Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Compounds
2.2. Cell Lines and Culture
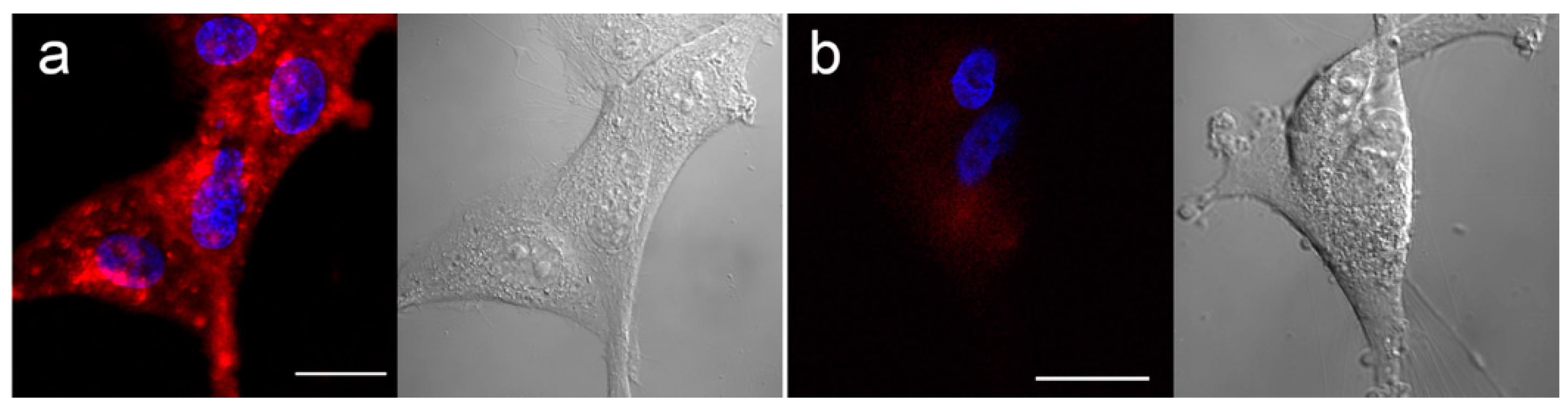
2.3. Fluorescence Microscopy
2.4. In Vivo Imaging Experiments
2.5. Neutron Exposure at the Institut Laue-Langevin
2.5.1. Neutron Beam Characteristics
2.5.2. BNCT Experiment on Cells
2.5.3. In Ovo BNCT Experiment
2.5.4. Statistical Analysis
2.6. Elemental Imaging Using Laser-Induced Breakdown Spectroscopy (LIBS)
2.7. In Ovo Fluorescence Imaging
2.8. Quantification of B Content by Inductively Coupled Plasma—Atomic Emission Spectrometry (ICP-AES)
3. Results
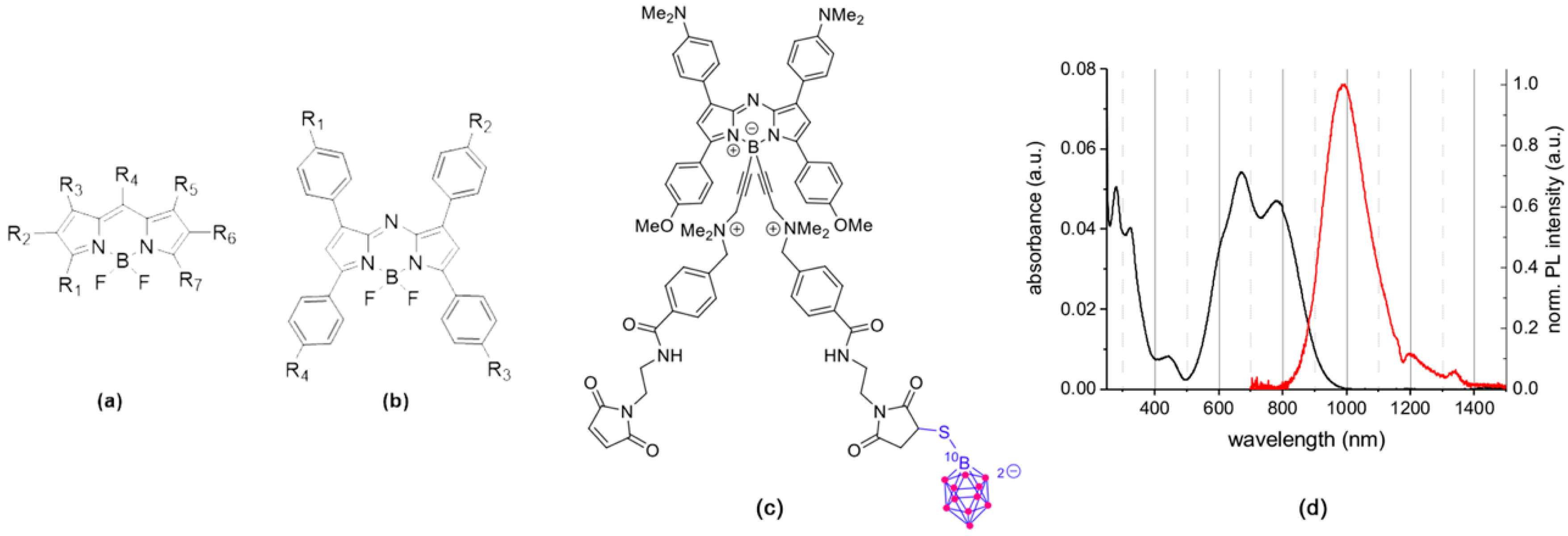
3.1. Rationale, Design, and Characterization of the Compounds
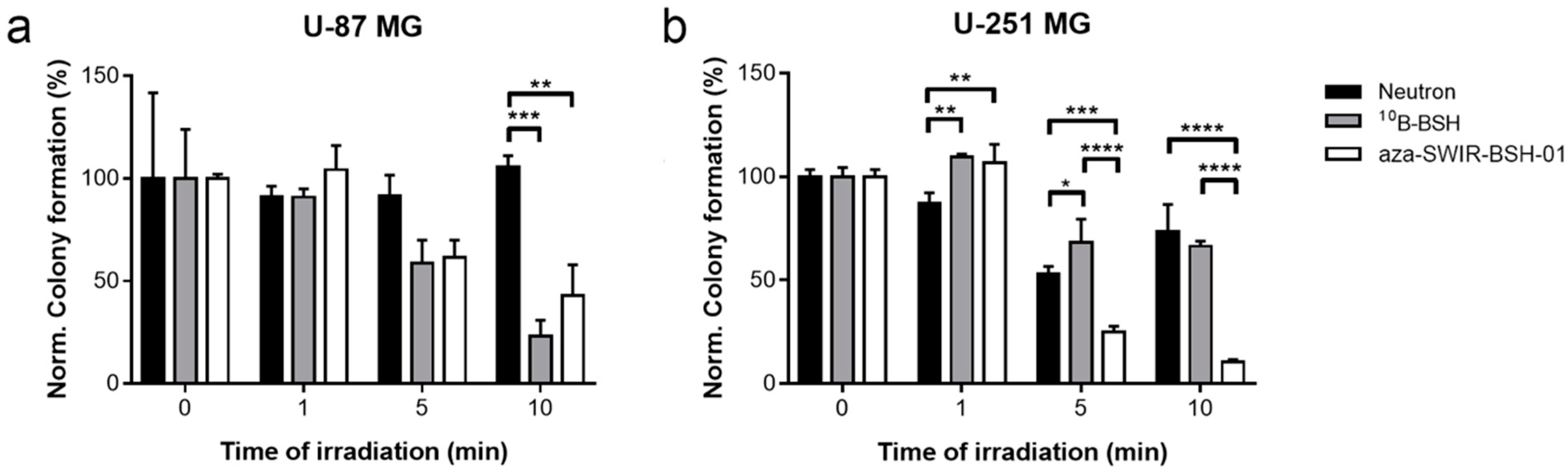
3.2. In Vitro Distribution and BNCT Efficacy
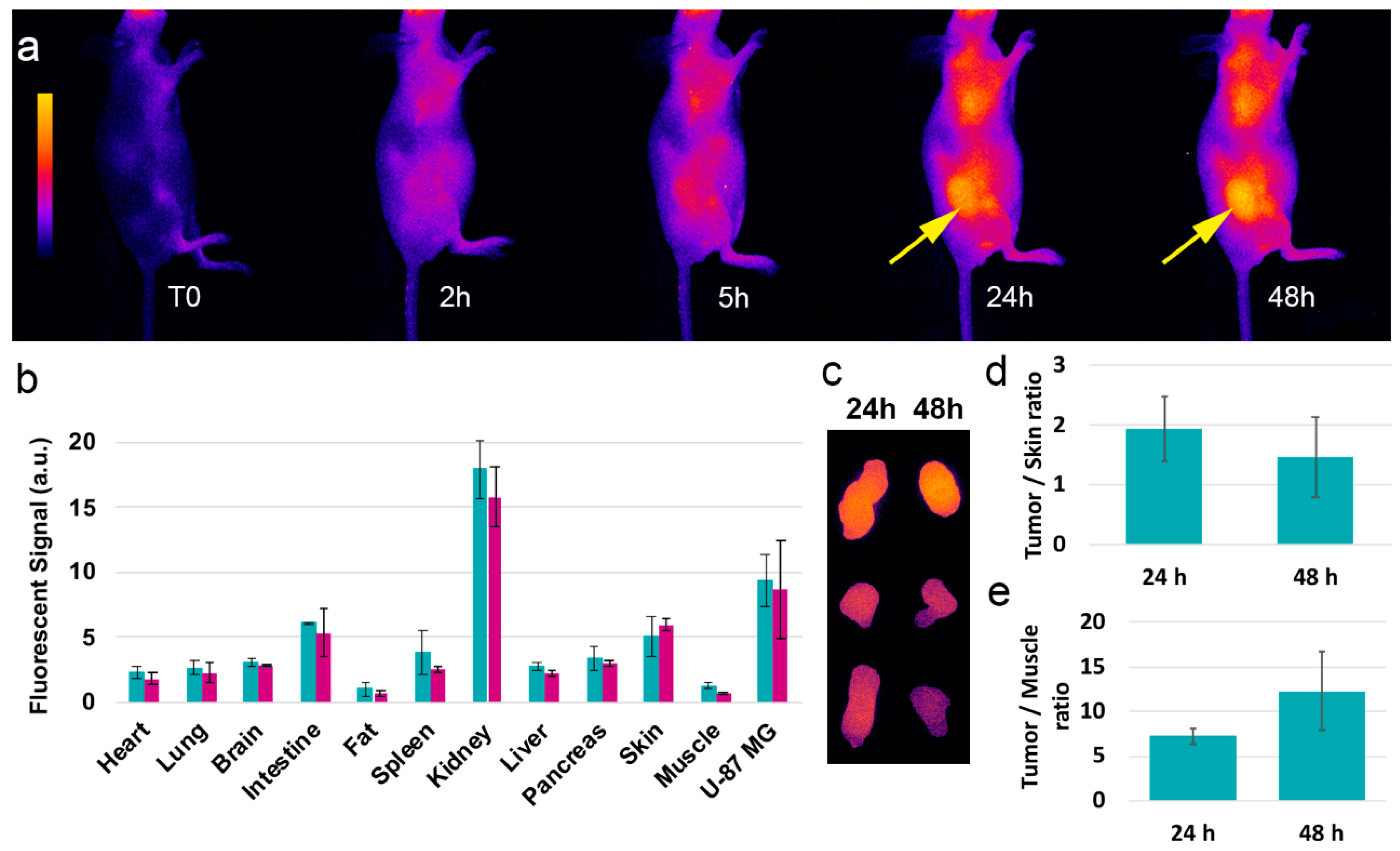
3.3. In Vivo Distribution and Behavior
3.4. In Ovo BNCT Assay and Distribution
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locher, G. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. Radium Ther. 1936, 36, 1–13. [Google Scholar]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aihara, T.; Morita, N.; Kamitani, N.; Kumada, H.; Ono, K.; Hiratsuka, J.; Harada, T. BNCT for advanced or recurrent head and neck cancer. Appl. Radiat. Isot. 2014, 88, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Aiyama, H.; Nakai, K.; Yamamoto, T.; Nariai, T.; Kumada, H.; Ishikawa, E.; Isobe, T.; Endo, K.; Takada, T.; Yoshida, F.; et al. A clinical trial protocol for second line treatment of malignant brain tumors with BNCT at University of Tsukuba. Appl. Radiat. Isot. 2011, 69, 1819–1822. [Google Scholar] [CrossRef]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef]
- Sauerwein, W.; Moss, R.; Rassow, J.; Stecher-Rasmussen, F.; Hideghety, K.; Wolbers, J.G.; Sack, H. Organisation and management of the first clinical trial of BNCT in Europe (EORTC protocol 11961).EORTC BNCT study group. Strahlenther. Onkol. 1999, 175 (Suppl. 2), 108–111. [Google Scholar] [CrossRef]
