Growth Factors, and Cytokines; Understanding the Role of Tyrosine Phosphatase SHP2 in Gametogenesis and Early Embryo Development
Abstract
:1. Introduction
2. Literature Review Procedure
2.1. Literature Review and Search Strategy
2.2. Inclusion and Exclusion Criteria
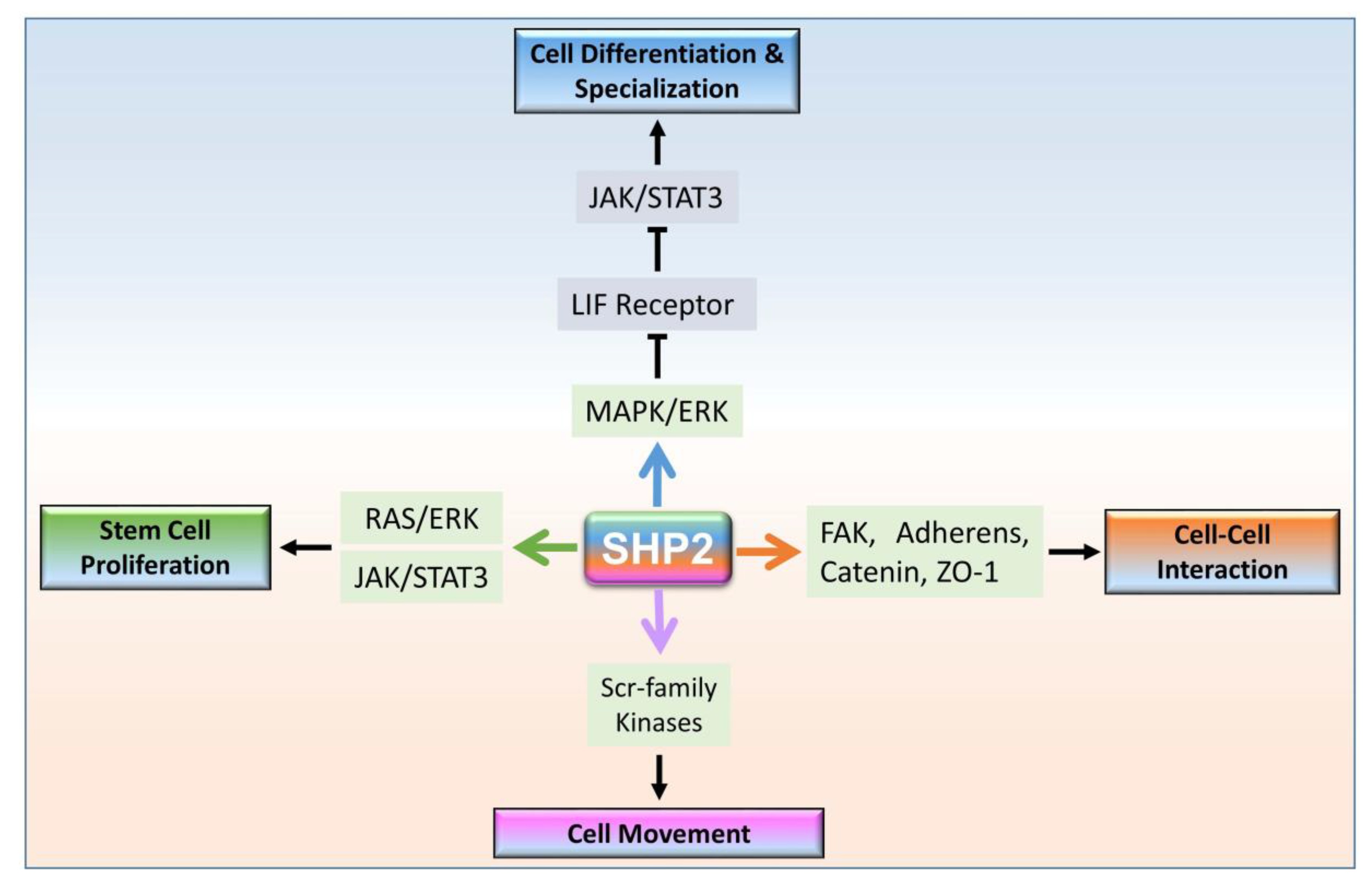
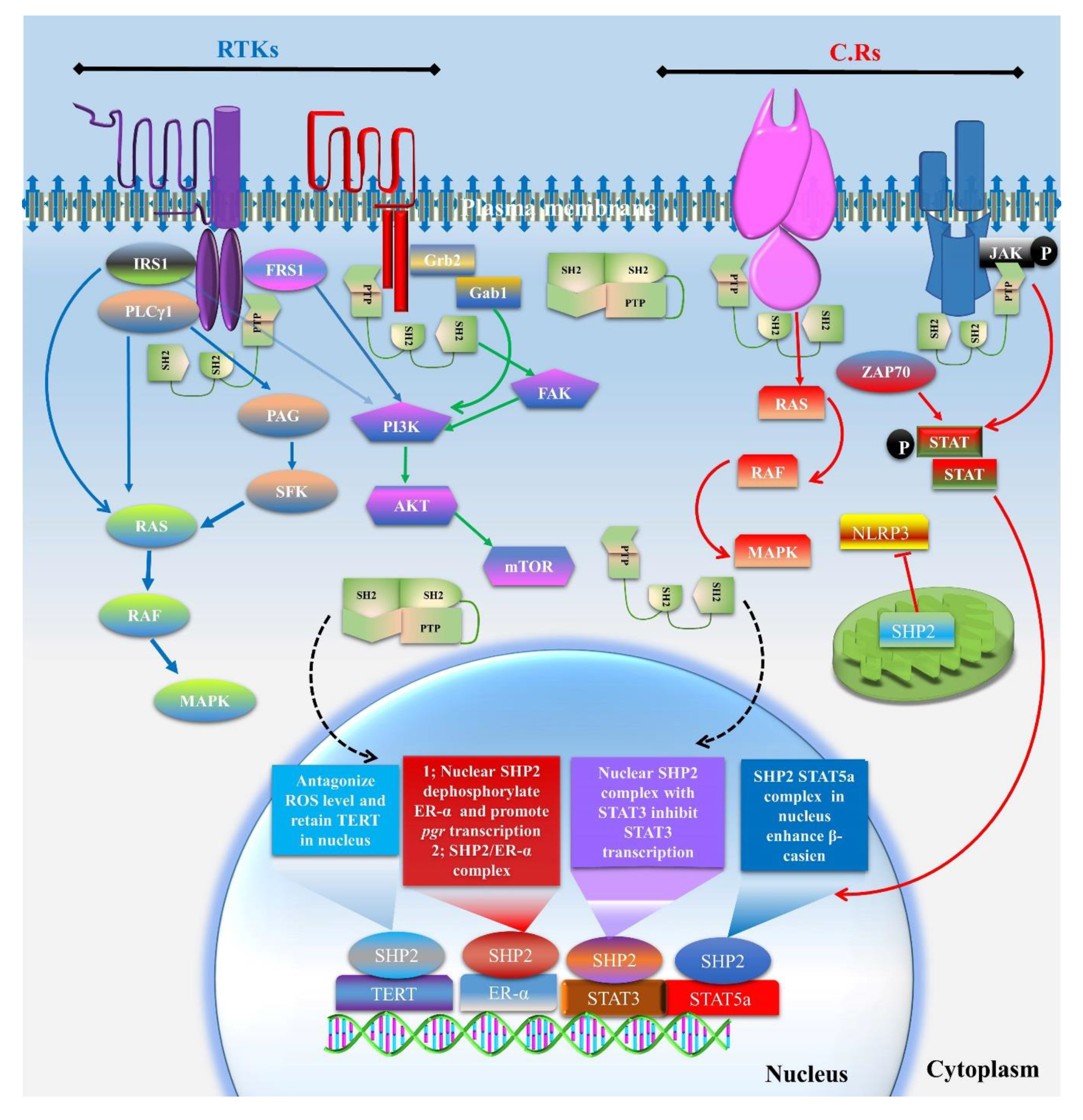
3. SHP2 Dependent Signaling in Multicellular Organism Development
3.1. Cytoplasmic Localized SHP2 Mechanisms
3.2. SHP2 Nuclear Localization and Role in Transcription
4. Growth Factors and Cytokines Dependent Signaling in Primordial Germ Cells (PGCs) and SHP2 Functions
4.1. Role of Growth Factors and Cytokine in PGCs Specification, Migration and Proliferation
4.2. SHP2 Expression and Interaction Prediction with Growth Factors and Cytokines Receptors Responsible for PGCs Specification, Migration and Proliferation
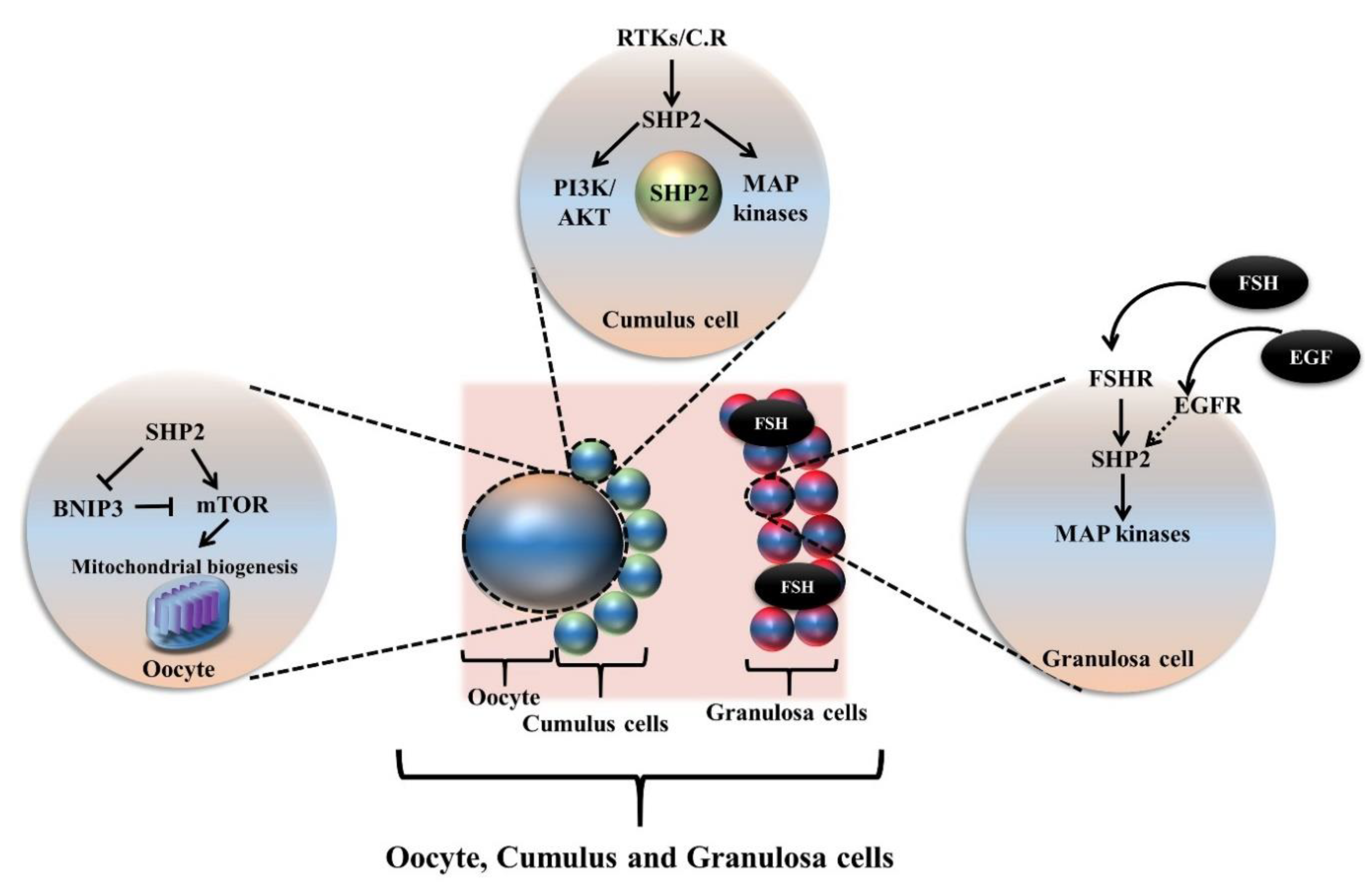
5. Oogenesis and SHP2 Dependent Growth Factors and Cytokines Signaling
5.1. SHP2 Dependent Growth Factors and Cytokines Role in Oocyte Meiotic Resumption, Maturation and Ovulation
5.1.1. Activation of Primordial Follicle and Role of Growth Factors and Cytokines
5.1.2. SHP2 Dependent Growth Factors and Cytokines Signaling during Oocyte Meiotic Maturation
5.1.3. SHP2 Dependent Growth Factors and Cytokines Signaling during Oocyte Ovulation
6. The Contribution of SHP2 to Spermatogenesis, Spermatogonia Stem Cells (SSCs) Self-Renewal and Differentiation
Spermatogonia Stem Cells (SSCs)
7. Early Embryonic Development and SHP2 Mediated Signaling Network
SHP2 Role and Mechanism in Embryonic Stem Cells (ESCs)
8. Nuclear/Cytoplasmic Localization of SHP2 and Embryo Implantation
9. Conclusions and Future Directions of SHP2 Research
Funding
Conflicts of Interest
References
- White, M.D.; Zenker, J.; Bissiere, S.; Plachta, N. Instructions for Assembling the Early Mammalian Embryo. Dev. Cell 2018, 45, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, K.A.; Hage, W.J. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found. Symp. 1994, 182, 68–84, discussion 84–91. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Barton, S.C.; Surani, M.A. A molecular programme for the specification of germ cell fate in mice. Nature 2002, 418, 293–300. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Magnusdottir, E.; Surani, M.A. How to make a primordial germ cell. Development 2014, 141, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef] [Green Version]
- Hubner, K.; Fuhrmann, G.; Christenson, L.K.; Kehler, J.; Reinbold, R.; De La Fuente, R.; Wood, J.; Strauss, J.F., 3rd; Boiani, M.; Scholer, H.R. Derivation of oocytes from mouse embryonic stem cells. Science 2003, 300, 1251–1256. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Gearing, D.P.; White, L.S.; Compton, D.L.; Schooley, K.; Donovan, P.J. Role of leukemia inhibitory factor and its receptor in mouse primordial germ cell growth. Development 1994, 120, 3145–3153. [Google Scholar] [PubMed]
- Miyahara, D.; Oishi, I.; Makino, R.; Kurumisawa, N.; Nakaya, R.; Ono, T.; Kagami, H.; Tagami, T. Chicken stem cell factor enhances primordial germ cell proliferation cooperatively with fibroblast growth factor 2. J. Reprod. Dev. 2016, 62, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawase, E.; Yamamoto, H.; Hashimoto, K.; Nakatsuji, N. Tumor necrosis factor-alpha (TNF-alpha) stimulates proliferation of mouse primordial germ cells in culture. Dev. Biol. 1994, 161, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Toksoz, D.; Nishikawa, S.; Nishikawa, S.; Williams, D.; Zsebo, K.; Hogan, B.L. Effect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature 1991, 353, 750–752. [Google Scholar] [CrossRef]
- Dolci, S.; Williams, D.E.; Ernst, M.K.; Resnick, J.L.; Brannan, C.I.; Lock, L.F.; Lyman, S.D.; Boswell, H.S.; Donovan, P.J. Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 1991, 352, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Lunenfeld, E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J. Androl. 2004, 6, 259–268. [Google Scholar]
