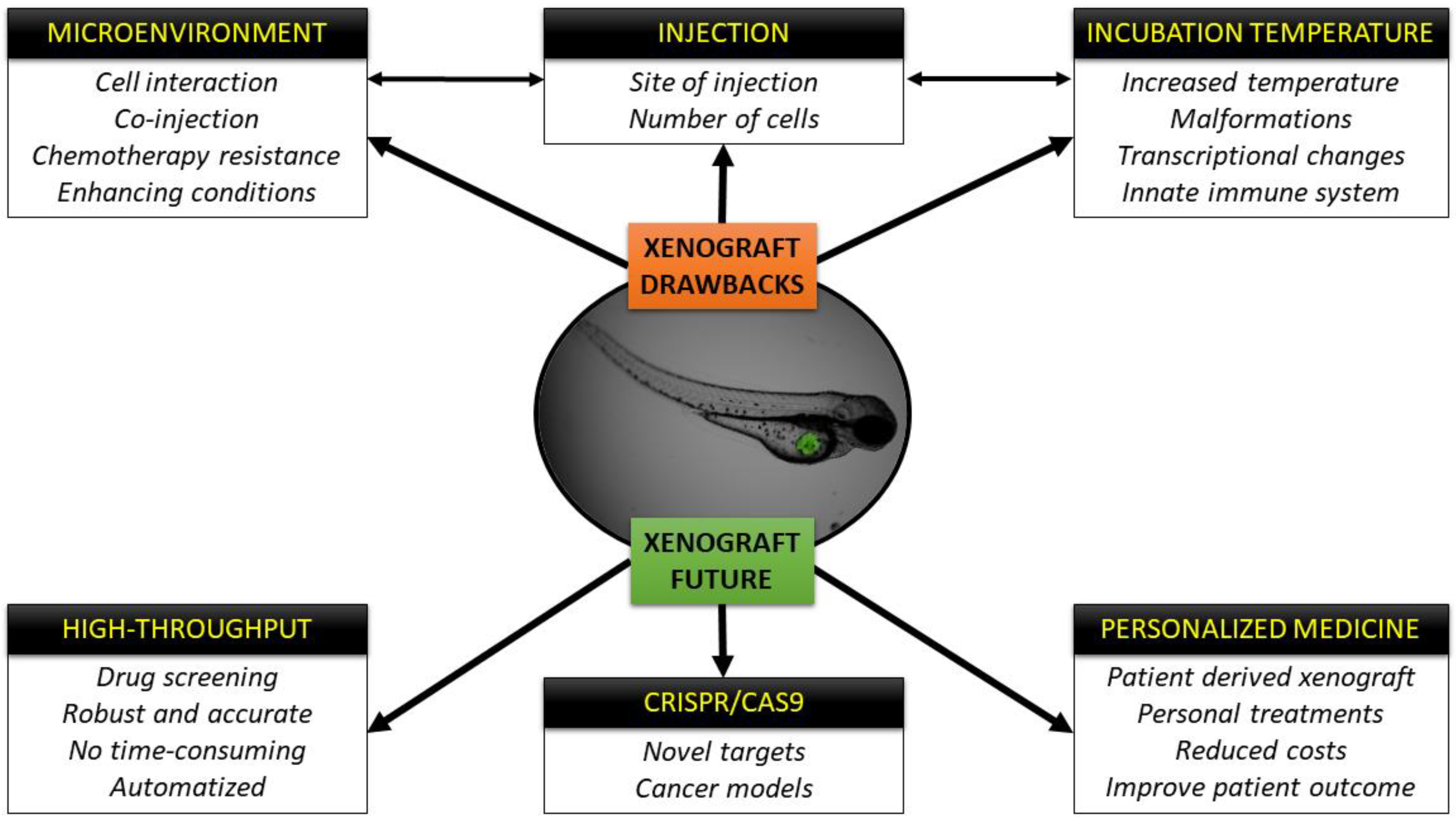
Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment
Abstract
1. From the Problem to the Solution: Cancer and Personalized Medicine
1.1. The Objective of the Modeling: Cancer
1.2. A Powerful Tool for Modeling Cancer: Zebrafish
1.2.1. Modeling Cancer through Xenotransplantation
- The zebrafish adaptive immune system is not mature until 4-6 weeks post-fertilization, and during the first 12–14 days of development, only innate immune cells are present. Thus, during the first 2 weeks post birth, induced immunosuppression is unnecessary, and cancer cells can efficiently survive, proliferate, and metastasize in an unaltered host, and even communicate and polarize innate immune cells such as macrophages [22,23].
