Neuroprotective Effects of a Novel Inhibitor of c-Jun N-Terminal Kinase in the Rat Model of Transient Focal Cerebral Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Equipment
2.3. Chemicals and Drugs
2.4. Study Molecule
2.5. Cerebral Ischemia Model
2.6. Experimental Protocol
2.7. Neurological Deficit Evaluation
2.8. Assessment of Cerebral Infarct Size
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
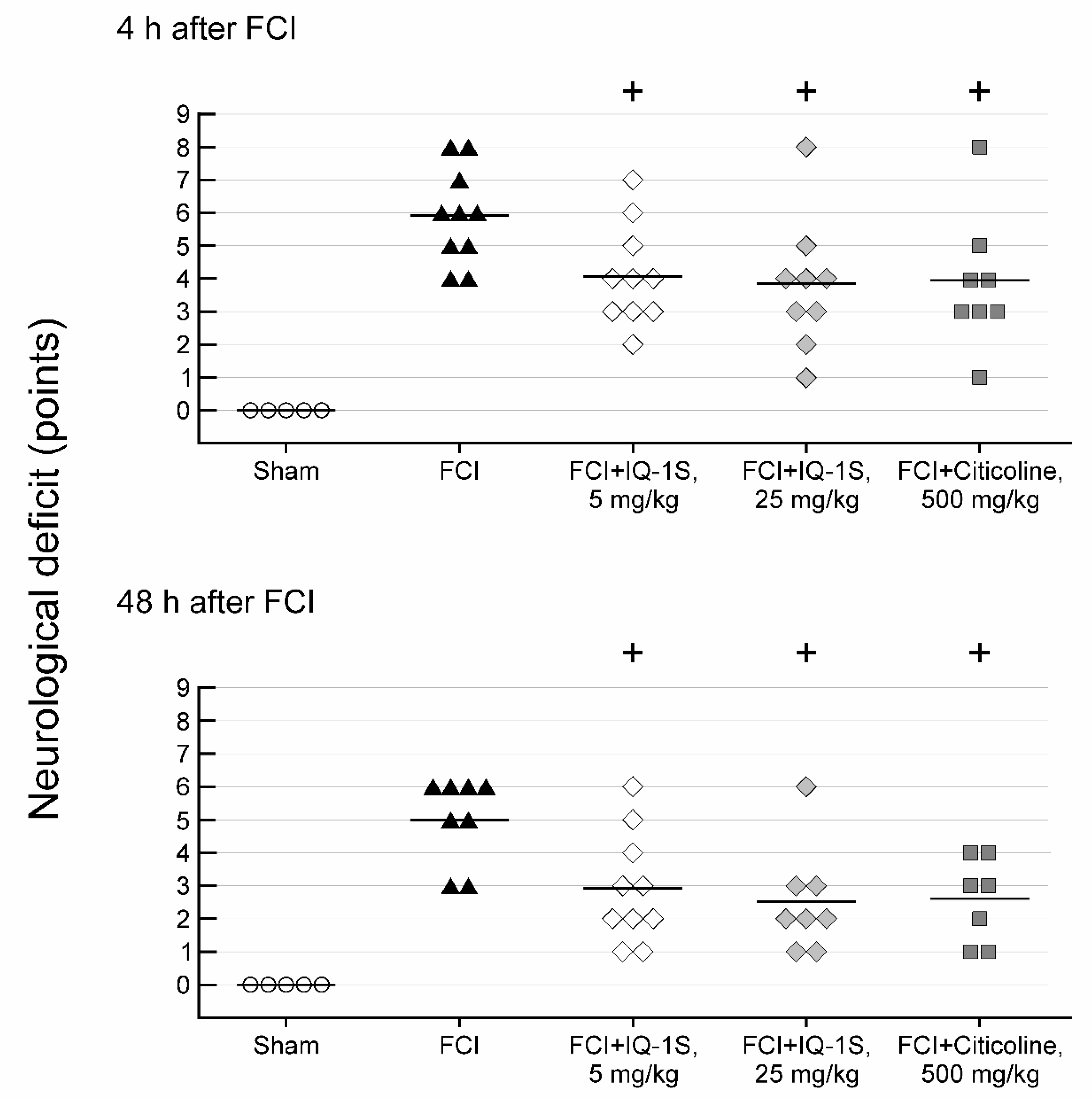
3.1. Effects of IQ-1S and Citicoline on Neurological Status in Rats with FCI
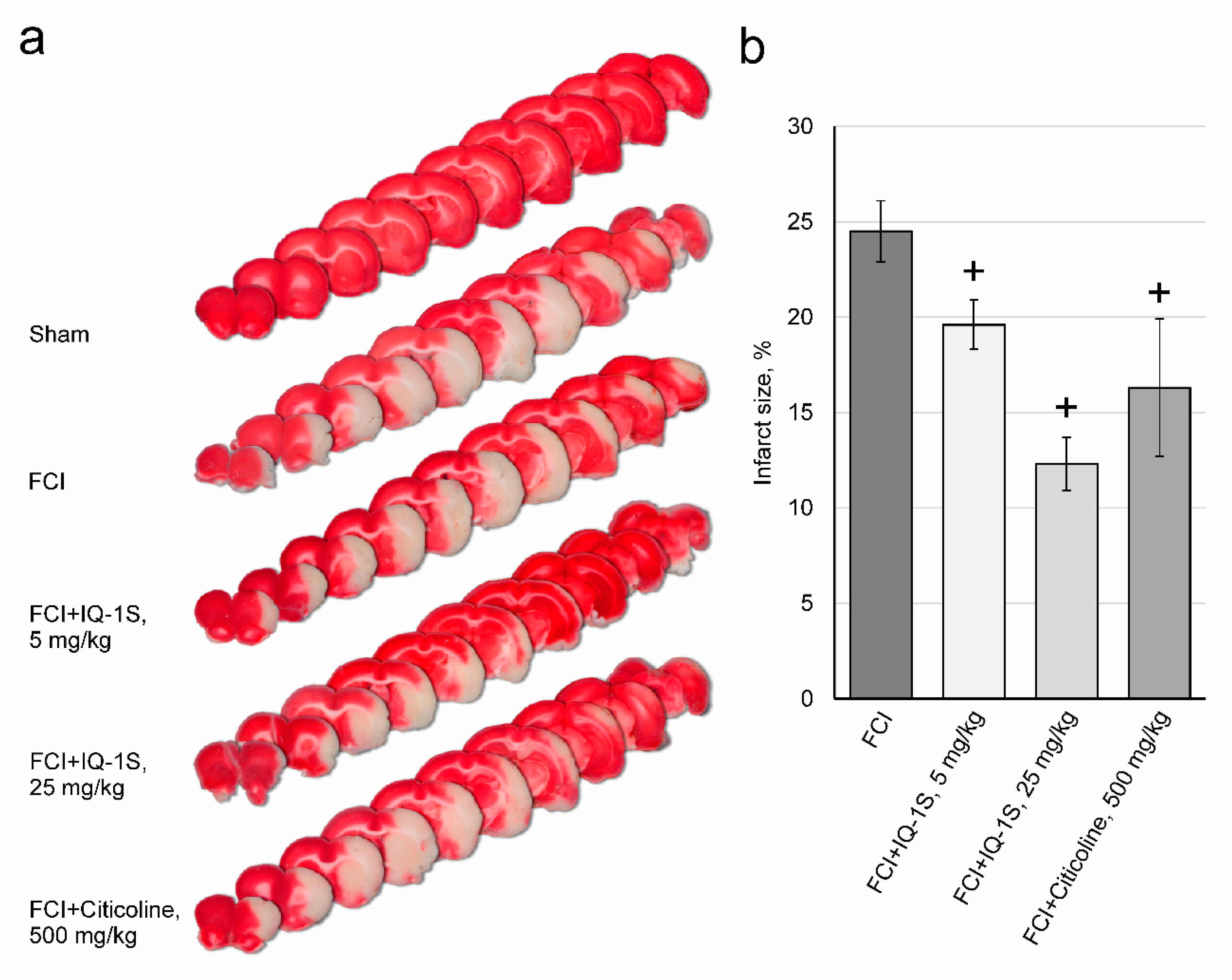
3.2. Effects of IQ-1S on Infarct Size in Rats with FCI
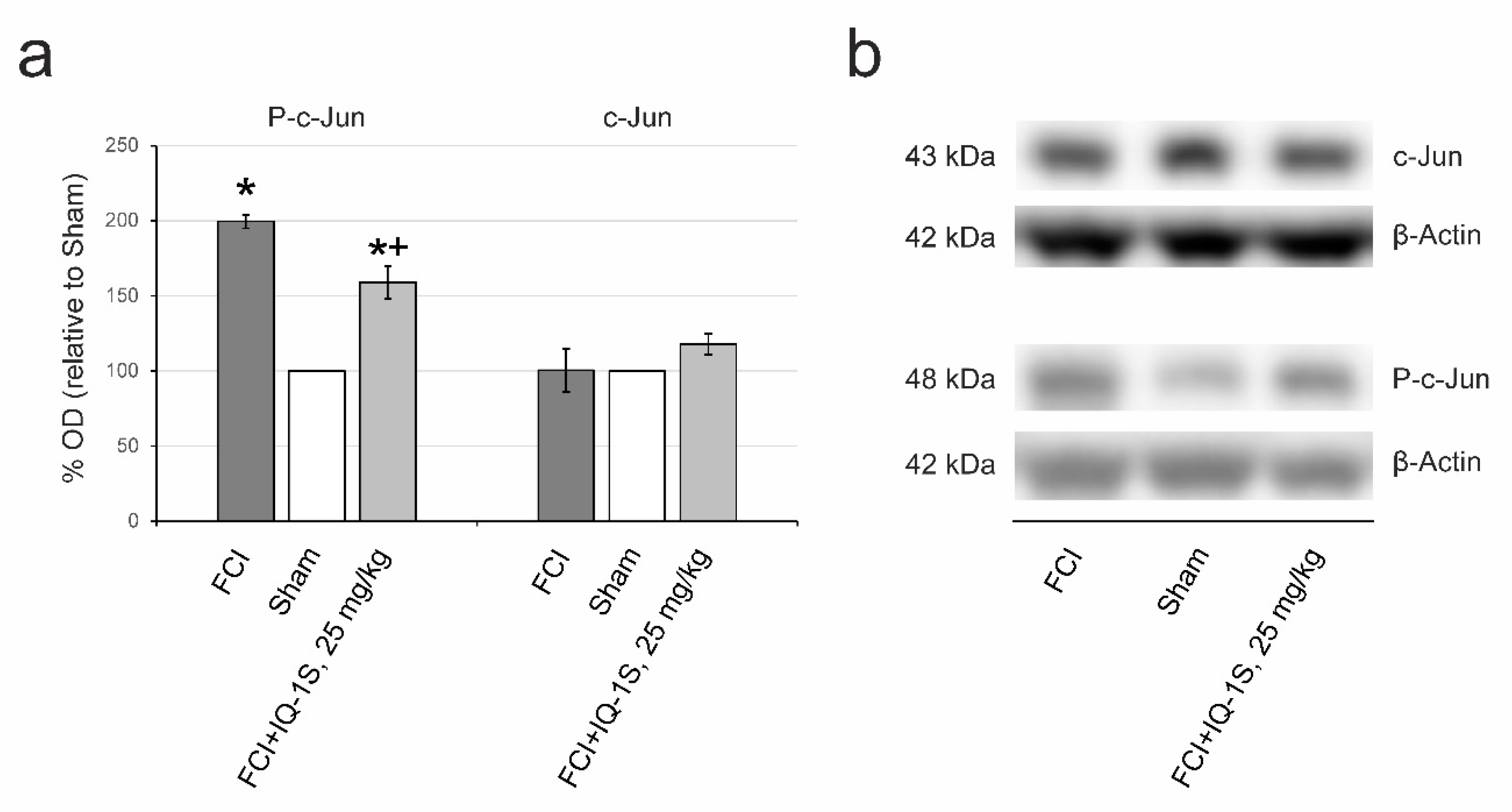
3.3. Inhibition of JNK
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, S.L.; Manhas, N.; Raghubir, R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007, 54, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Barrett, T.; Whitmarsh, A.J.; Cavanagh, J.; Sluss, H.K.; Dérijard, B.; Davis, R.J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996, 15, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Kawasaki, H.; Nishina, H. Diverse roles of JNK and MKK pathways in the brain. J. Signal. Transduct. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Brecht, S.; Kirchhof, R.; Chromik, A.; Willesen, M.; Nicolaus, T.; Raivich, G.; Wessig, J.; Waetzig, V.; Goetz, M.; Claussen, M.; et al. Specific pathophysiological functions of JNK isoforms in the brain. Eur. J. Neurosci. 2005, 21, 363–377. [Google Scholar] [CrossRef]
- Hu, B.R.; Liu, C.L.; Park, D.J. Alteration of MAP kinase pathways after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2000, 20, 1089–1095. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, H.; Qu, Y.; Xiao, X.; Zhu, Y. The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 2014, 1544, 45–53. [Google Scholar] [CrossRef]
- Gao, Y.; Signore, A.P.; Yin, W.; Cao, G.; Yin, X.M.; Sun, F.; Luo, Y.; Graham, S.H.; Chen, J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J. Cereb. Blood Flow Metab. 2005, 25, 694–712. [Google Scholar] [CrossRef]