- Idrees, M.; Xu, L.; Song, S.H.; Joo, M.D.; Lee, K.L.; Muhammad, T.; El Sheikh, M.; Sidrat, T.; Kong, I.K. PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development. Cells 2019, 8, 1272. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Wu, G.; Yuan, D.; Jia, B.; Liu, C.; Zhu, S.; Hou, Y. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2014, 81, 608–618. [Google Scholar] [CrossRef]
- Neira, J.A.; Tainturier, D.; Pena, M.A.; Martal, J. Effect of the association of IGF-I, IGF-II, bFGF, TGF-beta1, GM-CSF, and LIF on the development of bovine embryos produced in vitro. Theriogenology 2010, 73, 595–604. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Kong, N.; Zhang, Y.; Zhao, Y.; Xia, G.; Zhang, M. Epidermal Growth Factor-Mobilized Intracellular Calcium of Cumulus Cells Decreases Natriuretic Peptide Receptor 2 Affinity for Natriuretic Peptide Type C and Induces Oocyte Meiotic Resumption in the Mouse. Biol. Reprod. 2016, 95, 45. [Google Scholar] [CrossRef] [Green Version]
- Raheem, K.A. Cytokines, growth factors and macromolecules as mediators of implantation in mammalian species. Int. J. Vet. Sci. Med. 2018, 6, S6–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiler, P.; Plenz, G.; Deng, M.C. The interleukin-6 cytokine system in embryonic development, embryo-maternal interactions and cardiogenesis. Eur. Cytokine Netw. 2001, 12, 15–21. [Google Scholar] [PubMed]
- Yang, W.; Klaman, L.D.; Chen, B.; Araki, T.; Harada, H.; Thomas, S.M.; George, E.L.; Neel, B.G. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell 2006, 10, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, K.S.; Rosario, M.; Birchmeier, C.; Birchmeier, W. The tyrosine phosphatase Shp2 in development and cancer. Adv. Cancer Res. 2010, 106, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Tsiaras, W.G.; Araki, T.; Wen, G.; Minichiello, L.; Klein, R.; Neel, B.G. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 2002, 22, 4062–4072. [Google Scholar] [CrossRef] [Green Version]
- Cha, Y.; Park, K.S. SHP2 is a downstream target of ZAP70 to regulate JAK1/STAT3 and ERK signaling pathways in mouse embryonic stem cells. FEBS Lett. 2010, 584, 4241–4246. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Lee, H.C.; Chou, C.C.; Hur, S.S.; Osterday, K.; Del Alamo, J.C.; Lasheras, J.C.; Chien, S. Shp2 plays a crucial role in cell structural orientation and force polarity in response to matrix rigidity. Proc. Natl. Acad. Sci. USA 2013, 110, 2840–2845. [Google Scholar] [CrossRef] [Green Version]
- Puri, P.; Walker, W.H. The tyrosine phosphatase SHP2 regulates Sertoli cell junction complexes. Biol. Reprod. 2013, 88, 59. [Google Scholar] [CrossRef]
- Dance, M.; Montagner, A.; Salles, J.P.; Yart, A.; Raynal, P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 2008, 20, 453–459. [Google Scholar] [CrossRef]
- Neel, B.G.; Gu, H.; Pao, L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef]
- Araki, T.; Nawa, H.; Neel, B.G. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J. Biol. Chem. 2003, 278, 41677–41684. [Google Scholar] [CrossRef] [Green Version]
- Ran, H.; Kong, S.; Zhang, S.; Cheng, J.; Zhou, C.; He, B.; Xin, Q.; Lydon, J.P.; DeMayo, F.J.; Feng, G.S.; et al. Nuclear Shp2 directs normal embryo implantation via facilitating the ERalpha tyrosine phosphorylation by the Src kinase. Proc. Natl. Acad. Sci. USA 2017, 114, 4816–4821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonks, N.K.; Neel, B.G. From form to function: Signaling by protein tyrosine phosphatases. Cell 1996, 87, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Arregui, C.O.; Balsamo, J.; Lilien, J. Regulation of signaling by protein-tyrosine phosphatases: Potential roles in the nervous system. Neurochem. Res. 2000, 25, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu. Rev. Biochem. 1987, 56, 881–914. [Google Scholar] [CrossRef] [PubMed]