- Human cancer cells can communicate with the zebrafish embryo cells due to the conserved cell intercommunication shared between these two species. Cell–host interactions, such as the interaction between cancer cells and immune system, are active as can be inferred from neutrophil and macrophage recruitment to the tumor area [32].
(A) Yolk Sac
(B) Duct of Cuvier (Common Cardinal Vein)
(C) Perivitelline Space
(D) Intraperitoneal Cavity
1.2.2. Every Powerful Tool Has Its Own Drawbacks
2. The Cells and the Host: Is There a Perfect Temperature for Both?
2.1. Finding a Balance between Time and Temperature
2.2. Temperature in the Host: Are the Embryos Suffering Hyperthermia?
2.2.1. Is the Morphology and Mortality of the Embryo Influenced by Temperature?
2.2.2. How Are the Embryos Reacting to the Temperature at the Transcriptomic Level?
2.3. Chemotherapeutic Compounds Trials: Does Temperature Matter?
3. Microenvironment
3.1. CAFS, Endothelial Cells, and Perycites
3.2. TAMs
3.3. Mimicking the Tumor Microenvironment
3.3.1. Interaction between Tumor and Immune Cells—Zebrafish Transgenic Lines
3.3.2. Mimicking the Tissue Niche—Orthotopic Xenografts
3.3.3. Addressing Intratumoral Heterogeneity—Zebrafish PDX (zPDX)
4. The Future of the Zebrafish Xenografts Is Already Here
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAFs | Cancer associated fibroblasts |
| CHT | Caudal hematopoietic tissue |
| CSCs | Cancer stem cells |
| CTCs | Circulating tumor cells |
| CR | Conditional reprogrammed |
| Crispr/Cas9 | Clustered regularly interspaced short palindromic repeats/Cas9 |
| CSF-1 | Colony stimulating factor 1 |
| CSF1R | Colony stimulating factor 1 receptor |
| dpi | Days post-fertilization |
| EGFR | Epidermal growth factor receptor |
| EGF | Epithelial growth factor |
| EMT | Epithelial-mesenchymal transition |
| ECM | Extracellular matrix |
| FAP | Fibroblast-activating protein |
| GPF | Green fluorescent protein |
| hpf | Hours post-fertilization |
| hpi | Hours post-injection |
| IGF-1 | Insulin-like growth factor |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| M-CSF | Macrophage stimulation factor-1 |
| MET | Mesenchymal-epithelial transition |
| NET | Neuroendocrine tumors |
| NO | Nitric oxide |
| PDX | Patient-derived xenograft |
| RFP | Red fluorescent protein |
| SIVs | Sub-intestinal vessels |
| TEMs | Tie2-expressing macrophages |
| TGF-β | Transforming growth factor- β |
| TAMs | Tumor associated macrophages |
| VEGF | Vascular endothelial growth factor |
| WT | Wild-type |
| zPDX | Zebrafish patient-derived xenograft |
References
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Dotto, G.-P. Racial differences in cancer susceptibility and survival: More than the color of the skin? Trends Cancer 2017, 3, 181–197. [Google Scholar] [CrossRef]
- Li, S.; Garrett-Bakelman, F.E.; Chung, S.S.; Sanders, M.A.; Hricik, T.; Rapaport, F.; Patel, J.; Dillon, R.; Vijay, P.; Brown, A.L.; et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat. Med. 2016, 22, 792–799. [Google Scholar] [CrossRef]
- Almendro, V.; Marusyk, A.; Polyak, K. Cellular heterogeneity and molecular evolution in cancer. Annu. Rev. Pathol. 2013, 8, 277–302. [Google Scholar] [CrossRef]
- Li, J.; Ge, W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Mol. Cell. Endocrinol. 2020, 507, 110778. [Google Scholar] [CrossRef] [PubMed]
