Nalepa, I.; Zelek-Molik, A.; Rafa-Zablocka, K.; Budziszewska, B.; Kubera, M.; et al. Prenatal stress affects insulin-like growth factor-1 (IGF-1) level and IGF-1 receptor phosphorylation in the brain of adult rats. Eur. Neuropsychopharmacol. 2014, 24, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Duda, W.; Kubera, M.; Kreiner, G.; Curzytek, K.; Detka, J.; Głombik, K.; Ślusarczyk, J.; Basta-Kaim, A.; Budziszewska, B.; Lasoń, W.; et al. Suppression of pro-inflammatory cytokine expression and lack of anti-depressant-like effect of fluoxetine in lipopolysaccharide-treated old female mice. Int. Immunopharmacol. 2017, 48, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Głombik, K.; Stachowicz, A.; Trojan, E.; Ślusarczyk, J.; Suski, M.; Chamera, K.; Kotarska, K.; Olszanecki, R.; Basta-Kaim, A. Mitochondrial proteomics investigation of frontal cortex in an animal model of depression: Focus on chronic antidepressant drugs treatment. Pharmacol. Rep. 2018, 70, 322–330. [Google Scholar] [CrossRef]
- Sowa, J.; Bobula, B.; Glombik, K.; Slusarczyk, J.; Basta-Kaim, A.; Hess, G. Prenatal stress enhances excitatory synaptic transmission and impairs long-term potentiation in the frontal cortex of adult offspring rats. PLoS ONE 2015, 10, e0119407. [Google Scholar] [CrossRef]
- Trojan, E.; Głombik, K.; Ślusarczyk, J.; Budziszewska, B.; Kubera, M.; Roman, A.; Lasoń, W.; Basta-Kaim, A. The Beneficial Impact of Antidepressant Drugs on Prenatal Stress-Evoked Malfunction of the Insulin-Like Growth Factor-1 (IGF-1) Protein Family in the Olfactory Bulbs of Adult Rats. Neurotox. Res. 2016, 29, 288–298. [Google Scholar] [CrossRef]
- Bator, E.; Latusz, J.; Wędzony, K.; Maćkowiak, M. Adolescent environmental enrichment prevents the emergence of schizophrenia-like abnormalities in a neurodevelopmental model of schizophrenia. Eur. Neuropsychopharmacol. 2018, 28, 97–108. [Google Scholar] [CrossRef]
- Mällo, T.; Alttoa, A.; Kõiv, K.; Tõnissaar, M.; Eller, M.; Harro, J. Rats with persistently low or high exploratory activity: Behaviour in tests of anxiety and depression, and extracellular levels of dopamine. Behav. Brain Res. 2007, 177, 269–281. [Google Scholar] [CrossRef]
- Kumari, V.; Peters, E.R.; Fannon, D.; Premkumar, P.; Aasen, I.; Cooke, M.A.; Anilkumar, A.P.; Kuipers, E. Uncontrollable voices and their relationship to gating deficits in schizophrenia. Schizophr. Res. 2008, 101, 185–194. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Bhakta, S.; Chou, H.H.; Talledo, J.A.; Balvaneda, B.; Light, G.A. Memantine Effects On Sensorimotor Gating and Mismatch Negativity in Patients with Chronic Psychosis. Neuropsychopharmacology 2016, 41, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Dziwota, E.; Stepulak, M.Z.; Włoszczak-Szubzda, A.; Olajossy, M. Social functioning and the quality of life of patients diagnosed with schizophrenia. Ann. Agric. Environ. Med. 2018, 25, 50–55. [Google Scholar] [CrossRef]
- Henniger, M.S.H.; Ohl, F.; Hö Lter, S.M.; Weißenbacher, P.; Toschi, N.; Lö, P.; Wigger, A.; Spanagel, R.; Landgraf, R. Unconditioned anxiety and social behaviour in two rat lines selectively bred for high and low anxiety-related behaviour. Behav. Brain Res. 2000, 111, 153–163. [Google Scholar] [CrossRef]
- Kopec, A.M.; Smith, C.J.; Bilbo, S.D. Neuro-Immune mechanisms regulating social behavior: Dopamine as mediator? Trends Neurosci. 2019, 42, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Braff, D.L.; Grillon, C.; Geyer, M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry 1992, 49, 206–215. [Google Scholar] [CrossRef]
