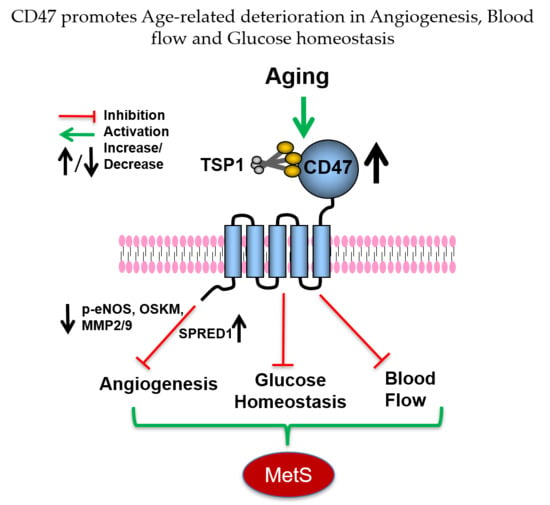
CD47 Promotes Age-Associated Deterioration in Angiogenesis, Blood Flow and Glucose Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Human Mesenteric Arteries
2.4. mRNA Isolation and Quantitative Reverse-Transcription PCR
2.5. Endothelial Cell (EC) Isolation and Culture
2.6. Protein Extraction and Western Blotting
2.7. Immunofluorescence
2.8. In Vivo Matrigel Plug Assay and Immunohistochemistry
2.9. Tube Formation Assay
2.10. Arterial Ring Assay
2.11. Scratch Wound Healing Assay
2.12. Cell Proliferation Assay
2.13. Laser Doppler Blood Flow Analysis
2.14. Glucose (GTT) and Insulin (ITT) Tolerance Test
2.15. Statistical Analysis
3. Results
3.1. Expression of CD47 Is Increased and Self-Renewal Transcription Factors Decreased in Aged Arteries
3.2. Age-Associated Induction of TSP1 Is Attenuated and OSKM Sustained in the Absence of CD47
3.3. Age-Associated Decrease in Arterial Endothelial Cell Proliferation is Abrogated in the Absence of CD47
3.4. CD47 Contributes to Age-Associated Decrease in Endothelial Cell Migration
3.5. CD47 Limits Tube Formation by Endothelial Cells from Aged Mice
3.6. Sprouting Angiogenesis in Aged Human and Murine Arterial Rings Is Hindered by CD47
3.7. CD47 Suppresses In Vivo Angiogenesis in Aged Mice
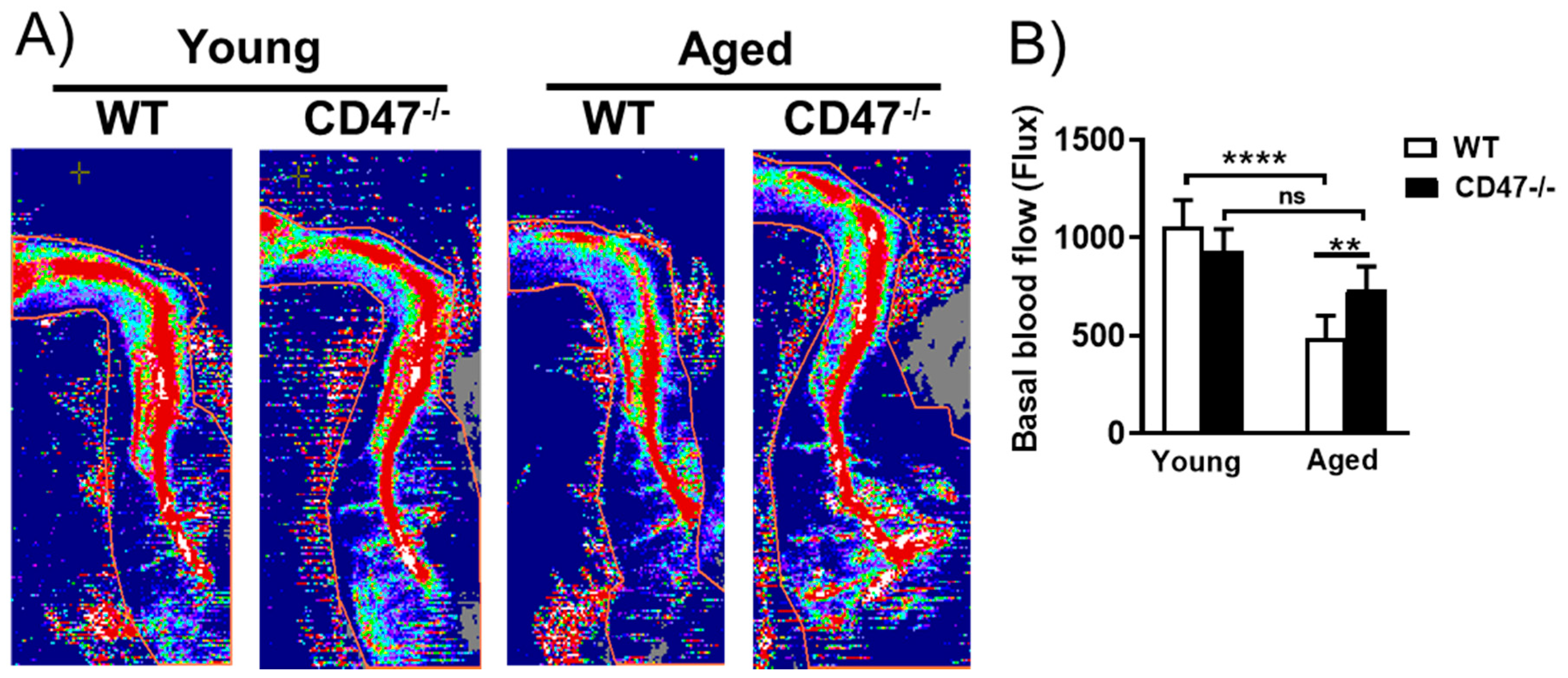
3.8. Youthful Blood Flow Is Maintained in Aged CD47-Null Mice
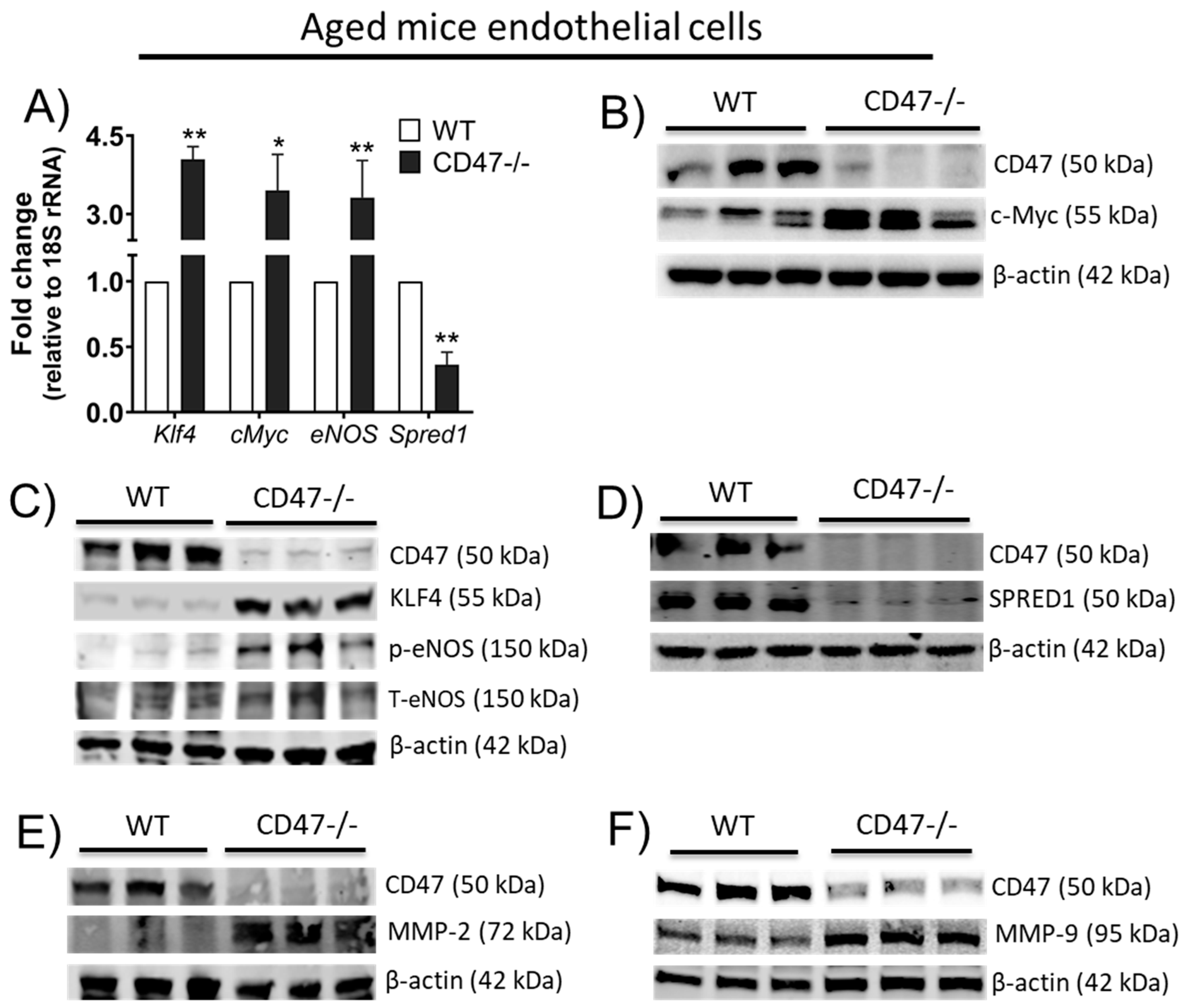
3.9. CD47 Limits Angiogenic Gene Expression and Matrix Metalloproteinases in Endothelial Cells from Older Animals
3.10. Age-Related Aspects of MetS Are Mitigated in the Absence of CD47
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the Metabolic Syndrome in the United States, 2003-2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Wong, C.; Rocha, M.; Jones, D.L. Decline in Self-Renewal Factors Contributes to Aging of the Stem Cell Niche in the Drosophila Testis. Cell Stem Cell 2007, 1, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Boil. 2015, 50, 212–230. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Dimitry, J.; Frazier, W.A.; Wink, D.A.; Roberts, D.D. CD47 Is Necessary for Inhibition of Nitric Oxide-stimulated Vascular Cell Responses by Thrombospondin-1. J. Boil. Chem. 2006, 281, 26069–26080. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 Inhibits VEGF Receptor-2 Signaling by Disrupting Its Association with CD47*. J. Boil. Chem. 2010, 285, 38923–38932. [Google Scholar] [CrossRef]