- Wang, L.W.; Chen, Y.W.; Ho, C.Y.; Hsueh Liu, Y.W.; Chou, F.I.; Liu, Y.H.; Liu, H.M.; Peir, J.J.; Jiang, S.H.; Chang, C.W.; et al. Fractionated BNCT for locally recurrent head and neck cancer: Experience from a phase I/II clinical trial at Tsing Hua Open-Pool Reactor. Appl. Radiat. Isot. 2014, 88, 23–27. [Google Scholar] [CrossRef]
- Haritz, D.; Gabel, D.; Huiskamp, R. Clinical phase-I study of Na2B12H11SH (BSH) in patients with malignant glioma as precondition for boron neutron capture therapy (BNCT). Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 1175–1181. [Google Scholar] [CrossRef]
- Takagaki, M.; Oda, Y.; Miyatake, S.; Kikuchi, H.; Kobayashi, T.; Sakurai, Y.; Osawa, M.; Mori, K.; Ono, K. Boron neutron capture therapy: Preliminary study of BNCT with sodium borocaptate (Na2B1 2H1 1SH) on glioblastoma. J. Neurooncol. 1997, 35, 177–185. [Google Scholar] [CrossRef]
- Gonzalez, S.J.; Bonomi, M.R.; Santa Cruz, G.A.; Blaumann, H.R.; Calzetta Larrieu, O.A.; Menendez, P.; Jimenez Rebagliati, R.; Longhino, J.; Feld, D.B.; Dagrosa, M.A.; et al. First BNCT treatment of a skin melanoma in Argentina: Dosimetric analysis and clinical outcome. Appl. Radiat. Isot. 2004, 61, 1101–1105. [Google Scholar] [CrossRef]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet 1989, 2, 388–389. [Google Scholar] [CrossRef]
- Kato, T.; Hirose, K.; Tanaka, H.; Mitsumoto, T.; Motoyanagi, T.; Arai, K.; Harada, T.; Takeuchi, A.; Kato, R.; Yajima, S.; et al. Design and construction of an accelerator-based boron neutron capture therapy (AB-BNCT) facility with multiple treatment rooms at the Southern Tohoku BNCT Research Center. Appl. Radiat. Isot. 2020, 156, 108961. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, A.J.; Baldo, M.; Bergueiro, J.R.; Cartelli, D.; Castell, W.; Thatar Vento, V.; Gomez Asoia, J.; Mercuri, D.; Padulo, J.; Suarez Sandin, J.C.; et al. Accelerator-based BNCT. Appl. Radiat. Isot. 2014, 88, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slatkin, D.N. A history of boron neutron capture therapy of brain tumours. Postulation of a brain radiation dose tolerance limit. Brain 1991, 114 Pt 4, 1609–1629. [Google Scholar] [CrossRef]