- Murata, Y.; Fujiwara, N.; Seo, J.H.; Yan, F.; Liu, X.; Terasaki, Y.; Luo, Y.; Arai, K.; Ji, X.; Lo, E.H. Delayed inhibition of c-Jun N-terminal kinase worsens outcomes after focal cerebral ischemia. J. Neurosci. 2012, 32, 8112–8115. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Hanks, T.S.; Kochetkova, I.; Pascual, D.W.; Jutila, M.A.; Quinn, M.T. Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors. Mol. Pharmacol. 2012, 81, 832–845. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Hammaker, D.; Kochetkova, I.; Khlebnikov, A.I.; Lyakhov, S.A.; Firestein, G.S.; Quinn, M.T. Anti-inflammatory effects and joint protection in collagen-induced arthritis after treatment with IQ-1S, a selective c-Jun N-terminal kinase inhibitor. J. Pharmacol. Exp. Ther. 2015, 353, 505–516. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Atochin, D.N.; Zanoza, S.O.; Gaidarzhy, N.M.; Lyakhov, S.A.; et al. Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors. Eur. J. Med. Chem. 2018, 161, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Atochin, D.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Seledtsov, V.I.; Swanson, H.; Quinn, M.T.; Huang, P.L. A novel dual NO-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci. Lett. 2016, 618, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Chernysheva, G.A.; Aliev, O.I.; Smol’iakova, V.I.; Fomina, T.I.; Osipenko, A.N.; Rydchenko, V.S.; Anfinogenova, Y.J.; Khlebnikov, A.I.; Schepetkin, I.A.; et al. Protective effects of new c-Jun N-terminal kinase inhibitor in the model of global cerebral ischemia in rats. Molecules 2019, 24, 1722. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Malashchenko, V.V.; Meniailo, M.E.; Atochin, D.N.; Seledtsova, G.V.; Schepetkin, I.A. Inhibitory effect of IQ-1S, a selective c-Jun N-terminal kinase (JNK) inhibitor, on phenotypical and cytokine-producing characteristics in human macrophages and T-cells. Eur. J. Pharmacol. 2020, 878, 173116. [Google Scholar] [CrossRef]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H.; STAIR Group. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef]
- Pearson, B.D. Indenoquinolines. III. Derivatives of 11H-indeno-[1,2-b]quinoxaline and related indenoquinolines. J. Org. Chem. 1962, 27, 1674–1678. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Wang, W.; Gao, H. Rat model of focal cerebral ischemia in the dominant hemisphere. Int. J. Clin. Exp. Med. 2015, 8, 504–511. [Google Scholar]
