Effects of Cryogenic Storage on Human Amnion Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. hAEC Isolation Procedure
2.2. Cryopreservation Procedure
2.3. Thawing Procedure
2.4. Flow Cytometry Analysis
2.5. Gene Profiling by qPCR
2.6. Statistical Analysis
3. Results
3.1. Quality Control of Cryopreserved Cell Products
3.2. Immunomodulatory Molecules
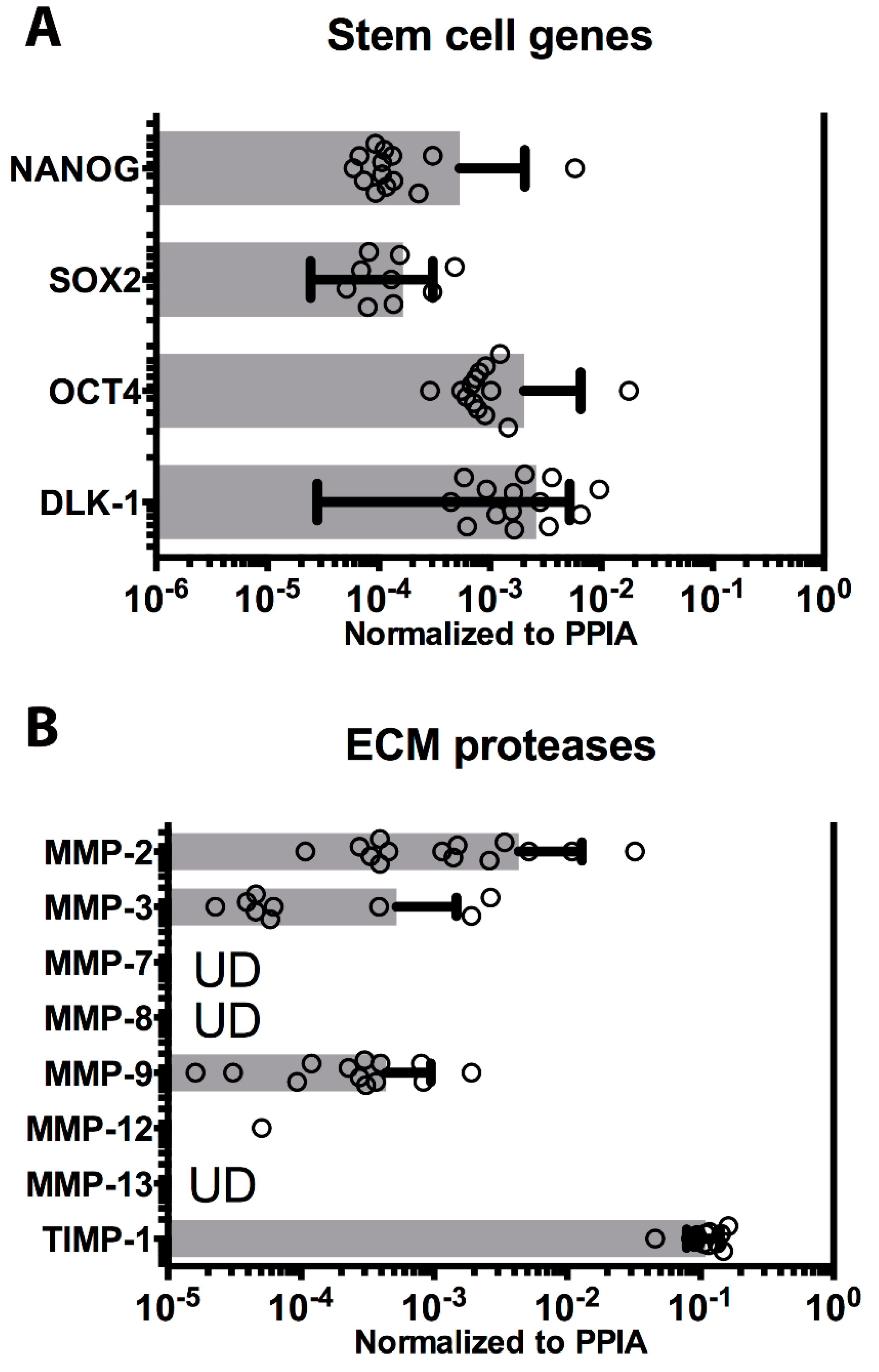
3.3. Pluripotency Genes
3.4. Cell Engraftment Enzymes
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Miki, T. A Rational Strategy for the Use of Amniotic Epithelial Stem Cell Therapy for Liver Diseases. Stem Cells Transl. Med. 2016, 5, 405–409. [Google Scholar] [CrossRef]
- Ilancheran, S.; Michalska, A.; Peh, G.S.L.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem Cells Derived from Human Fetal Membranes Display Multilineage Differentiation Potential. Boil. Reprod. 2007, 77, 577–588. [Google Scholar] [CrossRef]
- Takashima, S.; Ise, H.; Zhao, P.; Akaike, T.; Nikaido, T. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct. Funct. 2004, 29, 73–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, N.B.; Strom, S. Stem Cell Characteristics of Amniotic Epithelial Cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, F.; Gramignoli, R.; Dorko, K.; Miki, T.; Ranade, A.R.; Serra, M.P.; Doratiotto, S.; Sini, M.; Sharma, S.; Mitamura, K.; et al. Hepatic differentiation of amniotic epithelial cells. Hepatology 2011, 53, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Zhang, T.S.; Kawa, S.; Aizawa, T.; Ota, M.; Akaike, T.; Kato, K.; Konishi, I.; Nikaido, T. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003, 12, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-H.; Jin, J.; Joe, J.-H.; Song, Y.-S.; So, B.-I.; Lim, S.M.; Cheon, G.J.; Woo, S.-K.; Ra, J.-C.; Lee, Y.-Y.; et al. In Vivo Differentiation of Human Amniotic Epithelial Cells into Cardiomyocyte-Like Cells and Cell Transplantation Effect on Myocardial Infarction in Rats: Comparison with Cord Blood and Adipose Tissue-Derived Mesenchymal Stem Cells. Cell Transplant. 2012, 21, 1687–1696. [Google Scholar] [CrossRef]
- McDonald, C.; Siatskas, C.; Bernard, C.C.A. The emergence of amnion epithelial stem cells for the treatment of Multiple Sclerosis. Inflamm. Regen. 2011, 31, 256–271. [Google Scholar] [CrossRef][Green Version]
- Lebreton, F.; Bellofatto, K.; Wassmer, C.H.; Perez, L.; Lavallard, V.; Parnaud, G.; Cottet-Dumoulin, D.; Kerr-Conte, J.; Pattou, F.; Bosco, D.; et al. Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Arab. Archaeol. Epigr. 2020, 20, 1551–1561. [Google Scholar] [CrossRef]
- Moodley, Y.P.; Ilancheran, S.; Samuel, C.; Vaghjiani, V.; Atienza, D.; Williams, E.D.; Jenkin, G.; Wallace, E.M.; Trounson, A.; Manuelpillai, U. Human Amnion Epithelial Cell Transplantation Abrogates Lung Fibrosis and Augments Repair. Am. J. Respir. Crit. Care Med. 2010, 182, 643–651. [Google Scholar] [CrossRef]