- Chandra, S.; Barth, R.F.; Haider, S.A.; Yang, W.; Huo, T.; Shaikh, A.L.; Kabalka, G.W. Biodistribution and subcellular localization of an unnatural boron-containing amino acid (cis-ABCPC) by imaging secondary ion mass spectrometry for neutron capture therapy of melanomas and gliomas. PLoS ONE 2013, 8, e75377. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Shaikh, A.L.; Barth, R.F.; Huo, T.; Yang, W.; Gordnier, P.M.; Chandra, S. Boronated unnatural cyclic amino acids as potential delivery agents for neutron capture therapy. Appl. Radiat. Isot. 2011, 69, 1778–1781. [Google Scholar] [CrossRef] [Green Version]
- Futamura, G.; Kawabata, S.; Nonoguchi, N.; Hiramatsu, R.; Toho, T.; Tanaka, H.; Masunaga, S.I.; Hattori, Y.; Kirihata, M.; Ono, K.; et al. Evaluation of a novel sodium borocaptate-containing unnatural amino acid as a boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat. Oncol. 2017, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Masunaga, S.; Harada, T.; Kawamura, Y.; Ueda, S.; Okuda, K.; Nagasawa, H. Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy. Bioorg. Med. Chem. 2011, 19, 1721–1728. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Lerchen, H.G.; Kobberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem. 2020, 85, 1446–1457. [Google Scholar] [CrossRef]
- Kellert, M.; Hoppenz, P.; Lonnecke, P.; Worm, D.J.; Riedl, B.; Koebberling, J.; Beck-Sickinger, A.G.; Hey-Hawkins, E. Tuning a modular system-synthesis and characterisation of a boron-rich s-triazine-based carboxylic acid and amine bearing a galactopyranosyl moiety. Dalton Trans. 2020, 49, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellert, M.; Worm, D.J.; Hoppenz, P.; Sarosi, M.B.; Lonnecke, P.; Riedl, B.; Koebberling, J.; Beck-Sickinger, A.G.; Hey-Hawkins, E. Modular triazine-based carborane-containing carboxylic acids-synthesis and characterisation of potential boron neutron capture therapy agents made of readily accessible building blocks. Dalton Trans. 