- Rogers, N.M.; Roberts, D.D.; Isenberg, J.S. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann. Surg. 2013, 258, 184–191. [Google Scholar] [CrossRef]
- Kaur, S.; Soto-Pantoja, D.R.; Stein, E.V.; Liu, C.; Elkahloun, A.G.; Pendrak, M.L.; Nicolae, A.; Singh, S.P.; Nie, Z.; Levens, D.; et al. Thrombospondin-1 Signaling through CD47 Inhibits Self-renewal by Regulating c-Myc and Other Stem Cell Transcription Factors. Sci. Rep. 2013, 3, 1673. [Google Scholar] [CrossRef]
- Wang, J.-M.; Chen, A.F.; Zhang, K. Isolation and Primary Culture of Mouse Aortic Endothelial Cells. J. Vis. Exp. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Zaric, J.; Alday-Parejo, B.; Seebach, J.; Bousquenaud, M.; Stalin, J.; Bieler, G.; Schnittler, H.-J.; Rüegg, C. MAGI1 Mediates eNOS Activation and NO Production in Endothelial Cells in Response to Fluid Shear Stress. Cells 2019, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Chiba, T.; Minhas, N.; Meijles, D.N.; Lu, B.; O’Connell, P.; Rogers, N.M. Deficiency in SIRP-α cytoplasmic recruitment confers protection from acute kidney injury. FASEB J. 2019, 33, 11528–11540. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Baker, M.; Robinson, S.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’Amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K.M.; Vojnovic, B. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef]
- Kapetanaki, M.G.; Mora, A.L.; Rojas, M. Influence of age on wound healing and fibrosis. J. Pathol. 2012, 229, 310–322. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Rogers, N.M.; Sharifi-Sanjani, M.; Yao, M.; Ghimire, K.; Bienes-Martinez, R.; Mutchler, S.M.; Knupp, H.E.; Baust, J.; Novelli, E.M.; Ross, M.; et al. TSP1–CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc. Res. 2016, 113, 15–29. [Google Scholar] [CrossRef]
- Meijles, D.N.; Sahoo, S.; Al Ghouleh, I.; Amaral, J.H.; Bienes-Martinez, R.; Knupp, H.E.; Attaran, S.; Sembrat, J.; Nouraie, M.; Rojas, M.M.; et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 2017, 10, eaaj1784. [Google Scholar] [CrossRef]
- Norton, K.-A.; Popel, A.S. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci. Rep. 2016, 6, 36992. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Perruccio, E.M.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 13141–13146. [Google Scholar] [CrossRef]