- Cagan, R.L.; Zon, L.I.; White, R.M. Modeling Cancer with Flies and Fish. Dev. Cell 2019, 49, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Estrada, M.F.; Mendes, R.V.; Fior, R. Zebrafish Avatars towards Personalized Medicine—A Comparative Review between Avatar Models. Cells 2020, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Malaney, P.; Nicosia, S.V.; Dave, V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014, 344, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, F. An Account of the Fishes Found in the River Ganges and Its Branches; Bishen Singh Mahendra Pal Singh: Dehra Dun, India, 1822. [Google Scholar]
- Brown, J.M.; Recht, L.; Strober, S. The promise of targeting macrophages in cancer therapy. Clin. Cancer Res. 2017, 23, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Kirchberger, S.; Sturtzel, C.; Pascoal, S.; Distel, M. Quo natas, Danio?—Recent progress in modeling cancer in zebrafish. Front. Oncol. 2017, 7, 186. [Google Scholar] [CrossRef]
- Zon, L.I. Zebrafish: A new model for human disease. Genome Res. 1999, 9, 99–100. [Google Scholar]
- Konantz, M.; Balci, T.B.; Hartwig, U.F.; Dellaire, G.; André, M.C.; Berman, J.N.; Lengerke, C. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann. N. Y. Acad. Sci. 2012, 1266, 124–137. [Google Scholar] [CrossRef]
- Kozol, R.A.; Abrams, A.J.; James, D.M.; Buglo, E.; Yan, Q.; Dallman, J.E. Function Over Form: Modeling Groups of Inherited Neurological Conditions in Zebrafish. Front. Mol. Neurosci. 2016, 9, 55. [Google Scholar] [CrossRef]
- Lam, S.; Chua, H.; Gong, Z.; Lam, T.; Sin, Y. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Trede, N.S. A model 450 million years in the making: Zebrafish and vertebrate immunity. Dis. Models Mech. 2012, 5, 38–47. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Ye, J. A fresh look at zebrafish from the perspective of cancer research. J. Exp. Clin. Cancer Res. 2015, 34, 80. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R. The new age of chemical screening in zebrafish. Zebrafish 2010, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.; Kim, C.H. Innate Immune System of the Zebrafish, Danio rerio. In Innate Immunity of Plants, Animals, and Humans; Heine, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 113–133. [Google Scholar] [CrossRef]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Nicoli, S.; Ribatti, D.; Cotelli, F.; Presta, M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007, 67, 2927–2931. [Google Scholar] [CrossRef]
- Nicoli, S.; Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007, 2, 2918. [Google Scholar] [CrossRef]
- Lee, L.M.; Seftor, E.A.; Bonde, G.; Cornell, R.A.; Hendrix, M.J. The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 1560–1570. [Google Scholar] [CrossRef]
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9, 139–151. [Google Scholar] [CrossRef]
- Tulotta, C.; He, S.; Chen, L.; Groenewoud, A.; van der Ent, W.; Meijer, A.H.; Spaink, H.P.; Snaar-Jagalska, B.E. Imaging of human cancer cell proliferation, invasion, and micrometastasis in a zebrafish xenogeneic engraftment model. In Zebrafish; Kawakami, K., Pattons, E.E., Orger, M., Eds.; Springer: New York, NY, USA, 2016; pp. 155–169. [Google Scholar]
- Ikonomopoulou, M.P.; Fernandez-Rojo, M.A.; Pineda, S.S.; Cabezas-Sainz, P.; Winnen, B.; Morales, R.A.; Brust, A.; Sánchez, L.; Alewood, P.F.; Ramm, G.A. Gomesin inhibits melanoma growth by manipulating key signaling cascades that control cell death and proliferation. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Fior, R.; Póvoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243. [Google Scholar] [CrossRef] [PubMed]