- Kheilova, K.; Petr, J.; Zalmanova, T.; Kucerova-Chrpova, V.; Rehak, D. Src family kinases are involved in the meiotic maturation of porcine oocytes. Reprod. Fertil. Dev. 2015, 27, 1097–1105. [Google Scholar] [CrossRef]
- Agazie, Y.M.; Hayman, M.J. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol. Cell. Biol. 2003, 23, 7875–7886. [Google Scholar] [CrossRef] [Green Version]
- Agazie, Y.M.; Hayman, M.J. Development of an efficient “substrate-trapping” mutant of Src homology phosphotyrosine phosphatase 2 and identification of the epidermal growth factor receptor, Gab1, and three other proteins as target substrates. J. Biol. Chem. 2003, 278, 13952–13958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.Q.; Yang, W.; Kontaridis, M.I.; Bivona, T.G.; Wen, G.; Araki, T.; Luo, J.; Thompson, J.A.; Schraven, B.L.; Philips, M.R.; et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 2004, 13, 341–355. [Google Scholar] [CrossRef]
- Hanafusa, H.; Torii, S.; Yasunaga, T.; Matsumoto, K.; Nishida, E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 2004, 279, 22992–22995. [Google Scholar] [CrossRef] [Green Version]
- Cabrita, M.A.; Christofori, G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis 2008, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadari, Y.R.; Kouhara, H.; Lax, I.; Schlessinger, J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 1998, 18, 3966–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanke, S.; Mann, M. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2. Mol. Cell Proteom. 2009, 8, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef]
- Ali, S.; Chen, Z.; Lebrun, J.J.; Vogel, W.; Kharitonenkov, A.; Kelly, P.A.; Ullrich, A. PTP1D is a positive regulator of the prolactin signal leading to beta-casein promoter activation. EMBO J. 1996, 15, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, S.; Volarevic, S.; Moriggl, R.; Mercep, M.; Groner, B. Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol. Endocrinol. 1998, 12, 556–567. [Google Scholar] [CrossRef]
- Zehender, A.; Huang, J.; Gyorfi, A.H.; Matei, A.E.; Trinh-Minh, T.; Xu, X.; Li, Y.N.; Chen, C.W.; Lin, J.; Dees, C.; et al. The tyrosine phosphatase SHP2 controls TGFbeta-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat. Commun. 2018, 9, 3259. [Google Scholar] [CrossRef]
- Kim, H.; Baumann, H. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol. Cell. Biol. 1999, 19, 5326–5338. [Google Scholar] [CrossRef] [Green Version]
- Tauchi, T.; Feng, G.S.; Marshall, M.S.; Shen, R.; Mantel, C.; Pawson, T.; Broxmeyer, H.E. The ubiquitously expressed Syp phosphatase interacts with c-kit and Grb2 in hematopoietic cells. J. Biol. Chem. 1994, 269, 25206–25211. [Google Scholar]
- Sette, C.; Dolci, S.; Geremia, R.; Rossi, P. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int. J. Dev. Biol. 2000, 44, 599–608. [Google Scholar]
- Songyang, Z.; Shoelson, S.E.; Chaudhuri, M.; Gish, G.; Pawson, T.; Haser, W.G.; King, F.; Roberts, T.; Ratnofsky, S.; Lechleider, R.J.; et al. SH2 domains recognize specific phosphopeptide sequences. Cell 1993, 72, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, E.Y.; Ko, J.J.; Lee, K.A. Gas6 is a reciprocal regulator of mitophagy during mammalian oocyte maturation. Sci. Rep. 2019, 9, 10343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Liu, W.; Chen, Z.; Gu, Y.; Peng, S.; Shen, L.; Shen, Y.; Wang, X.; Feng, G.S.; Sun, Y.; et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis. Nat. Commun. 2017, 8, 2168. [Google Scholar] [CrossRef] [PubMed]