- Bruno, S.; Darzynkiewicz, Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Betancur, P.A.; Abraham, B.J.; Yiu, Y.Y.; Willingham, S.B.; Khameneh, F.; Zarnegar, M.; Kuo, A.H.; McKenna, K.; Kojima, Y.; Leeper, N.J.; et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 2017, 8, 14802. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.M. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc. Res. 2007, 74, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yu, H.; Chen, X.; Shen, T.; Cui, Z.; Si, G.; Zhang, J.; Cheng, Y.; Jia, S.; Song, S.; et al. PDGF-BB/KLF4/VEGF Signaling Axis in Pulmonary Artery Endothelial Cell Angiogenesis. Cell Physiol. Biochem. 2017, 41, 2333–2349. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; Castaneda, J.L.; Manns, J.M.; Uknis, A.B.; Sainz, I.M.; Safadi, F.F.; Popoff, S.N.; Cadena, R.A.D. Amelioration of inflammation, angiogenesis and CTGF expression in an arthritis model by a TSP1-derived peptide treatment. J. Cell. Physiol. 2007, 211, 504–512. [Google Scholar] [CrossRef]
- Kastana, P.; Zahra, F.T.; Ntenekou, D.; Katraki-Pavlou, S.; Beis, D.; Lionakis, M.S.; Mikelis, C.M.; Papadimitriou, E. Matrigel Plug Assay for In Vivo Evaluation of Angiogenesis. Breast Cancer 2019, 1952, 219–232. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Hyodo, F.; Pappan, L.K.; Abu-Asab, M.; Tsokos, M.; Krishna, M.C.; Frazier, W.A.; Roberts, D.D. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Lawler, J. Molecular Basis for the Regulation of Angiogenesis by Thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef]
- Kaur, S.; Chang, T.; Singh, S.P.; Lim, L.; Mannan, P.; Garfield, S.H.; Pendrak, M.L.; Soto-Pantoja, D.R.; Rosenberg, A.Z.; Jin, S.; et al. CD47 signaling regulates the immunosuppressive activity of VEGF in T cells. J. Immunol. 2014, 193, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Skorczewski, J.; Feng, X.; Mei, L.; Murphy-Ullrich, J.E. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J. Biol. Chem. 2004, 279, 34311–34322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Isenberg, J.S.; Billiar, T.R.; Chen, A.F. Thrombospondin-1/CD36 pathway contributes to bone marrow-derived angiogenic cell dysfunction in type 1 diabetes via Sonic hedgehog pathway suppression. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1464–E1472. [Google Scholar] [CrossRef] [PubMed]
- Maile, L.A.; Capps, B.E.; Miller, E.C.; Allen, L.B.; Veluvolu, U.; Aday, A.W.; Clemmons, D.R. Glucose Regulation of Integrin-Associated Protein Cleavage Controls the Response of Vascular Smooth Muscle Cells to Insulin-Like Growth Factor-I. Mol. Endocrinol. 2008, 22, 1226–1237. [Google Scholar] [CrossRef][Green Version]