- Fraher, D.; Sanigorski, A.; Mellett, N.A.; Meikle, P.J.; Sinclair, A.J.; Gibert, Y. Zebrafish Embryonic Lipidomic Analysis Reveals that the Yolk Cell Is Metabolically Active in Processing Lipid. Cell Rep. 2016, 14, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Veinotte, C.J.; Dellaire, G.; Berman, J.N. Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis. Models Mech. 2014, 7, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Mercatali, L.; La Manna, F.; Groenewoud, A.; Casadei, R.; Recine, F.; Miserocchi, G.; Pieri, F.; Liverani, C.; Bongiovanni, A.; Spadazzi, C. Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. Int. J. Mol. Sci. 2016, 17, 1375. [Google Scholar] [CrossRef]
- Drabsch, Y.; He, S.; Zhang, L.; Snaar-Jagalska, B.E.; ten Dijke, P. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013, 15, R106. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lamers, G.E.; Beenakker, J.-W.M.; Cui, C.; Ghotra, V.P.; Danen, E.H.; Meijer, A.H.; Spaink, H.P.; Snaar-Jagalska, B.E. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012, 227, 431–445. [Google Scholar] [CrossRef]
- Hill, D.; Chen, L.; Snaar-Jagalska, E.; Chaudhry, B. Embryonic zebrafish xenograft assay of human cancer metastasis. F1000Research 2018, 7, 1682. [Google Scholar] [CrossRef]
- Drabsch, Y.; Snaar-Jagalska, B.E.; Ten Dijke, P. Fish tales: The use of zebrafish xenograft human cancer cell models. Histol. Histopathol. 2017, 32, 673–686. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin. Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef]
- Tang, Q.; Abdelfattah, N.S.; Blackburn, J.S.; Moore, J.C.; Martinez, S.A.; Moore, F.E.; Lobbardi, R.; Tenente, I.M.; Ignatius, M.S.; Berman, J.N. Optimized cell transplantation using adult rag2 mutant zebrafish. Nat. Methods 2014, 11, 821–824. [Google Scholar] [CrossRef]
- Stoletov, K.; Montel, V.; Lester, R.D.; Gonias, S.L.; Klemke, R. High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 17406–17411. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mahajan, N.K.; Sinha, P.; Jayandharan, G.R. An efficient method to generate xenograft tumor models of acute myeloid leukemia and hepatocellular carcinoma in adult zebrafish. Blood Cells Mol. Dis. 2019, 75, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Li, L.; Li, Q.; He, X.; Cui, Z. Transcriptomic characterization of temperature stress responses in larval zebrafish. PLoS ONE 2012, 7, e37209. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Cao, Z.; Hosaka, K.; Jensen, L.; Yang, H.; Sun, Y.; Zhuang, R.; Liu, Y.; Cao, Y. Invasiveness and metastasis of retinoblastoma in an orthotopic zebrafish tumor model. Sci. Rep. 2015, 5, 10351. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.; Charan, M.; Welker, A.M.; Dravid, P.; Studebaker, A.W.; Leonard, J.R.; Pierson, C.R.; Nakano, I.; Beattie, C.E.; Hwang, E.I.; et al. DeltaNp73/ETS2 complex drives glioblastoma pathogenesis- targeting downstream mediators by rebastinib prolongs survival in preclinical models of glioblastoma. Neuro Oncol. 2020, 22, 345–356. [Google Scholar] [CrossRef]
- Sounni, N.E.; Noel, A. Targeting the tumor microenvironment for cancer therapy. Clin. Chem. 2013, 59, 85–93. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for The Laboratory Use of Zebrafish. 2000. Available online: http://zfin.org/zf_info/zfbook/zfbk.html (accessed on 21 November 2019).