- Khatib, H.; Monson, R.L.; Schutzkus, V.; Kohl, D.M.; Rosa, G.J.; Rutledge, J.J. Mutations in the STAT5A gene are associated with embryonic survival and milk composition in cattle. J. Dairy Sci. 2008, 91, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, N.; Schimchowitsch, S.; Lebrun, J.J.; Ali, S. Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells. J. Biol. Chem. 2002, 277, 31107–31114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wang, J.; Cao, F.; Jiang, H.; Li, A.; Li, J.; Qiu, L.; Shen, H.; Chang, W.; Zhou, C.; et al. SHP2 associates with nuclear localization of STAT3: Significance in progression and prognosis of colorectal cancer. Sci. Rep. 2017, 7, 17597. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kang, Y.; Wei, L.; Liu, W.; Tian, Y.; Chen, B.; Lin, X.; Li, Y.; Feng, G.S.; Lu, Z. Tyrosine phosphatase Shp2 mediates the estrogen biological action in breast cancer via interaction with the estrogen extranuclear receptor. PLoS ONE 2014, 9, e102847. [Google Scholar] [CrossRef] [Green Version]
- Jakob, S.; Schroeder, P.; Lukosz, M.; Buchner, N.; Spyridopoulos, I.; Altschmied, J.; Haendeler, J. Nuclear protein tyrosine phosphatase Shp-2 is one important negative regulator of nuclear export of telomerase reverse transcriptase. J. Biol. Chem. 2008, 283, 33155–33161. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, M.; Snow, M.H.; McLaren, A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990, 110, 521–528. [Google Scholar]
- Ohinata, Y.; Ohta, H.; Shigeta, M.; Yamanaka, K.; Wakayama, T.; Saitou, M. A signaling principle for the specification of the germ cell lineage in mice. Cell 2009, 137, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Runyan, C.; Shoemaker, A.; Surani, A.; Wylie, C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development 2009, 136, 1295–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef] [Green Version]
- Donovan, P.J. Growth factor regulation of mouse primordial germ cell development. Curr. Top. Dev. Biol. 1994, 29, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Miyauchi, H. Gametogenesis from Pluripotent Stem Cells. Cell Stem Cell 2016, 18, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Molyneaux, K.; Runyan, C.; Schaible, K.; Wylie, C. The roles of FGF signaling in germ cell migration in the mouse. Development 2005, 132, 5399–5409. [Google Scholar] [CrossRef] [Green Version]
- De Rooij, D.G. The spermatogonial stem cell niche. Microsc. Res. Tech. 2009, 72, 580–585. [Google Scholar] [CrossRef]
- Anderson, R.; Copeland, T.K.; Scholer, H.; Heasman, J.; Wylie, C. The onset of germ cell migration in the mouse embryo. Mech. Dev. 2000, 91, 61–68. [Google Scholar] [CrossRef]
- Leerberg, D.M.; Sano, K.; Draper, B.W. Fibroblast growth factor signaling is required for early somatic gonad development in zebrafish. PLoS Genet. 2017, 13, e1006993. [Google Scholar] [CrossRef]
- Lamb, D.J. Growth factors and testicular development. J. Urol. 1993, 150, 583–592. [Google Scholar] [CrossRef]
- Pangas, S.A. Growth factors in ovarian development. Semin. Reprod. Med. 2007, 25, 225–234. [Google Scholar] [CrossRef]
- Resnick, J.L.; Bixler, L.S.; Cheng, L.; Donovan, P.J. Long-term proliferation of mouse primordial germ cells in culture. Nature 1992, 359, 550–551. [Google Scholar] [CrossRef]
- McCoshen, J.A.; McCallion, D.J. A study of the primordial germ cells during their migratory phase in Steel mutant mice. Experientia 1975, 31, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Shirayama, M.; Conte, D., Jr.; Vasale, J.; Batista, P.J.; Claycomb, J.M.; Moresco, J.J.; Youngman, E.M.; Keys, J.; Stoltz, M.J.; et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol. Cell 2009, 36, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, Y.; Miyoshi, S.; Fukuda, K.; Nishiyama, N.; Ikegami, Y.; Tanimoto, K.; Murata, M.; Takahashi, E.; Shimoda, K.; Hirano, T.; et al. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J. Mol. Cell Cardiol. 2007, 43, 710–716. [Google Scholar] [CrossRef]