2019, 48, 10834–10844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savolainen, S.; Kortesniemi, M.; Timonen, M.; Reijonen, V.; Kuusela, L.; Uusi-Simola, J.; Salli, E.; Koivunoro, H.; Seppala, T.; Lonnroth, N.; et al. Boron neutron capture therapy (BNCT) in Finland: Technological and physical prospects after 20 years of experiences. Phys. Med. 2013, 29, 233–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivunoro, H.; Hippelainen, E.; Auterinen, I.; Kankaanranta, L.; Kulvik, M.; Laakso, J.; Seppala, T.; Savolainen, S.; Joensuu, H. Biokinetic analysis of tissue boron ((1)(0)B) concentrations of glioma patients treated with BNCT in Finland. Appl. Radiat. Isot. 2015, 106, 189–194. [Google Scholar] [CrossRef]
- Atallah, I.; Milet, C.; Henry, M.; Josserand, V.; Reyt, E.; Coll, J.L.; Hurbin, A.; Righini, C.A. Near-infrared fluorescence imaging-guided surgery improves recurrence-free survival rate in novel orthotopic animal model of head and neck squamous cell carcinoma. Head Neck 2016, 38 (Suppl. 1), E246–E255. [Google Scholar] [CrossRef] [Green Version]
- Jacquart, A.; Keramidas, M.; Vollaire, J.; Boisgard, R.; Pottier, G.; Rustique, E.; Mittler, F.; Navarro, F.P.; Boutet, J.; Coll, J.L.; et al. LipImage 815: Novel dye-loaded lipid nanoparticles for long-term and sensitive in vivo near-infrared fluorescence imaging. J. Biomed. Opt. 2013, 18, 101311. [Google Scholar] [CrossRef]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef]
- Ge, Y.; O’Shea, D.F. Azadipyrromethenes: From traditional dye chemistry to leading edge applications. Chem. Soc. Rev. 2016, 45, 3846–3864. [Google Scholar] [CrossRef] [PubMed]
- Godard, A.; Kalot, G.; Pliquett, J.; Busser, B.; Le Guevel, X.; Wegner, K.D.; Resch-Genger, U.; Rousselin, Y.; Coll, J.L.; Denat, F.; et al. Water-Soluble Aza-BODIPYs: Biocompatible Organic Dyes for High Contrast In Vivo NIR-II Imaging. Bioconj. Chem. 2020, 31, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Bodio, E.; Denat, F.; Goze, C. BODIPYS and aza-BODIPY derivatives as promising fluorophores for in vivo molecular imaging and theranostic applications. J. Porphyr. Phthalocyanines 2019, 23, 1159–1183. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, B.; Passador, K.; Goze, C.; Denat, F.; Bodio, E.; Salmain, M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord. Chem. Rev. 2018, 358, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Lhenry, D.; Larrouy, M.; Bernhard, C.; Goncalves, V.; Raguin, O.; Provent, P.; Moreau, M.; Collin, B.; Oudot, A.; Vrigneaud, J.M.; et al. BODIPY: A Highly Versatile Platform for the Design of Bimodal Imaging Probes. Chem. Eur. J. 2015, 21, 13091–13099. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C.; Goze, C.; Rousselin, Y.; Denat, F. First bodipy-DOTA derivatives as probes for bimodal imaging. Chem. Commun. 2010, 46, 8267–8269. [Google Scholar] [CrossRef] [PubMed]
- Bodio, E.; Goze, C. Investigation of B-F substitution on BODIPY and aza-BODIPY dyes: Development of B-O and B-C BODIPYs. Dye. Pigment 2019, 160, 700–710. [Google Scholar] [CrossRef]
- Flores, O.; Pliquett, J.; Abad Galan, L.; Lescure, R.; Denat, F.; Maury, O.; Pallier, A.; Bellaye, P.S.; Collin, B.; Meme, S.; et al. Aza-BODIPY Platform: Toward an Efficient Water-Soluble Bimodal Imaging Probe for MRI and Near-Infrared Fluorescence. Inorg. Chem. 2020, 59, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, J.; Amor, S.; Ponce-Vargas, M.; Laly, M.; Racoeur, C.; Rousselin, Y.; Denat, F.; Bettaieb, A.; Fleurat-Lessard, P.; Paul, C.; et al. Design of a multifunctionalizable BODIPY platform for the facile elaboration of a large series of gold(i)-based optical theranostics. Dalton Trans. 2018, 47, 11203–11218. [Google Scholar] [CrossRef]
- Flores, O.; Trommenschlager, A.; Amor, S.; Marques, F.; Silva, F.; Gano, L.; Denat, F.; Cabral Campello, M.; Goze, C.; Bodio, E.; et al. In vitro and in vivo trackable titanocene-based complexes using optical imaging or SPECT. Dalton Trans. 2017, 46, 14548–14555. [Google Scholar] [CrossRef]
- Xuan, S.; Zhao, N.; Zhou, Z.; Fronczek, F.R.; Vicente, M.G. Synthesis and in Vitro Studies of a Series of Carborane-Containing Boron Dipyrromethenes (BODIPYs). J. Med. Chem. 2016, 59, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Wang, H.; Bhupathiraju, N.V.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G. Synthesis and properties of a series of carboranyl-BODIPYs. J. Organomet. Chem. 2015, 798, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Nakata, E.; Koizumi, M.; Yamashita, Y.; Onaka, K.; Sakurai, Y.; Kondo, N.; Ono, K.; Uto, Y.; Hori, H. Design, synthesis and destructive dynamic effects of BODIPY-containing and curcuminoid boron tracedrugs for neutron dynamic therapy. Anticancer Res. 2011, 31, 2477–2481. [Google Scholar] [PubMed]