- Murphy, S.; Lim, R.; Dickinson, H.; Acharya, R.; Rosli, S.; Jenkin, G.; Wallace, E.M. Human Amnion Epithelial Cells Prevent Bleomycin-Induced Lung Injury and Preserve Lung Function. Cell Transplant. 2011, 20, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Vosdoganes, P.; Wallace, E.M.; Chan, S.T.; Acharya, R.; Moss, T.; Lim, R. Human Amnion Epithelial Cells Repair Established Lung Injury. Cell Transplant. 2013, 22, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Skvorak, K.J.; Dorko, K.; Marongiu, F.; Tahan, V.; Hansel, M.C.; Gramignoli, R.; Arning, E.; Bottiglieri, T.; Gibson, K.M.; Strom, S. Improved Amino Acid, Bioenergetic Metabolite and Neurotransmitter Profiles following Human Amnion Epithelial Cell Transplant in Intermediate Maple Syrup Urine Disease Mice. Mol. Genet. Metab. 2013, 109, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Skvorak, K.J.; Dorko, K.; Marongiu, F.; Tahan, V.; Hansel, M.C.; Gramignoli, R.; Gibson, K.M.; Strom, S.C. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology 2013, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Gramignoli, R. Therapeutic use of human amnion-derived products: Cell-based therapy for liver disease. Curr. Pathobiol. Rep. 2016, 4, 157–167. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Tchongue, J.; Lourensz, D.; Vaghjiani, V.; Samuel, C.S.; Liu, A.; Williams, E.D.; Sievert, W. Transplantation of Human Amnion Epithelial Cells Reduces Hepatic Fibrosis in Immunocompetent CCl4-Treated Mice. Cell Transplant. 2010, 19, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Vanosi, G.; Lindenmair, A.; Hennerbichler, S.; Peterbauer-Scherb, A.; Wolbank, S.; Cargnoni, A.; Signoroni, P.B.; Campagnol, M.; Gabriel, C.; et al. Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank 2012, 14, 475–488. [Google Scholar] [CrossRef]
- Malhotra, A.; Lim, R.; Mockler, J.C.; Wallace, E.M. Two-year outcomes of infants enrolled in the first-in-human study of amnion cells for bronchopulmonary dysplasia. Stem Cells Transl. Med. 2019, 9, 289–294. [Google Scholar] [CrossRef]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants with Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.-J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Strom, S.; Gramignoli, R. Human amnion epithelial cells expressing HLA-G as novel cell-based treatment for liver disease. Hum. Immunol. 2016, 77, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Kolanko, E.; Kopaczka, K.; Koryciak-Komarska, H.; Czech, E.; Szmytkowska, P.; Gramignoli, R.; Czekaj, P. Increased immunomodulatory capacity of human amniotic cells after activation by pro-inflammatory chemokines. Eur. J. Pharmacol. 2019, 859, 172545. [Google Scholar] [CrossRef] [PubMed]
- Tee, J.Y.; Vaghjiani, V.; Liu, Y.H.; Murthi, P.; Chan, J.; Manuelpillai, U. Immunogenicity and immunomodulatory properties of hepatocyte-like cells derived from human amniotic epithelial cells. Curr. Stem Cell Res. Ther. 2013, 8, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Banas, R.; Trumpower, C.; Bentlejewski, C.; Marshall, V.; Sing, G.; Zeevi, A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum. Immunol. 2008, 69, 321–328. [Google Scholar] [CrossRef]
- Morandi, F.; Horenstein, A.L.; Quarona, V.; Faini, A.C.; Castella, B.; Srinivasan, R.C.; Strom, S.; Malavasi, F.; Gramignoli, R. Ectonucleotidase Expression on Human Amnion Epithelial Cells: Adenosinergic Pathways and Dichotomic Effects on Immune Effector Cell Populations. J. Immunol. 2018, 202, 724–735. [Google Scholar] [CrossRef]
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006, 2, 133–142. [Google Scholar] [CrossRef]
- Gramignoli, R.; Srinivasan, R.C.; Kannisto, K.; Strom, S. Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures. Curr. Protoc. Stem Cell Boil. 2016, 37, 1E.10.1–1E.10.13. [Google Scholar] [CrossRef]