- Nabatadze, N.; Woolley, C. Measurement of inositol triphosphate levels from rat hippocampal slices. Bio-Protocol 2016, 6, e1780. [Google Scholar] [CrossRef]
- Belayev, L. Overcoming Barriers to Translation from Experimental Stroke Models. In Translational Stroke Research; Lapchak, P., Zhang, J., Eds.; Springer: New York, NY, USA, 2012; pp. 493–524. [Google Scholar]
- Wang, N.Q.; Wang, L.Y.; Zhao, H.P.; Liu, P.; Wang, R.L.; Song, J.X.; Gao, L.; Ji, X.M.; Luo, Y.M. Luoyutong treatment promotes functional recovery and neuronal plasticity after cerebral ischemia-reperfusion injury in rats. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Yuliani, S.; Mustofa; Partadiredja, G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr. Neurosci. 2019, 22, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Martynov, M.Y.; Gusev, E.I. Current knowledge on the neuroprotective and neuroregenerative properties of citicoline in acute ischemic stroke. J. Exp. Pharmacol. 2015, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Xu, J.; Li, C.; Zhou, C.; Zhang, F.; Han, D.; Zhang, G.Y. Coupling between neuronal nitric oxide synthase and glutamate receptor 6-mediated c-Jun N-terminal kinase signaling pathway via S-nitrosylationcontributes to ischemia neuronal death. Neuroscience 2008, 155, 1120–1132. [Google Scholar] [CrossRef]
- Hu, S.Q.; Ye, J.S.; Zong, Y.Y.; Sun, C.C.; Liu, D.H.; Wu, Y.P.; Song, T.; Zhang, G.Y. S-nitrosylation of mixed lineage kinase 3 contributes to its activation after cerebral ischemia. J. Biol. Chem. 2012, 287, 2364–2377. [Google Scholar] [CrossRef]
- Pei, D.S.; Song, Y.J.; Yu, H.M.; Hu, W.W.; Du, Y.; Zhang, G.Y. Exogenous nitric oxide negatively regulates c-Jun N-terminal kinase activation via inhibiting endogenous NO-induced S-nitrosylation during cerebral ischemia and reperfusion in rat hippocampus. J Neurochem. 2008, 106, 1952–1963. [Google Scholar] [CrossRef]
- Margaill, I.; Plotkine, M.; Lerouet, D. Antioxidant strategies in the treatment of stroke. Free Radic. Biol. Med. 2005, 39, 429–443. [Google Scholar] [CrossRef]