- Ahmed, Z.; Lin, C.C.; Suen, K.M.; Melo, F.A.; Levitt, J.A.; Suhling, K.; Ladbury, J.E. Grb2 controls phosphorylation of FGFR2 by inhibiting receptor kinase and Shp2 phosphatase activity. J. Cell Biol. 2013, 200, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Everingham, S.; Ramdas, B.; Kapur, R.; Craig, A.W. SHP2 phosphatase promotes mast cell chemotaxis toward stem cell factor via enhancing activation of the Lyn/Vav/Rac signaling axis. J. Immunol. 2014, 192, 4859–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Hurk, R.; Zhao, J. Formation of mammalian oocytes and their growth, differentiation and maturation within ovarian follicles. Theriogenology 2005, 63, 1717–1751. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.; Cabau, C.; Bouchez, O.; Sarry, J.; Marsaud, N.; Foissac, S.; Woloszyn, F.; Mulsant, P.; Mandon-Pepin, B. An overview of gene expression dynamics during early ovarian folliculogenesis: Specificity of follicular compartments and bi-directional dialog. BMC Genom. 2013, 14, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Sasseville, M.; Ritter, L.J.; Nguyen, T.M.; Liu, F.; Mottershead, D.G.; Russell, D.L.; Gilchrist, R.B. Growth differentiation factor 9 signaling requires ERK1/2 activity in mouse granulosa and cumulus cells. J. Cell Sci. 2010, 123, 3166–3176. [Google Scholar] [CrossRef]
- Hayashi, M.; McGee, E.A.; Min, G.; Klein, C.; Rose, U.M.; Van Duin, M.; Hsueh, A.J. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology 1999, 140, 1236–1244. [Google Scholar] [CrossRef]
- Wu, D.; Pang, Y.; Ke, Y.; Yu, J.; He, Z.; Tautz, L.; Mustelin, T.; Ding, S.; Huang, Z.; Feng, G.S. A conserved mechanism for control of human and mouse embryonic stem cell pluripotency and differentiation by shp2 tyrosine phosphatase. PLoS ONE 2009, 4, e4914. [Google Scholar] [CrossRef]
- Morohaku, K.; Tanimoto, R.; Sasaki, K.; Kawahara-Miki, R.; Kono, T.; Hayashi, K.; Hirao, Y.; Obata, Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9021–9026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, Y.; Manganaro, T.F.; Tao, X.J.; Martimbeau, S.; Donahoe, P.K.; Tilly, J.L. Requirement for phosphatidylinositol-3’-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology 1999, 140, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xia, G. Hormonal control of mammalian oocyte meiosis at diplotene stage. Cell Mol. Life Sci. 2012, 69, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Su, Y.Q.; Ariga, M.; Law, E.; Jin, S.L.; Conti, M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004, 303, 682–684. [Google Scholar] [CrossRef]
- Ahumada, C.J.; Salvador, I.; Cebrian-Serrano, A.; Lopera, R.; Silvestre, M.A. Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality. Animal 2013, 7, 455–462. [Google Scholar] [CrossRef]
- Idrees, M.; Xu, L.; El Sheikh, M.; Sidrat, T.; Song, S.H.; Joo, M.D.; Lee, K.L.; Kong, I.K. The PPARdelta Agonist GW501516 Improves Lipolytic/Lipogenic Balance through CPT1 and PEPCK during the Development of Pre-Implantation Bovine Embryos. Int. J. Mol. Sci. 2019, 20, 6066. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Li, J.; Gao, Q.; Wei, S.; Yang, B. SHP2 overexpression enhances the invasion and metastasis of ovarian cancer in vitro and in vivo. Onco Targets Ther 2017, 10, 3881–3891. [Google Scholar] [CrossRef] [Green Version]
- Donaubauer, E.M.; Law, N.C.; Hunzicker-Dunn, M.E. Follicle-Stimulating Hormone (FSH)-dependent Regulation of Extracellular Regulated Kinase (ERK) Phosphorylation by the Mitogen-activated Protein (MAP) Kinase Phosphatase MKP3. J. Biol. Chem. 2016, 291, 19701–19712. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, M. Signaling networks in somatic cells and oocytes activated during ovulation. Ann. D’endocrinologie 2010, 71, 189–190. [Google Scholar] [CrossRef] [PubMed]
- El-Hayek, S.; Demeestere, I.; Clarke, H.J. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc. Natl. Acad. Sci. USA 2014, 111, 16778–16783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunzicker-Dunn, M.E.; Lopez-Biladeau, B.; Law, N.C.; Fiedler, S.E.; Carr, D.W.; Maizels, E.T. PKA and GAB2 play central roles in the FSH signaling pathway to PI3K and AKT in ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2979–E2988. [Google Scholar] [CrossRef] [Green Version]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Tam, P.P.; Snow, M.H. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J. Embryol. Exp. Morphol. 1981, 64, 133–147. [Google Scholar]