- Musnier, B.; Wegner, K.D.; Comby-Zerbino, C.; Trouillet, V.; Jourdan, M.; Hausler, I.; Antoine, R.; Coll, J.L.; Resch-Genger, U.; Le Guevel, X. High photoluminescence of shortwave infrared-emitting anisotropic surface charged gold nanoclusters. Nanoscale 2019, 11, 12092–12096. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, S.; Yang, N.D.; Zhang, C.; Wu, Q.; Yu, C. Recent development of small-molecule organic fluorophores for multifunctional bioimaging in the second near-infrared window. J. Lumin. 2020, 225, 117338. [Google Scholar] [CrossRef]
- Sancey, L.; Motto-Ros, V.; Kotb, S.; Wang, X.; Lux, F.; Panczer, G.; Yu, J.; Tillement, O. Laser-induced breakdown spectroscopy: A new approach for nanoparticle’s mapping and quantification in organ tissue. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancey, L.; Motto-Ros, V.; Busser, B.; Kotb, S.; Benoit, J.M.; Piednoir, A.; Lux, F.; Tillement, O.; Panczer, G.; Yu, J. Laser spectrometry for multi-elemental imaging of biological tissues. Sci. Rep. 2014, 4, 6065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez, Y.; Busser, B.; Trichard, F.; Kulesza, A.; Laurent, J.M.; Zaun, V.; Lux, F.; Benoit, J.M.; Panczer, G.; Dugourd, P.; et al. 3D Imaging of Nanoparticle Distribution in Biological Tissue by Laser-Induced Breakdown Spectroscopy. Sci. Rep. 2016, 6, 29936. [Google Scholar] [CrossRef]
- Busser, B.; Moncayo, S.; Trichard, F.; Bonneterre, V.; Pinel, N.; Pelascini, F.; Dugourd, P.; Coll, J.L.; D’Incan, M.; Charles, J.; et al. Characterization of foreign materials in paraffin-embedded pathological specimens using in situ multi-elemental imaging with laser spectroscopy. Mod. Pathol. 2018, 31, 378–384. [Google Scholar] [CrossRef]
- Pliquett, J.; Dubois, A.; Racoeur, C.; Mabrouk, N.; Amor, S.; Lescure, R.; Bettaieb, A.; Collin, B.; Bernhard, C.; Denat, F.; et al. A Promising Family of Fluorescent Water-Soluble aza-BODIPY Dyes for in Vivo Molecular Imaging. Bioconj. Chem. 2019, 30, 1061–1066. [Google Scholar] [CrossRef]