- Green, A.R.; Ashwood, T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: Experimental and clinical studies with NXY-059 and free radical scavengers. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 109–118. [Google Scholar] [CrossRef]
- Rogalewski, A.; Schneider, A.; Ringelstein, E.B.; Schäbitz, W.R. Toward a multimodal neuroprotective treatment of stroke. Stroke 2006, 37, 1129–1136. [Google Scholar]
- Ahnstedt, H.; McCullough, L.D. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell Immunol. 2019, 345, 103960. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, R.; Zhu, X.; Smerin, D.; Zhong, Y.; Gu, L.; Fang, W.; Xiong, X. The involvement and therapy target of immune cells after ischemic stroke. Front. Immunol. 2019, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- Green, A.R.; Shuaib, A. Therapeutic strategies for the treatment of stroke. Drug Discov. Today 2006, 11, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, I.A.; Oslyakova, A.O.; Mikhailova, S.G. Microcirculation and blood rheology in patients with cerebrovascular disorders. Clin. Hemorheol. Microcirc. 2011, 49, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.Y.; Burke, R.E. Targeting the JNK signaling pathway for stroke and Parkinson’s diseases therapy. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 63–67. [Google Scholar] [CrossRef]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Hayashi, T.; Sakai, K.; Sasaki, C.; Zhang, W.R.; Warita, H.; Abe, K. c-Jun N-terminal kinase (JNK) and JNK interacting protein response in rat brain after transient middle cerebral artery occlusion. Neurosci. Lett. 2000, 284, 195–199. [Google Scholar] [CrossRef]
- Irving, E.A.; Bamford, M. Role of mitogen- and stress-activated kinases in ischemic injury. J. Cereb. Blood Flow Metab. 2002, 22, 631–647. [Google Scholar] [CrossRef]
- Borsello, T.; Clarke, P.G.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; Bogousslavsky, J.; Bonny, C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef]