- Maimaitiyiming, H.; Norman, H.; Zhou, Q.; Wang, S. CD47 Deficiency Protects Mice From Diet-induced Obesity and Improves Whole Body Glucose Tolerance and Insulin Sensitivity. Sci. Rep. 2015, 5, 8846. [Google Scholar] [CrossRef]
- Leiter, E.H.; Premdas, F.; Harrison, D.E.; Lipson, L.G. Aging and glucose homeostasis in C57BL/6J male mice. FASEB J. 1988, 2, 2807–2811. [Google Scholar] [CrossRef]
- Basu, R.; Breda, E.; Oberg, A.L.; Powell, C.C.; Man, C.D.; Basu, A.; Vittone, J.L.; Klee, G.G.; Arora, P.; Jensen, M.D.; et al. Mechanisms of the age-associated deterioration in glucose tolerance: Contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003, 52, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Anderson, S.; Kim, Y.G.; Mazzalli, M.; Suga, S.; A Jefferson, J.; Gordon, K.L.; Oyama, T.T.; Hughes, J.; Hugo, C.; et al. Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am. J. Kidney Dis. 2001, 37, 601–611. [Google Scholar] [CrossRef]
- Cai, H.; Yuan, Z.; Fei, Q.; Zhao, J. Investigation of thrombospondin-1 and transforming growth factor-beta expression in the heart of aging mice. Exp. Ther. Med. 2012, 3, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Tejedo, J.R.; Tapia-Limonchi, R.; Mora-Castilla, S.; Cahuana, G.M.; Hmadcha, A.; Martin, F.; Bedoya, F.J.; Soria, B. Low concentrations of nitric oxide delay the differentiation of embryonic stem cells and promote their survival. Cell Death Dis. 2010, 1, e80. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.M.; Qin, Y.; Miller, T.W.; Bandle, R.W.; Csanyi, G.; Pagano, P.J.; Bauer, P.M.; Schnermann, J.; Roberts, D.D.; Isenberg, J.S. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc. Res. 2010, 88, 471–481. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of nitric oxide signalling by thrombospondin 1: Implications for anti-angiogenic therapies. Nat. Rev. Cancer 2009, 9, 182–194. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Giuliani, A.; Recchioni, R.; Bonafè, M.; Marcheselli, F.; De Carolis, S.; Campanati, A.; Giuliodori, K.; Rippo, M.R.; Brugè, F.; et al. Anti-TNF-α treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells. Oncotarget 2016, 7, 11945–11958. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Dickman, K.G.; Zong, W. Akt and c-Myc differentially activate cellular metabolic programs and prime cells to bioenergetic inhibition. J. Biol. Chem. 2010, 285, 7324–7333. [Google Scholar] [CrossRef]