- Srinivasan, R.C.; Kannisto, K.; Strom, S.; Gramignoli, R. Evaluation of different routes of administration and biodistribution of human amnion epithelial cells in mice. Cytotherapy 2019, 21, 113–124. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Strom, S. Human Hepatocyte Transplantation: Worldwide Results. Transplantation 2006, 82, 441–449. [Google Scholar] [CrossRef]
- Gramignoli, R.; Vosough, M.; Kannisto, K.; Srinivasan, R.C.; Strom, S. Clinical Hepatocyte Transplantation: Practical Limits and Possible Solutions. Eur. Surg. Res. 2015, 54, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Iansante, V.; Mitry, R.R.; Filippi, C.; Fitzpatrick, E.; Dhawan, A. Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatr. Res. 2017, 83, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Quan-Wen, L.; Liu, Q.; Li, J.; Wei, L.; Ren, K.; Zhang, X.; Ding, T.; Xiao, L.; Zhang, W.; Xin, H.W.H. Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure. Stem Cell Res. Ther. 2018, 9, 321. [Google Scholar]
- Rajagopalan, S.; Long, E.O. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012, 3, 258. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Long, E.O. A Human Histocompatibility Leukocyte Antigen (HLA)-G–specific Receptor Expressed on All Natural Killer Cells. J. Exp. Med. 1999, 189, 1093–1100. [Google Scholar] [CrossRef]
- Burnstock, G.; Boeynaems, J.-M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar] [CrossRef]
- Morandi, F.; Morandi, B.; Horenstein, A.L.; Chillemi, A.; Quarona, V.; Zaccarello, G.; Carrega, P.; Ferlazzo, G.; Mingari, M.C.; Moretta, L.; et al. A non-canonical adenosinergic pathway led by CD38 in human melanoma cells induces suppression of T cell proliferation. Oncotarget 2015, 6, 25602–25618. [Google Scholar] [CrossRef]
- Tanimizu, N. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003, 116, 1775–1786. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, R.-J. Prevalence of Telomerase Activity in Human Cancer. J. Formos. Med Assoc. 2011, 110, 275–289. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Shirvaikar, N.; Marquez-Curtis, L.; Janowska-Wieczorek, A. Hematopoietic Stem Cell Mobilization and Homing after Transplantation: The Role of MMP-2, MMP-9, and MT1-MMP. Biochem. Res. Int. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, V. Cell Migration through Extracellular Matrix. J. Cell Boil. 2000, 149, 1167–1170. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. et Biophys. Acta (BBA)–Bioenerg. 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Bembi, B.; Comelli, M.; Scaggiante, B.; Pineschi, A.; Rapelli, S.; Gornati, R.; Montorfano, G.; Berra, B.; Agosti, E.; Romeo, D. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am. J. Med. Genet. 1992, 44, 527–533. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, R.C.; Strom, S.C.; Gramignoli, R. Effects of Cryogenic Storage on Human Amnion Epithelial Cells. Cells 2020, 9, 1696. https://doi.org/10.3390/cells9071696
Srinivasan RC, Strom SC, Gramignoli R. Effects of Cryogenic Storage on Human Amnion Epithelial Cells. Cells. 2020; 9(7):1696. https://doi.org/10.3390/cells9071696
Chicago/Turabian StyleSrinivasan, Raghuraman C., Stephen C. Strom, and Roberto Gramignoli. 2020. "Effects of Cryogenic Storage on Human Amnion Epithelial Cells" Cells 9, no. 7: 1696. https://doi.org/10.3390/cells9071696
APA StyleSrinivasan, R. C., Strom, S. C., & Gramignoli, R. (2020). Effects of Cryogenic Storage on Human Amnion Epithelial Cells. Cells, 9(7), 1696. https://doi.org/10.3390/cells9071696