- Hilscher, B.; Hilscher, W.; Bulthoff-Ohnolz, B.; Kramer, U.; Birke, A.; Pelzer, H.; Gauss, G. Kinetics of gametogenesis. I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974, 154, 443–470. [Google Scholar] [CrossRef]
- Ishikura, Y.; Yabuta, Y.; Ohta, H.; Hayashi, K.; Nakamura, T.; Okamoto, I.; Yamamoto, T.; Kurimoto, K.; Shirane, K.; Sasaki, H.; et al. In Vitro Derivation and Propagation of Spermatogonial Stem Cell Activity from Mouse Pluripotent Stem Cells. Cell Rep. 2016, 17, 2789–2804. [Google Scholar] [CrossRef] [Green Version]
- Matoba, S.; Ogura, A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult mice. Biol. Reprod. 2011, 84, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Ohinata, Y.; Payer, B.; O’Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Phillips, B.T.; Suzuki, H.; Orwig, K.E.; Rajkovic, A.; Lapinski, P.E.; King, P.D.; Feng, G.S.; Walker, W.H. The transition from stem cell to progenitor spermatogonia and male fertility requires the SHP2 protein tyrosine phosphatase. Stem Cells 2014, 32, 741–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politch, J.A.; Tucker, L.; Bowman, F.P.; Anderson, D.J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 2007, 22, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lindahl, M.; Hyvonen, M.E.; Parvinen, M.; De Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M.; et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493. [Google Scholar] [CrossRef]
- Nagano, M.; Ryu, B.Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef]
- Perrinjaquet, M.; Vilar, M.; Ibanez, C.F. Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J. Biol. Chem. 2010, 285, 31867–31875. [Google Scholar] [CrossRef] [Green Version]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Tang, Z.; Li, Y.; Liu, W.; Zhang, S.; Wang, B.; Tian, Y.; Zhao, Y.; Ran, H.; Liu, W.; et al. Deletion of the tyrosine phosphatase Shp2 in Sertoli cells causes infertility in mice. Sci. Rep. 2015, 5, 12982. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.X.; Wang, J.; Wu, J. Extrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiation. Asian J. Androl. 2015, 17, 347–354. [Google Scholar] [CrossRef]
- Wawersik, M.; Milutinovich, A.; Casper, A.L.; Matunis, E.; Williams, B.; Van Doren, M. Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 2005, 436, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.R.; Posenau, T.; Gumulak-Smith, J.J.; Matunis, E.; Van Doren, M.; Wawersik, M. Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev. Biol. 2009, 334, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, G.S. Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell Res. 2007, 17, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.L.; Beddington, R.S. Multi-lineage ‘stem’ cells in the mammalian embryo. J. Cell Sci. Suppl. 1988, 10, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, B.; Bonilla, L.; Block, J.; Fear, J.M.; Bonilla, A.Q.; Hansen, P.J. Colony-stimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 2009, 150, 5046–5054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, S.D.; Hansen, P.J.; Ealy, A.D. Fibroblast growth factor requirements for in vitro development of bovine embryos. Theriogenology 2011, 75, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Jack, G.D.; Zhang, L.; Friedman, A.D. M-CSF elevates c-Fos and phospho-C/EBPalpha(S21) via ERK whereas G-CSF stimulates SHP2 phosphorylation in marrow progenitors to contribute to myeloid lineage specification. Blood 2009, 114, 2172–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidder, B.L. Derivation and manipulation of trophoblast stem cells from mouse blastocysts. Methods Mol. Biol. 2014, 1150, 201–212. [Google Scholar] [CrossRef]