- Miller, T.W.; Soto-Pantoja, D.R.; Schwartz, A.L.; Sipes, J.M.; DeGraff, W.G.; Ridnour, L.A.; Wink, D.A.; Roberts, D.D. CD47 Receptor Globally Regulates Metabolic Pathways That Control Resistance to Ionizing Radiation. J. Boil. Chem. 2015, 290, 24858–24874. [Google Scholar] [CrossRef]
- Matsuo, Y.; Tanaka, M.; Yamakage, H.; Sasaki, Y.; Muranaka, K.; Hata, H.; Ikai, I.; Shimatsu, A.; Inoue, M.; Chun, T.-H.; et al. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metab. 2015, 64, 1490–1499. [Google Scholar] [CrossRef]
- Varma, V.; Yao-Borengasser, A.; Bodles, A.M.; Rasouli, N.; Phanavanh, B.; Nolen, G.T.; Kern, E.M.; Nagarajan, R.; Spencer, H.J.; Lee, M.-J.; et al. Thrombospondin-1 Is an Adipokine Associated with Obesity, Adipose Inflammation, and Insulin Resistance. Diabetes 2007, 57, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Gonzalez-Quesada, C.; Li, N.; Cavalera, M.; Lee, D.-W.; Frangogiannis, N.G. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am. J. Physiol. Metab. 2013, 305, E439–E450. [Google Scholar] [CrossRef] [PubMed]
- Schickel, J.; Stahn, K.; Zimmer, K.-P.; Sudbrak, R.; Størm, T.M.; Dürst, M.; Kiehntopf, M.; Deufel, T. Gene for integrin-associated protein (IAP, CD47): Physical mapping, genomic structure, and expression studies in skeletal muscle. Biochem. Cell Boil. 2002, 80, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.S.; Dong, Y.; Zhang, L.; Mitch, W.E. Signal regulatory protein-α interacts with the insulin receptor contributing to muscle wasting in chronic kidney disease. Kidney Int. 2013, 84, 308–316. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Hennington, B.S.; Moore, A.G.; Blanchard, E.J.; Cameron, J. Gender differences in the renal nitric oxide (NO) system: Dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am. J. Hypertens. 1998, 11, 97–104. [Google Scholar] [CrossRef]
- Desrois, M.; Lan, C.; Movassat, J.; Bernard, M. Reduced up-regulation of the nitric oxide pathway and impaired endothelial and smooth muscle functions in the female type 2 diabetic goto-kakizaki rat heart. Nutr. Metab. 2017, 14, 6. [Google Scholar] [CrossRef]
- Nevitt, C.; McKenzie, G.; Christian, K.; Austin, J.; Hencke, S.; Hoying, J.B.; Leblanc, A.J. Physiological levels of thrombospondin-1 decrease NO-dependent vasodilation in coronary microvessels from aged rats. Am. J. Physiol. Circ. Physiol. 2016, 310, H1842–H1850. [Google Scholar] [CrossRef]