- Mena, A.; Ruiz-Salas, J.C.; Puentes, A.; Dorado, I.; Ruiz-Veguilla, M.; De la Casa, L.G. Reduced prepulse inhibition as a biomarker of schizophrenia. Front. Behav. Neurosci. 2016, 10, 202. [Google Scholar] [CrossRef]
- Moriwaki, M.; Kishi, T.; Takahashi, H.; Hashimoto, R.; Kawashima, K.; Okochi, T.; Kitajima, T.; Furukawa, O.; Fujita, K.; Takeda, M.; et al. Prepulse inhibition of the startle response with chronic schizophrenia: A replication study. Neurosci. Res. 2009, 65, 259–262. [Google Scholar] [CrossRef]
- Borrell, J.; Vela, M.; Arévalo-Martin, A.; Molina-Holgado, E.; Guaza, C. Prenatal Immune Challenge Disrupts Sensorimotor Gating in Adult Rats: Implications for the Etiopathogenesis of Schizophrenia. Neuropsychopharmacology 2002, 26, 204–215. [Google Scholar] [CrossRef]
- Khan, A.; Powell, S.B. Sensorimotor gating deficits in “two-hit” models of schizophrenia risk factors. Schizophr. Res. 2018, 198, 68–83. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in schizophrenia: Pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef]
- Vidal, P.M.; Pacheco, R. The Cross-Talk Between the Dopaminergic and the Immune System Involved in Schizophrenia. Front. Pharmacol. 2020, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; Patterson, P.H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain. Behav. Immun. 2011, 25, 604–615. [Google Scholar] [CrossRef]
- Lyons, A.; Downer, E.J.; Crotty, S.; Nolan, Y.M.; Mills, K.H.G.; Lynch, M.A. CD200 ligand-receptor interaction modulates microglial activation in vivo and in vitro: A role for IL-4. J. Neurosci. 2007, 27, 8309–8313. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.; McQuillan, K.; Deighan, B.F.; O’Reilly, J.A.; Downer, E.J.; Murphy, A.C.; Watson, M.; Piazza, A.; O’Connell, F.; Griffin, R.; et al. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain. Behav. Immun. 2009, 23, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Lipska, B.K.; Weinberger, D.R. To Model a Psychiatric Disorder in Animals: Schizophrenia As a Reality Test. Neuropsychopharmacology 2000, 23, 223–239. [Google Scholar] [CrossRef]
- Wedzony, K.; Fijal, K.; Mackowiak, M.; Chocyk, A.; Zajaczkowski, W. Impact of postnatal blockade of N-methyl-d-aspartate receptors on rat behavior: A search for a new developmental model of schizophrenia. Neuroscience 2008, 153, 1370–1379. [Google Scholar] [CrossRef]
- Sachs, G.S. A review of agitation in mental illness: Burden of illness and underlying pathology. J. Clin. Psychiatry 2006, 67, 5–12. [Google Scholar]
- Buonocore, M.; Bosia, M.; Bechi, M.; Spangaro, M.; Cavedoni, S.; Cocchi, F.; Bianchi, L.; Guglielmino, C.; Mastromatteo, A.R.; Cavallaro, R. Targeting anxiety to improve quality of life in patients with schizophrenia. Eur. Psychiatry 2017, 45, 129–135. [Google Scholar] [CrossRef]
- Blumstein, L.K.; Crawley, J.N. Further Characterization of a Simple, Automated Exploratory Model for the Anxiolytic Effects of Benzodiazepines. Pharmacol. Biochem. Behav. 1983, 18, 37–40. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lin, S.Y.; Wang, S. Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain. Behav. Immun. 2012, 26, 459–468. [Google Scholar] [CrossRef]