- Abele, H.; Dubbers, D.; Haese, H.; Klein, M.; Knoepfler, A.; Kreuz, M.; Lauer, T.; Maerkisch, B.; Mund, D.; Nesvizhevsky, V.; et al. Characterization of a ballistic supermirror neutron guide. Nucl. Instrum. Methods Phys. Res. Sect. A 2006, 562, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa-Rivera, M.; Ruiz-Magana, M.J.; Porroas, I.; Praena, J.; Torres-Sanchez, P.; Sabariego, M.P.; Köester, U.; Forsyth, T.; Solden, T.; Haertlein, M.; et al. Neutron radiobiology studies with a pure cold neutron beam. Nucl. Instrum. Methods Phys. Res. Sect. B 2020, 462, 24–31. [Google Scholar] [CrossRef]
- Hideghety, K.; Sauerwein, W.; Wittig, A.; Gotz, C.; Paquis, P.; Grochulla, F.; Haselsberger, K.; Wolbers, J.; Moss, R.; Huiskamp, R.; et al. Tissue uptake of BSH in patients with glioblastoma in the EORTC 11961 phase I BNCT trial. J. Neurooncol. 2003, 62, 145–156. [Google Scholar] [CrossRef]
- Busser, B.; Moncayo, S.; Coll, J.L.; Sancey, L.; Motto-Ros, V. Elemental imaging using laser-induced breakdown spectroscopy: A new and promising approach for biological and medical applications. Coord. Chem. Rev. 2018, 358, 70–79. [Google Scholar] [CrossRef]
- Yokoyama, K.; Miyatake, S.; Kajimoto, Y.; Kawabata, S.; Doi, A.; Yoshida, T.; Asano, T.; Kirihata, M.; Ono, K.; Kuroiwa, T. Pharmacokinetic study of BSH and BPA in simultaneous use for BNCT. J. Neurooncol. 2006, 78, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tamanoi, F. Recent excitements in the study of the CAM assay. Enzymes 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Steiner, R. Angiostatic activity of anticancer agents in the chick embryo chorioallantoic membrane (CHE-CAM) assay. EXS 1992, 61, 449–454. [Google Scholar] [CrossRef]
- Tanaka, N.G.; Sakamoto, N.; Tohgo, A.; Nishiyama, Y.; Ogawa, H. Inhibitory effects of anti-angiogenic agents on neovascularization and growth of the chorioallantoic membrane (CAM). The possibility of a new CAM assay for angiogenesis inhibition. Exp. Pathol. 1986, 30, 143–150. [Google Scholar] [CrossRef]