- Short, S.M.; Derrien, A.; Narsimhan, R.P.; Lawler, J.; Ingber, D.E.; Zetter, B.R. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J. Cell Biol. 2005, 168, 643–653. [Google Scholar] [CrossRef]
- A Schwartz, M.; Brown, E.J.; Fazeli, B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J. Boil. Chem. 1993, 268, 19931–19934. [Google Scholar]
- Ugarte, G.; Santander, C.; Brandan, E. Syndecan-4 and beta1 integrin are regulated by electrical activity in skeletal muscle: Implications for cell adhesion. Matrix Biol. 2010, 29, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Bréchot, N.; Gómez, E.; Bignon, M.; Laschet, J.; Dussiot, M.; Cazes, A.; Alanio, C.; Durand, M.; Philippe, J.; Silvestre, J.-S.; et al. Modulation of Macrophage Activation State Protects Tissue from Necrosis during Critical Limb Ischemia in Thrombospondin-1-Deficient Mice. PLoS ONE 2008, 3, e3950. [Google Scholar] [CrossRef]
- Rogers, N.M.; Thomson, A.W.; Isenberg, J.S. Activation of Parenchymal CD47 Promotes Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2012, 23, 1538–1550. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, K.; Li, Y.; Chiba, T.; Julovi, S.M.; Li, J.; Ross, M.A.; Straub, A.C.; O’Connell, P.J.; Rüegg, C.; Pagano, P.J.; et al. CD47 Promotes Age-Associated Deterioration in Angiogenesis, Blood Flow and Glucose Homeostasis. Cells 2020, 9, 1695. https://doi.org/10.3390/cells9071695
Ghimire K, Li Y, Chiba T, Julovi SM, Li J, Ross MA, Straub AC, O’Connell PJ, Rüegg C, Pagano PJ, et al. CD47 Promotes Age-Associated Deterioration in Angiogenesis, Blood Flow and Glucose Homeostasis. Cells. 2020; 9(7):1695. https://doi.org/10.3390/cells9071695
Chicago/Turabian StyleGhimire, Kedar, Yao Li, Takuto Chiba, Sohel M. Julovi, Jennifer Li, Mark A. Ross, Adam C. Straub, Philip J. O’Connell, Curzio Rüegg, Patrick J. Pagano, and et al. 2020. "CD47 Promotes Age-Associated Deterioration in Angiogenesis, Blood Flow and Glucose Homeostasis" Cells 9, no. 7: 1695. https://doi.org/10.3390/cells9071695
APA StyleGhimire, K., Li, Y., Chiba, T., Julovi, S. M., Li, J., Ross, M. A., Straub, A. C., O’Connell, P. J., Rüegg, C., Pagano, P. J., Isenberg, J. S., & Rogers, N. M. (2020). CD47 Promotes Age-Associated Deterioration in Angiogenesis, Blood Flow and Glucose Homeostasis. Cells, 9(7), 1695. https://doi.org/10.3390/cells9071695