- Ferrer, I.; Friguls, B.; Dalfó, E.; Planas, A.M. Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. ActaNeuropathol. 2003, 105, 425–437. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, Q.G.; Zhu, G.X.; Pei, D.S.; Guan, Q.H.; Zhang, G.Y. Activation of c-Jun NH2-terminal kinase 3 is mediated by the GluR6.PSD-95.MLK3 signaling module following cerebral ischemia in rat hippocampus. Brain Res. 2005, 1061, 57–66. [Google Scholar] [CrossRef]
- Jaros, F.; Straka, T.; Dobesová, Z.; Pintérová, M.; Chalupský, K.; Kunes, J.; Entlicher, G.; Zicha, J. Vasorelaxant activity of some oxime derivatives. Eur. J. Pharmacol. 2007, 575, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, Q.G.; Zhang, G.Y. The neuroprotective effects of K252a through inhibiting MLK3/MKK7/JNK3 signaling pathway on ischemic brain injury in rat hippocampal CA1 region. Neuroscience 2005, 131, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Carboni, S.; Boschert, U.; Gaillard, P.; Gotteland, J.P.; Gillon, J.Y.; Vitte, P.A. AS601245, a c-Jun NH2-terminal kinase (JNK) inhibitor, reduces axon/dendrite damage and cognitive deficits after global cerebral ischaemia in gerbils. Br. J. Pharmacol. 2008, 153, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Krenitsky, V.P.; Delgado, M.; Nadolny, L.; Sahasrabudhe, K.; Ayala, L.; Clareen, S.S.; Hilgraf, R.; Albers, R.; Kois, A.; Hughes, K.; et al. Aminopurine based JNK inhibitors for the prevention of ischemia reperfusion injury. Bioorg. Med. Chem. Lett. 2012, 22, 1427–1432. [Google Scholar] [CrossRef]
- Gehringer, M.; Muth, F.; Koch, P.; Laufer, S.A. c-Jun N-terminal kinase inhibitors: A patent review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 849–872. [Google Scholar] [CrossRef]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-terminal kinases and their modulators in myocardial ischemia/reperfusion injury. Front. Pharmacol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Carboni, S.; Hiver, A.; Szyndralewiez, C.; Gaillard, P.; Gotteland, J.P.; Vitte, P.A. AS601245 (1,3-benzothiazol-2-yl (2-[[2-(3-pyridinyl) ethyl] amino]-4 pyrimidinyl) acetonitrile): A c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J. Pharmacol. Exp. Ther. 2004, 310, 25–32. [Google Scholar] [CrossRef]
- Guan, Q.H.; Pei, D.S.; Liu, X.M.; Wang, X.T.; Xu, T.L.; Zhang, G.Y. Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res. 2006, 1092, 36–46. [Google Scholar] [CrossRef]