- Dunker, N.; Jendrossek, V. Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research. Cancers 2019, 11, 1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Moon, Y.H.; Kim, D.J.; Kim, S.A.; Lee, J.B.; Ahn, S.G.; Yoon, J.H. Photodynamic therapy with hexenyl ester of 5-aminolevulinic acid induces necrotic cell death in salivary gland adenocarcinoma cells. Oncol. Rep. 2010, 24, 177–181. [Google Scholar]
- Zuo, Z.; Syrovets, T.; Wu, Y.; Hafner, S.; Vernikouskaya, I.; Liu, W.; Ma, G.; Weil, T.; Simmet, T.; Rasche, V. The CAM cancer xenograft as a model for initial evaluation of MR labelled compounds. Sci. Rep. 2017, 7, 46690. [Google Scholar] [CrossRef] [Green Version]
- Warnock, G.; Turtoi, A.; Blomme, A.; Bretin, F.; Bahri, M.A.; Lemaire, C.; Libert, L.C.; Seret, A.E.; Luxen, A.; Castronovo, V.; et al. In vivo PET/CT in a human glioblastoma chicken chorioallantoic membrane model: A new tool for oncology and radiotracer development. J. Nucl. Med. 2013, 54, 1782–1788. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalot, G.; Godard, A.; Busser, B.; Pliquett, J.; Broekgaarden, M.; Motto-Ros, V.; Wegner, K.D.; Resch-Genger, U.; Köster, U.; Denat, F.; et al. Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications. Cells 2020, 9, 1953. https://doi.org/10.3390/cells9091953
Kalot G, Godard A, Busser B, Pliquett J, Broekgaarden M, Motto-Ros V, Wegner KD, Resch-Genger U, Köster U, Denat F, et al. Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications. Cells. 2020; 9(9):1953. https://doi.org/10.3390/cells9091953
Chicago/Turabian StyleKalot, Ghadir, Amélie Godard, Benoît Busser, Jacques Pliquett, Mans Broekgaarden, Vincent Motto-Ros, Karl David Wegner, Ute Resch-Genger, Ulli Köster, Franck Denat, and et al. 2020. "Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications" Cells 9, no. 9: 1953. https://doi.org/10.3390/cells9091953
APA StyleKalot, G., Godard, A., Busser, B., Pliquett, J., Broekgaarden, M., Motto-Ros, V., Wegner, K. D., Resch-Genger, U., Köster, U., Denat, F., Coll, J.-L., Bodio, E., Goze, C., & Sancey, L. (2020). Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications. Cells, 9(9), 1953. https://doi.org/10.3390/cells9091953