- Makinson, R.; Lloyd, K.; Rayasam, A.; McKee, S.; Brown, A.; Barila, G.; Grissom, N.; George, R.; Marini, M.; Fabry, Z.; et al. Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain. Behav. Immun. 2017, 66, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Pei, D.E.; Yang, R.D.; Wan, C.L.; Ye, Y.M.; Peng, S.S.; Zeng, Q.Q.; Yu, Y. Prenatal maternal vaginal inflammation increases anxiety and alters HPA axis signalling in adult male mice. Int. J. Dev. Neurosci. 2019, 75, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, V.T.; de Castro Medeiros, D.; Ropke, J.; Guidine, P.A.; Rezende, G.H.; Moraes, M.F.D.; Mendes, E.M.A.; Macedo, D.; Moreira, F.A.; de Oliveira, A.C.P. Effects of early or late prenatal immune activation in mice on behavioral and neuroanatomical abnormalities relevant to schizophrenia in the adulthood. Int. J. Dev. Neurosci. 2017, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Taricano, M.; Maiorka, P.C.; Palermo-Neto, J.; Bernardi, M.M. Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation 2010, 17, 240–251. [Google Scholar] [CrossRef]
- Powell, S.B.; Newman, H.A.; Mcdonald, T.A.; Bugenhagen, P.; Lewis, M.H. Development of Spontaneous Stereotyped Behavior in Deer Mice: Effects of Early and Late Exposure to a More Complex Environment. Dev. Psychobiol. 2000, 37, 100–108. [Google Scholar] [CrossRef]
- Rink, L.; Pagel, T.; Franklin, J.; Baethge, C. Characteristics and heterogeneity of schizoaffective disorder compared with unipolar depression and schizophrenia—A systematic literature review and meta-analysis. J. Affect. Disord. 2016, 191, 8–14. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 2015, e52587. [Google Scholar] [CrossRef]
- Babri, S.; Doosti, M.H.; Salari, A.A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain. Behav. Immun. 2014, 37, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Solati, J.; Hosseini, M.H.; Shahi, H.R.; Saki, G.; Salari, A.A. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res. Bull. 2012, 87, 295–302. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav. Brain Res. 2014, 259, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Taghzouti, K.; Lamarque, S.; Kharouby, M.; Simon, H. Interindividual Differences in Active and Passive Behaviors in the Forced-Swimming Test: Implications for Animal Models of Psychopathology. Biol Psychiatry 1999, 45, 750–758. [Google Scholar] [CrossRef]
- Rybnikova, E.A.; Vetrovoi, O.V.; Zenko, M.Y. Comparative Characterization of Rat Strains (Wistar, Wistar–Kyoto, Sprague Dawley, Long Evans, LT, SHR, BD-IX) by Their Behavior, Hormonal Level and Antioxidant Status. J. Evol. Biochem. Physiol. 2018, 54, 374–382. [Google Scholar] [CrossRef]
- Zhan, Y. Theta frequency prefrontal-hippocampal driving relationship during free exploration in mice. Neuroscience 2015, 300, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Ressler, K.; Binder, E.; Nemeroff, C. The Neurobiology of Anxiety Disorders: Brain Imaging, Genetics, and Psychoneuroendocrinology. Psychiatr. Clin. N. Am. 2009, 32, 549–575. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Liu, L.; Wei, J.L.; Hu, Z.L.; Li, L.; Wang, S.; Xu, J.M.; Zhou, X.F.; Li, C.Q.; Yang, Z.Y.; et al. Brain-derived neurotrophic factor precursor in the hippocampus regulates both depressive and anxiety-like behaviors in rats. Front. Psychiatry 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sierakowiak, A.; Mattsson, A.; Gómez-Galán, M.; Feminía, T.; Graae, L.; Aski, S.N.; Damberg, P.; Lindskog, M.; Brené, S.; Åberg, E. Hippocampal Morphology in a Rat Model of Depression: The Effects of Physical Activity. Open Neuroimag. J. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Johnson, A.; Varberg, Z.; Benhardus, J.; Maahs, A.; Schrater, P. The hippocampus and exploration: Dynamically evolving behavior and neural representations. Front. Hum. Neurosci. 2012, 6, 1–17. [Google Scholar] [CrossRef]