- Dakappa, P.H.; Mahabala, C. Analysis of long-term temperature variations in the human body™. Crit. Rev. Biomed. Eng. 2015, 43, 385–399. [Google Scholar] [CrossRef]
- Eguiara, A.; Holgado, O.; Beloqui, I.; Abalde, L.; Sanchez, Y.; Callol, C.; Martin, A.G. Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification. Cell Cycle 2011, 10, 3751–3757. [Google Scholar] [CrossRef]
- Ghotra, V.P.; He, S.; De Bont, H.; van Der Ent, W.; Spaink, H.P.; van De Water, B.; Snaar-Jagalska, B.E.; Danen, E.H. Automated whole animal bio-imaging assay for human cancer dissemination. PLoS ONE 2012, 7, e31281. [Google Scholar] [CrossRef]
- Ban, J.; Aryee, D.N.; Fourtouna, A.; Van Der Ent, W.; Kauer, M.; Niedan, S.; Machado, I.; Rodriguez-Galindo, C.; Tirado, O.M.; Schwentner, R. Suppression of deacetylase SIRT1 mediates tumor-suppressive NOTCH response and offers a novel treatment option in metastatic Ewing sarcoma. Cancer Res. 2014, 74, 6578–6588. [Google Scholar] [CrossRef]
- Van der Ent, W.; Burrello, C.; de Lange, M.J.; van der Velden, P.A.; Jochemsen, A.G.; Jager, M.J.; Snaar-Jagalska, B.E. Embryonic zebrafish: Different phenotypes after injection of human uveal melanoma cells. Ocul. Oncol. Pathol. 2015, 1, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Chen, Q.; Campbell, F.; Snaar-Jagalska, E.; Kros, A. Light-Triggered Cancer Cell Specific Targeting and Liposomal Drug Delivery in a Zebrafish Xenograft Model. Adv. Healthc. Mater. 2020, 9, e1901489. [Google Scholar] [CrossRef] [PubMed]
- Pype, C.; Verbueken, E.; Saad, M.A.; Casteleyn, C.R.; Van Ginneken, C.J.; Knapen, D.; Van Cruchten, S.J. Incubation at 32.5 C and above causes malformations in the zebrafish embryo. Reprod. Toxicol. 2015, 56, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Sainz, P.; Guerra-Varela, J.; Carreira, M.J.; Mariscal, J.; Roel, M.; Rubiolo, J.A.; Sciara, A.A.; Abal, M.; Botana, L.M.; López, R. Improving zebrafish embryo xenotransplantation conditions by increasing incubation temperature and establishing a proliferation index with ZFtool. BMC Cancer 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fan, Y.; Pei, X. Fangjihuangqi Decoction inhibits MDA-MB-231 cell invasion in vitro and decreases tumor growth and metastasis in triple-negative breast cancer xenografts tumor zebrafish model. Cancer Med. 2020, 9, 2564–2578. [Google Scholar] [CrossRef]
- Pascoal, S.; Salzer, B.; Scheuringer, E.; Wenninger-Weinzierl, A.; Sturtzel, C.; Holter, W.; Taschner-Mandl, S.; Lehner, M.; Distel, M. A Preclinical Embryonic Zebrafish Xenograft Model to Investigate CAR T Cells in Vivo. Cancers 2020, 12, 567. [Google Scholar] [CrossRef]
- Usai, A.; Di Franco, G.; Colucci, P.; Pollina, L.E.; Vasile, E.; Funel, N.; Palmeri, M.; Dente, L.; Falcone, A.; Morelli, L. A Model of a Zebrafish Avatar for Co-Clinical Trials. Cancers 2020, 12, 677. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, Y.; Jonsson, P.; Wang, J.; Xu, L.; Rouhi, P.; Sinha, I.; Cao, Y.; Williams, C.; Dahlman-Wright, K. Genome-wide profiling of AP-1–regulated transcription provides insights into the invasiveness of triple-negative breast cancer. Cancer Res. 2014, 74, 3983–3994. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef]
- Zhang, B.; Shimada, Y.; Kuroyanagi, J.; Umemoto, N.; Nishimura, Y.; Tanaka, T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS ONE 2014, 9, e85439. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Q.; Zhang, S.; Liu, H.; Zhao, B.; Du, B.; Wang, W.; Lin, P.; Zhang, Z.; Zhong, Y. Digoxin Enhances the Anticancer Effect on Non-Small Cell Lung Cancer While Reducing the Cardiotoxicity of Adriamycin. Front. Pharmacol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Orlova, V.V.; Drabsch, Y.; Freund, C.; Petrus-Reurer, S.; van den Hil, F.E.; Muenthaisong, S.; Dijke, P.t.; Mummery, C.L. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sensi, F.; D’Angelo, E.; Piccoli, M.; Pavan, P.; Mastrotto, F.; Caliceti, P.; Biccari, A.; Corallo, D.; Urbani, L.; Fassan, M. Recellularized Colorectal Cancer Patient-derived Scaffolds as in vitro Pre-clinical 3D Model for Drug Screening. Cancers 2020, 12, 681. [Google Scholar] [CrossRef]