- Saxton, T.M.; Henkemeyer, M.; Gasca, S.; Shen, R.; Rossi, D.J.; Shalaby, F.; Feng, G.S.; Pawson, T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997, 16, 2352–2364. [Google Scholar] [CrossRef]
- Ralston, A.; Rossant, J. How signaling promotes stem cell survival: Trophoblast stem cells and Shp2. Dev. Cell 2006, 10, 275–276. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A.; Rossant, J.; Nagy, R.; Abramow-Newerly, W.; Roder, J.C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 1993, 90, 8424–8428. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.K.; Feng, G.S. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene 1998, 17, 433–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.E.; Moon, S.H.; Kim, D.K.; Choi, C.; Song, J.; Park, K.S. Sprouty1 regulates neural and endothelial differentiation of mouse embryonic stem cells. Stem Cells Dev. 2012, 21, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Nakamura, T.; Nakao, K.; Arai, T.; Katsuki, M.; Heike, T.; Yokota, T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999, 18, 4261–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, M.; Oates, A.; Dunn, A.R. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J. Biol. Chem. 1996, 271, 30136–30143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, L.C.; Saleh, W. Growth factors in reproduction. Trends Endocrinol. Metab. TEM 1995, 6, 60–69. [Google Scholar] [CrossRef]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Kontgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- Robb, L.; Li, R.; Hartley, L.; Nandurkar, H.H.; Koentgen, F.; Begley, C.G. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 1998, 4, 303–308. [Google Scholar] [CrossRef]
- Xie, H.; Wang, H.; Tranguch, S.; Iwamoto, R.; Mekada, E.; Demayo, F.J.; Lydon, J.P.; Das, S.K.; Dey, S.K. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 18315–18320. [Google Scholar] [CrossRef] [Green Version]
- Large, M.J.; Wetendorf, M.; Lanz, R.B.; Hartig, S.M.; Creighton, C.J.; Mancini, M.A.; Kovanci, E.; Lee, K.F.; Threadgill, D.W.; Lydon, J.P.; et al. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014, 10, e1004451. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.H.; Qu, C.K.; Henegariu, O.; Lu, X.; Feng, G.S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 1998, 273, 21125–21131. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Sharma, R.; Durairajanayagam, D.; Ayaz, A.; Cui, Z.; Willard, B.; Gopalan, B.; Sabanegh, E. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod. Biol. Endocrinol. RB&E 2015, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Yu, Y.; Zheng, S.; Zhao, X.; Dong, Q.; He, Z.; Liang, Y.; Lu, Q.; Fang, Y.; Gan, X.; et al. Overexpression of Shp2 tyrosine phosphatase is implicated in leukemogenesis in adult human leukemia. Blood 2005, 106, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.; Mehler, E.L.; Goldberg, R.; Zampino, G.; Brunner, H.G.; Kremer, H.; Van der Burgt, I.; Crosby, A.H.; Ion, A.; Jeffery, S.; et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 2001, 29, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Quintana, E.; Rodriguez-Gonzalez, F. LEOPARD Syndrome: Clinical Features and Gene Mutations. Mol. Syndromol. 2012, 3, 145–157. [Google Scholar] [CrossRef] [Green Version]


| Growth Factors | Signaling Cascade | Signaling Target in Gametogenesis and Early Embryo Development | SHP2 Role in Signaling Identified in Other Tissues | Key References |
|---|---|---|---|---|
| EGF | EGFR/Grb2/SHP2/p85 PI3K/AKT RAS/MAPK | Play a role in early ovarian folliculogenesis | SHP2 make complex with Grb2 and p85 to activate PI3K/AKT signaling | [17,30,77,78] |
| Resume meiosis and mediate FSH signaling in the oocyte. Play a role in embryo implantation | Dephosphorylate EGFR on Tyr 922 to activate RAS/MAPK. | |||