- Marcotte, E.R.; Pearson, D.M.; Srivastava, L.K. Animal models of schizophrenia: A critical review. J. Psychiatry Neurosci. 2001, 26, 395–410. [Google Scholar]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 2001, 156, 234–258. [Google Scholar] [CrossRef]
- Simões, L.R.; Sangiogo, G.; Tashiro, M.H.; Generoso, J.S.; Faller, C.J.; Dominguini, D.; Mastella, G.A.; Scaini, G.; Giridharan, V.V.; Michels, M.; et al. Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J. Psychiatr. Res. 2018, 100, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Heidinger, L.; Reilly, J.L.; Wang, L.; Goldman, M.B. Circuit activity underlying a distinct modulator of prepulse inhibition. Psychiatry Res. Neuroimaging 2019, 288, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, C.; Wiedermann, D.; Neumaier, B.; Drzezga, A.; Timmermann, L.; Graf, R.; Leweke, F.M.; Endepols, H. The functional networks of prepulse inhibition: Neuronal connectivity analysis based on fdg-pet in awake and unrestrained rats. Front. Behav. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Mosher, L.J.; Frau, R.; Pardu, A.; Pes, R.; Devoto, P.; Bortolato, M. Selective activation of D1dopamine receptors impairs sensorimotor gating in Long–Evans rats. Br. J. Pharmacol. 2016, 173, 2122–2134. [Google Scholar] [CrossRef]
- Sipes, T.A.; Geyer, M.A. Multiple Serotonin Receptor Subtypes Modulate Prepulse Inhibition of the Startle Response in Rats. Neuropharmacology 1994, 33, 441–448. [Google Scholar] [CrossRef]
- Jensen, K.S.; Oranje, B.; Wienberg, M.; Glenthøj, B.Y. The effects of increased central serotonergic activity on prepulse inhibition and habituation of the human startle response. Neuropsychopharmacology 2007, 32, 2117–2124. [Google Scholar] [CrossRef]
- Shoji, H.; Miyakawa, T. Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: A large-scale meta-analytic study. Mol. Brain 2018, 11, 42. [Google Scholar] [CrossRef]
- Williamson, L.L.; Sholar, P.W.; Mistry, R.S.; Smith, S.H.; Bilbo, S.D. Microglia and memory: Modulation by early-life infection. J. Neurosci. 2011, 31, 15511–15521. [Google Scholar] [CrossRef]
- Maynard, T.M.; Sikich, L.; Lieberman, J.A.; LaMantia, A.-S. Neural Development, Cell-Cell Signaling, and the ‘Two-Hit’ Hypothesis of Schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef]
- Deslauriers, J.; Racine, W.; Sarret, P.; Grignon, S. Preventive effect of α-lipoic acid on prepulse inhibition deficits in a juvenile two-hit model of schizophrenia. Neuroscience 2014, 272, 261–270. [Google Scholar] [CrossRef]
- Fortier, M.E.; Luheshi, G.N.; Boksa, P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav. Brain Res. 2007, 181, 270–277. [Google Scholar] [CrossRef]
- Juszczak, G.R.; Blaszczyk, J.; Sadowski, B.; Sliwa, A.T.; Wolak, P.; Tymosiak-Zielinska, A.; Lisowski, P.; Swiergiel, A.H. Lipopolysaccharide does not affect acoustic startle reflex in mice. Brain. Behav. Immun. 2008, 22, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lockey, A.J.; Kavaliers, M.; Ossenkopp, K.P. Lipopolysaccharide produces dose-dependent reductions of the acoustic startle response without impairing prepulse inhibition in male rats. Brain. Behav. Immun. 2009, 23, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Serrano, M.; Tonelli, L.; Listwak, S.; Sternberg, E.; Riley, A.L. Effects of Cross Fostering on Open-Field Behavior, Acoustic Startle, Lipopolysaccharide-Induced Corticosterone Release, and Body Weight in Lewis and Fischer Rats. Behav. Genet. 2001, 31, 427–436. [Google Scholar] [CrossRef]