- Corkery, D.P.; Dellaire, G.; Berman, J.N. Leukaemia xenotransplantation in zebrafish–chemotherapy response assay in vivo. Br. J. Haematol. 2011, 153, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Bentley, V.L.; Veinotte, C.J.; Corkery, D.P.; Pinder, J.B.; LeBlanc, M.A.; Bedard, K.; Weng, A.P.; Berman, J.N.; Dellaire, G. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Cabezas-Sainz, P.; Garcia-Caballero, L.; Gonzalez, L.O.; Macia, M.; Sanchez, L.; Vizoso, F.; Perez-Fernandez, R. POU1F1 transcription factor promotes breast cancer metastasis via recruitment and polarization of macrophages. J. Pathol. 2019, 249, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.; Keerthisinghe, P.; Du, L.; Sanderson, L.; Crosier, K.; Crosier, P.; Hall, C. Innate immune cells and bacterial infection in zebrafish. In Methods in Cell Biology; Detrich, H.W., III, Westerfield, M., Zon, L.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 138, pp. 31–60. [Google Scholar]
- Novoa, B.; Figueras, A. Zebrafish: Model for the study of inflammation and the innate immune response to infectious diseases. In Current Topics in Innate Immunity II; Lambris, J.D., Hajishengallis, G., Eds.; Springer: New York, NY, USA, 2012; pp. 253–275. [Google Scholar]
- Schirone, R.C.; Gross, L. Effect of temperature on early embryological development of the zebra fish, Brachydanio rerio. J. Exp. Zool. 1968, 169, 43–52. [Google Scholar] [CrossRef]
- Ørnsrud, R.; Gil, L.; Waagbø, R. Teratogenicity of elevated egg incubation temperature and egg vitamin A status in Atlantic salmon, Salmo salar L. J. Fish Dis. 2004, 27, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Brenig, B.; Tetens, J.; Sharifi, A.R. Phenotypic plasticity induced using high ambient temperature during embryogenesis in domesticated zebrafish, Danio rerio. Reprod. Domest. Anim. 2019, 54, 435–444. [Google Scholar] [CrossRef]
- Lahiri, K.; Vallone, D.; Gondi, S.B.; Santoriello, C.; Dickmeis, T.; Foulkes, N.S. Temperature regulates transcription in the zebrafish circadian clock. PLoS Biol. 2005, 3, e351. [Google Scholar] [CrossRef]
- Zhang, Q.; Kopp, M.; Babiak, I.; Fernandes, J.M. Low incubation temperature during early development negatively affects survival and related innate immune processes in zebrafish larvae exposed to lipopolysaccharide. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.C.F.; Hunt von Herbing, I. Metabolic plasticity in development: Synergistic responses to high temperature and hypoxia in zebrafish, Danio rerio. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2017, 327, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Song, G.; Yan, J.; He, X.; Li, Q.; Cui, Z. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genom. 2013, 14, 612. [Google Scholar] [CrossRef]
- Long, Y.; Yan, J.; Song, G.; Li, X.; Li, X.; Li, Q.; Cui, Z. Transcriptional events co-regulated by hypoxia and cold stresses in Zebrafish larvae. BMC Genom. 2015, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- Levesque, K.D.; Wright, P.A.; Bernier, N.J. Cross Talk without Cross Tolerance: Effect of Rearing Temperature on the Hypoxia Response of Embryonic Zebrafish. Physiol. Biochem. Zool. 2019, 92, 349–364. [Google Scholar] [CrossRef]
- Meijer, A.H. Protection and pathology in TB: Learning from the zebrafish model. In Seminars in Immunopathology; Hussell, T., Grabiec, A.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 38, pp. 261–273. [Google Scholar]