| bFGF | FGFR/ Spry/Grb2 MAPK | bFGF plays a role in PGCs specification, migration, and proliferation | SHP2 dephosphorylate Spry and detach it from Grb2 and activated MAP kinases. | [61,63,66,69,79] |
| PGCs proliferation and self-renewal. Enhance in vitro oocyte maturation and embryo development. | FRS1,Grb2 & SHP2 make complex to activate RAS/MAPK | |||
| IGF | IGFR/ IRS/MAP K/ERK | Play a role in early ovarian folliculogenesis | SHP2 dephosphorylate IRS1/2 and recruit its binding with PI3K and PLCγ | [19,40,80] |
| Enhance invitro oocyte maturation and embryo development | ||||
| GDNF | GDNF RET/PI3K/AKT | Spermatogonia stem cells self-renewal and proliferation | SHP2 interact with RET and activate PI3K/AKT signaling | [60,81,82] |
| Cytokines | Signaling Cascade | Signaling Target in Gametogenesis and Early Embryo Development | SHP2 Role in Signaling Identified in Other Tissues | Key References |
|---|---|---|---|---|
| BMPs | BMP receptors type I and type2 SMAD4/1/5/8 | BMPs induce the formation of PGCs from epiblast of the embryo | Interaction between BMP receptors and SHP2 is yet not completely explored, SHP2 deletion in human ES cells impair SMAD signaling. | [58,60,61,83] |
| LIF | LIFR Gp-130/zap-70 JAK/STAT | PGCs development, proliferation, and oocyte in vitro maturation | SHP2 interact with gp-130 and also with Zap-70 for the activation of JAK/STAT and MAP kinases | [65,69,72,78,79,83,84] |
| Play a role in embryo implantation | ||||
| KL/SCF | Kit receptor PI3K/AKT RAS/MAPK PLCγ/Adherine | PGCs proliferation, self-renewal, and oocyte maturation. | SHP2 bind with c-Kit at Tyr567 located in the c-Kit juxta membrane region | [46,47,59,70,71,74,85] |
| Colony stimulating factor CSF-1 | CSF1R MAPK/ERK | Play a role in oocyte meiotic resumption, and maturation | SHP2 become phosphorylated and activated by CSF-1 receptor for ERK pathway activation | [79,86,87] |
| Interleukin | ILR Gp-130 JAK/STAT | Enhance inner cell mass of embryo during in vitro development | SHP2 bind with gp-130 a receptor sub-unite of interleukins and suppress JAK/STAT pathway via downregulating JAK activity | [22,45,88] |
| Play a role in embryo implantation | ||||
| TGF-β | TGFR JAK2/STAT3 | Gonadal and testicular development | TGF-β activate SHP2 and recruit it to JAK2 for dephosphorylation at Y570, resulting in activation of STAT3 | [19,44,64] |
| Play a role in early embryo development | ||||
| GDF9 | BMPR2/ ALKA4/5/7 SMAD2/3/4 | Play a role in follicle formation during the early stage, and its deletion inhibit somatic cell differentiation with complete infertility. | Interaction between GDF9 receptor and SHP2 is yet unidentified, but both the proteins show expression in granulosa cells and downstream pathway is MAP kinases | [89,90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, M.; Oh, S.-H.; Muhammad, T.; El-Sheikh, M.; Song, S.-H.; Lee, K.-L.; Kong, I.-K. Growth Factors, and Cytokines; Understanding the Role of Tyrosine Phosphatase SHP2 in Gametogenesis and Early Embryo Development. Cells 2020, 9, 1798. https://doi.org/10.3390/cells9081798
Idrees M, Oh S-H, Muhammad T, El-Sheikh M, Song S-H, Lee K-L, Kong I-K. Growth Factors, and Cytokines; Understanding the Role of Tyrosine Phosphatase SHP2 in Gametogenesis and Early Embryo Development. Cells. 2020; 9(8):1798. https://doi.org/10.3390/cells9081798
Chicago/Turabian StyleIdrees, Muhammad, Seon-Hwa Oh, Tahir Muhammad, Marwa El-Sheikh, Seok-Hwan Song, Kyeong-Lim Lee, and Il-Keun Kong. 2020. "Growth Factors, and Cytokines; Understanding the Role of Tyrosine Phosphatase SHP2 in Gametogenesis and Early Embryo Development" Cells 9, no. 8: 1798. https://doi.org/10.3390/cells9081798
APA StyleIdrees, M., Oh, S.-H., Muhammad, T., El-Sheikh, M., Song, S.-H., Lee, K.-L., & Kong, I.-K. (2020). Growth Factors, and Cytokines; Understanding the Role of Tyrosine Phosphatase SHP2 in Gametogenesis and Early Embryo Development. Cells, 9(8), 1798. https://doi.org/10.3390/cells9081798