- Taylor, A.N.; Tio, D.L.; Romeo, H.E. The febrile response to intraperitoneal lipopolysaccharide: Strain and gender differences in rats. J. Neuroimmunol. 2005, 158, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Glowa, J.R.; Hansen, C.T. Differences in Response to an Acoustic Startle Stimulus Among Forty-Six Rat Strains. Behav. Genet. 1994, 24, 79–84. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Weber, M.; Qu, Y.; Light, G.A.; Braff, D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology 2008, 199, 331–388. [Google Scholar] [CrossRef]
- Faraday, M.M. Rat sex and strain differences in responses to stress. Physiol. Behav. 2002, 75, 507–522. [Google Scholar] [CrossRef]
- Liu, Y.; Bando, Y.; Vargas-Lowy, D.; Elyaman, W.; Khoury, S.J.; Huang, T.; Reif, K.; Chitnis, T. CD200R1 agonist attenuates mechanisms of chronic disease in a murine model of multiple sclerosis. J. Neurosci. 2010, 30, 2025–2038. [Google Scholar] [CrossRef]
- Carter, D.A.; Dick, A.D. CD200 maintains microglial potential to migrate in adult human retinal explant model. Curr. Eye Res. 2004, 28, 427–436. [Google Scholar] [CrossRef]
- Lyons, A.; Downer, E.J.; Costello, D.A.; Murphy, N.; Lynch, M.A. Dok2 mediates the CD200Fc attenuation of Aβ-induced changes in glia. J. Neuroinflammation 2012, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Schubert, I.; Ahlbrand, R.; Winter, A.; Vollmer, L.; Lewkowich, I.; Sah, R. Enhanced fear and altered neuronal activation in forebrain limbic regions of CX3CR1-deficient mice. Brain. Behav. Immun. 2018, 68, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bolós, M.; Perea, J.R.; Terreros-Roncal, J.; Pallas-Bazarra, N.; Jurado-Arjona, J.; Ávila, J.; Llorens-Martín, M. Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain. Behav. Immun. 2018, 68, 76–89. [Google Scholar] [CrossRef]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Corona, A.W.; Huang, Y.; O’Connor, J.C.; Dantzer, R.; Kelley, K.W.; Popovich, P.G.; Godbout, J.P. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J. Neuroinflammation 2010, 7, 1–14. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W.G.M. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bisht, K.; Tremblay, M.È. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014, 8, 129. [Google Scholar] [CrossRef]
- Denieffe, S.; Kelly, R.J.; McDonald, C.; Lyons, A.; Lynch, M.A. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain. Behav. Immun. 2013, 34, 86–97. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain. Behav. Immun. 2018, 69, 62–73. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, S.; Yan, Z.Q.; Zhao, Y.X.; Zhou, H.Y.; Wang, Y.; Lu, G.Q.; Zhang, J.D. Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: Roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic. Biol. Med. 2011, 50, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Montgomery, A.J.; Asselin, M.C.; Murray, R.M.; Grasby, P.M.; Mcguire, P.K. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br. J. Psychiatry 2007, 191, s13–s18. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.H.; Jarskog, L.F.; Vadlamudi, S.; Lauder, J.M. Prenatal infection and risk for schizophrenia: IL-1β, IL-6, and TNFα inhibit cortical neuron dendrite development. Neuropsychopharmacology 2004, 29, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Behrens, M.M.; Ali, S.S.; Dugan, L.L. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J. Neurosci. 2008, 28, 13957–13966. [Google Scholar] [CrossRef]