- Yang, X.-J.; Chen, G.-L.; Yu, S.-C.; Xu, C.; Xin, Y.-H.; Li, T.-T.; Shi, Y.; Gu, A.; Duan, J.-J.; Qian, C. TGF-β1 enhances tumor-induced angiogenesis via JNK pathway and macrophage infiltration in an improved zebrafish embryo/xenograft glioma model. Int. Immunopharmacol. 2013, 15, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.V.; Abrahamsson, A.; Jensen, L.D.E.; Dabrosin, C. Estradiol promotes breast cancer cell migration via recruitment and activation of neutrophils. Cancer Immunol. Res. 2017, 5, 234–247. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Wertman, J.; Veinotte, C.J.; Dellaire, G.; Berman, J.N. The zebrafish xenograft platform: Evolution of a novel cancer model and preclinical screening tool. In Cancer and Zebrafish; Langenau, M.D., Ed.; Springer: New York, NY, USA, 2016; pp. 289–314. [Google Scholar]
- Letrado, P.; de Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding up the Cancer Drug Discovery Process. Cancer Res. 2018, 78, 6048. [Google Scholar] [CrossRef]
- Lee, H.J.; Yang, Y.J.; Jeong, S.; Lee, J.D.; Choi, S.Y.; Jung, D.W.; Moon, I.S. Development of a vestibular schwannoma xenograft zebrafish model for in vivo antitumor drug screening. Laryngoscope 2016, 126, E409–E415. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tanner, K. Recapitulating the Tumor Ecosystem along the Metastatic Cascade Using 3D Culture Models. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Augsten, M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 2014, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. (Landmark Ed.) 2010, 15, 166–179. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science (N. Y.) 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Nagasaki, T.; Hara, M.; Nakanishi, H.; Takahashi, H.; Sato, M.; Takeyama, H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: Anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br. J. Cancer 2014, 110, 469–478. [Google Scholar] [CrossRef]
- Sennino, B.; Falcon, B.L.; McCauley, D.; Le, T.; McCauley, T.; Kurz, J.C.; Haskell, A.; Epstein, D.M.; McDonald, D.M. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res 2007, 67, 7358–7367. [Google Scholar] [CrossRef]
- Chouaib, S.; Kieda, C.; Benlalam, H.; Noman, M.Z.; Mami-Chouaib, F.; Ruegg, C. Endothelial cells as key determinants of the tumor microenvironment: Interaction with tumor cells, extracellular matrix and immune killer cells. Crit. Rev. Immunol. 2010, 30, 529–545. [Google Scholar] [CrossRef]
- Policastro, L.L.; Ibanez, I.L.; Notcovich, C.; Duran, H.A.; Podhajcer, O.L. The tumor microenvironment: Characterization, redox considerations, and novel approaches for reactive oxygen species-targeted gene therapy. Antioxid. Redox Signal. 2013, 19, 854–895. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mosser, D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2008, 214, 161–178. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Wang, L.; Wang, F.; Zhang, Z.; Li, J.; Yue, D.; Chen, X.; Ping, Y.; Huang, L.; et al. Impaired T cell function in malignant pleural effusion is caused by TGF-beta derived predominantly from macrophages. Int. J. Cancer 2016, 139, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Han, D.; Cao, J.; Tian, J. Tumour-associated macrophages mediate the invasion and metastasis of bladder cancer cells through CXCL8. PeerJ 2020, 8, e8721. [Google Scholar] [CrossRef]
- Schmid, M.C.; Varner, J.A. Myeloid cells in the tumor microenvironment: Modulation of tumor angiogenesis and tumor inflammation. J. Oncol. 2010, 2010, 201026. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Wyckoff, J.B.; Wang, Y.; Lin, E.Y.; Li, J.F.; Goswami, S.; Stanley, E.R.; Segall, J.E.; Pollard, J.W.; Condeelis, J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007, 67, 2649–2656. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Zea, A.H.; Rodriguez, P.C.; Atkins, M.B.; Hernandez, C.; Signoretti, S.; Zabaleta, J.; McDermott, D.; Quiceno, D.; Youmans, A.; O’Neill, A.; et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: A mechanism of tumor evasion. Cancer Res. 2005, 65, 3044–3048. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.; Chia, C.P.Z.; Chen, W.; Zhang, D.; Park, S.A.; Jin, W.; Wang, D.; Alegre, M.-L.; Zhang, Y.E.; Sun, L.; et al. T Cell Receptor-Regulated TGF-β Type I Receptor Expression Determines T Cell Quiescence and Activation. Immunity 2018, 48, 745–759.e746. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.-Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef]