- Koch, P.; Gehringer, M.; Laufer, S.A. Inhibitors of c-Jun N-terminal kinases: An update. J. Med. Chem. 2015, 58, 72–95. [Google Scholar]
- Ginsberg, M.D. Life after cerovive: A personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke 2007, 38, 1967–1972. [Google Scholar] [CrossRef]
- Chernysheva, G.A.; Smolyakova, V.I.; Yanovskaya, E.A.; Plotnikiov, M.B. Pharmacokinetics of a novel specific inhibitor of c-Jun N-terminal kinases 11H-indeno[1,2-b]quinoxalin-11-one oxime sodium salt. Manuscript in preparation.
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikov, M.B.; Chernysheva, G.A.; Smolyakova, V.I.; Aliev, O.I.; Trofimova, E.S.; Sherstoboev, E.Y.; Osipenko, A.N.; Khlebnikov, A.I.; Anfinogenova, Y.J.; Schepetkin, I.A.; et al. Neuroprotective Effects of a Novel Inhibitor of c-Jun N-Terminal Kinase in the Rat Model of Transient Focal Cerebral Ischemia. Cells 2020, 9, 1860. https://doi.org/10.3390/cells9081860
Plotnikov MB, Chernysheva GA, Smolyakova VI, Aliev OI, Trofimova ES, Sherstoboev EY, Osipenko AN, Khlebnikov AI, Anfinogenova YJ, Schepetkin IA, et al. Neuroprotective Effects of a Novel Inhibitor of c-Jun N-Terminal Kinase in the Rat Model of Transient Focal Cerebral Ischemia. Cells. 2020; 9(8):1860. https://doi.org/10.3390/cells9081860
Chicago/Turabian StylePlotnikov, Mark B., Galina A. Chernysheva, Vera I. Smolyakova, Oleg I. Aliev, Eugene S. Trofimova, Eugene Y. Sherstoboev, Anton N. Osipenko, Andrei I. Khlebnikov, Yana J. Anfinogenova, Igor A. Schepetkin, and et al. 2020. "Neuroprotective Effects of a Novel Inhibitor of c-Jun N-Terminal Kinase in the Rat Model of Transient Focal Cerebral Ischemia" Cells 9, no. 8: 1860. https://doi.org/10.3390/cells9081860
APA StylePlotnikov, M. B., Chernysheva, G. A., Smolyakova, V. I., Aliev, O. I., Trofimova, E. S., Sherstoboev, E. Y., Osipenko, A. N., Khlebnikov, A. I., Anfinogenova, Y. J., Schepetkin, I. A., & Atochin, D. N. (2020). Neuroprotective Effects of a Novel Inhibitor of c-Jun N-Terminal Kinase in the Rat Model of Transient Focal Cerebral Ischemia. Cells, 9(8), 1860. https://doi.org/10.3390/cells9081860