- Meyer, U. Developmental neuroinflammation and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 42, 20–34. [Google Scholar] [CrossRef]
- Garner, K.M.; Amin, R.; Johnson, R.W.; Scarlett, E.J.; Burton, M.D. Microglia priming by interleukin-6 signaling is enhanced in aged mice. J. Neuroimmunol. 2018, 324, 90–99. [Google Scholar] [CrossRef]
- Costello, D.A.; Lyons, A.; Denieffe, S.; Browne, T.C.; Cox, F.F.; Lynch, M.A. Long term potentiation is impaired in membrane glycoprotein CD200-deficient mice: A role for toll-like receptor activation. J. Biol. Chem. 2011, 286, 34722–34732. [Google Scholar] [CrossRef]
- Wynne, A.M.; Henry, C.J.; Huang, Y.; Cleland, A.; Godbout, J.P. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain. Behav. Immun. 2010, 24, 1190–1201. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Jia, X.; Wang, Q.; Li, Y.; Hu, M.; Tian, L.; Yang, J.; Xing, W.; Zhang, W.; et al. Neuroprotection by IFN-γ via astrocyte-secreted IL-6 in acute neuroinflammation. Oncotarget 2017, 8, 40065–40078. [Google Scholar] [CrossRef]
- Walker, D.G.; Dalsing-Hernandez, J.E.; Campbell, N.A.; Lue, L.F. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: A potential mechanism leading to chronic inflammation. Exp. Neurol. 2009, 215, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010, 207, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine To Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.H.; Zhang, E.; Kim, J.J.; Baek, H.; Shin, N.; Kim, S.; Kim, S.R.; Kim, H.R.; Lee, S.J.; Park, J.B.; et al. CD200R/Foxp3-mediated signalling regulates microglial activation. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]



| Group | Type of Social Interaction | |||
|---|---|---|---|---|
| Aggressive | Non-Aggressive | |||
| Number of Events | Time [s] | Number of Events | Time [s] | |
| control | 4.83 ± 2.17 | 11.33 ± 4.52 | 29.67 ± 6.71 | 82.83 ± 15.92 |
| MIA | 2.00 ± 1.63 | 4.50 ± 3.18 | 42.33 ± 7.10 | 124.67 ± 20.49 |
| Prepulse Intensity | Group | |||
|---|---|---|---|---|
| PND30 | PND60 | |||
| Control | MIA | Control | MIA | |
| 70 dB | 16.21 ± 9.67 | 25.08 ± 5.27 | 38.07 ± 3.95 | 44.66 ± 5.01 |
| 75 dB | 21.45 ± 8.45 | 43.85 ± 3.22 * | 58.60 ± 4.22 | 59.33 ± 4.02 |
| 80 dB | 33.93 ± 9.45 | 37.34 ± 5.69 | 52.00 ± 4.25 | 61.12 ± 3.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamera, K.; Szuster-Głuszczak, M.; Trojan, E.; Basta-Kaim, A. Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200–CD200R and CX3CL1–CX3CR1 Systems. Cells 2020, 9, 1676. https://doi.org/10.3390/cells9071676
Chamera K, Szuster-Głuszczak M, Trojan E, Basta-Kaim A. Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200–CD200R and CX3CL1–CX3CR1 Systems. Cells. 2020; 9(7):1676. https://doi.org/10.3390/cells9071676
Chicago/Turabian StyleChamera, Katarzyna, Magdalena Szuster-Głuszczak, Ewa Trojan, and Agnieszka Basta-Kaim. 2020. "Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200–CD200R and CX3CL1–CX3CR1 Systems" Cells 9, no. 7: 1676. https://doi.org/10.3390/cells9071676
APA StyleChamera, K., Szuster-Głuszczak, M., Trojan, E., & Basta-Kaim, A. (2020). Maternal Immune Activation Sensitizes Male Offspring Rats to Lipopolysaccharide-Induced Microglial Deficits Involving the Dysfunction of CD200–CD200R and CX3CL1–CX3CR1 Systems. Cells, 9(7), 1676. https://doi.org/10.3390/cells9071676