- De Palma, M.; Venneri, M.A.; Galli, R.; Sergi Sergi, L.; Politi, L.S.; Sampaolesi, M.; Naldini, L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005, 8, 211–226. [Google Scholar] [CrossRef]
- Zhang, B.; Xuan, C.; Ji, Y.; Zhang, W.; Wang, D. Zebrafish xenotransplantation as a tool for in vivo cancer study. Fam. Cancer 2015, 14, 487–493. [Google Scholar] [CrossRef]
- Renshaw, S.; Loynes, C.; Trushell, D.; Elworthy, S.; Ingham, P.; Whyte, M. A transgenic zebrafish model of neutrophilic inflammation. Blood 2007, 108, 3976–3978. [Google Scholar] [CrossRef]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef]
- Bernut, A.; Herrmann, J.-L.; Kissa, K.; Dubremetz, J.-F.; Gaillard, J.-L.; Lutfalla, G.; Kremer, L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. USA 2014, 111, E943–E952. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-W.; Beis, D.; Mitchell, T.; Chen, J.-N.; Stainier, D.Y.R. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 2005, 132, 5199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kaiser, M.S.; Larson, J.D.; Nasevicius, A.; Clark, K.J.; Wadman, S.A.; Roberg-Perez, S.E.; Ekker, S.C.; Hackett, P.B.; McGrail, M.; et al. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 2010, 137, 3119. [Google Scholar] [CrossRef] [PubMed]
- Roh-Johnson, M.; Shah, A.N.; Stonick, J.A.; Poudel, K.R.; Kargl, J.; Yang, G.H.; di Martino, J.; Hernandez, R.E.; Gast, C.E.; Zarour, L.R.; et al. Macrophage-Dependent Cytoplasmic Transfer during Melanoma Invasion in vivo. Dev. Cell 2017, 43, 549–562.e546. [Google Scholar] [CrossRef]
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis. Models Mech. 2018, 11, dmm035998. [Google Scholar] [CrossRef]
- Chen, Q.; Ramu, V.; Aydar, Y.; Groenewoud, A.; Zhou, X.-Q.; Jager, M.J.; Cole, H.; Cameron, C.G.; McFarland, S.A.; Bonnet, S.; et al. TLD1433 Photosensitizer Inhibits Conjunctival Melanoma Cells in Zebrafish Ectopic and Orthotopic Tumour Models. Cancers 2020, 12, 587. [Google Scholar] [CrossRef]
- Williams, J.A. Using PDX for Preclinical Cancer Drug Discovery: The Evolving Field. J. Clin. Med. 2018, 7, 41. [Google Scholar] [CrossRef]
- Noël, A.; Gutiérrez-Fernández, A.; Sounni, N.E.; Behrendt, N.; Maquoi, E.; Lund, I.K.; Cal, S.; Hoyer-Hansen, G.; López-Otín, C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front. Pharmacol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Detry, B.; Erpicum, C.; Paupert, J.; Blacher, S.; Maillard, C.; Bruyère, F.; Pendeville, H.; Remacle, T.; Lambert, V.; Balsat, C.; et al. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood 2012, 119, 5048–5056. [Google Scholar] [CrossRef]
- Wehmas, L.; Tanguay, R.; Punnoose, A.; Greenwood, J. Developing a Novel Embryo-Larval Zebrafish Xenograft Assay to Prioritize Human Glioblastoma Therapeutics. Zebrafish 2016, 13. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sanz-Arriazu, L.; Lorenzoni, R.; Blanco-Prieto, M.J. Glioblastoma chemotherapeutic agents used in the clinical setting and in clinical trials: Nanomedicine approaches to improve their efficacy. Int. J. Pharm. 2020, 119283. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; La Du, J.; Tanguay, R.L.; Greenwood, J.A. Calpain 2 is required for the invasion of glioblastoma cells in the zebrafish brain microenvironment. J. Neurosci. Res. 2012, 90, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Farin, A.; Suzuki, S.O.; Weiker, M.; Goldman, J.E.; Bruce, J.N.; Canoll, P. Transplanted glioma cells migrate and proliferate on host brain vasculature: A dynamic analysis. Glia 2006, 53, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.T.; Reed-Harris, Y.; Barton, C.L.; La Du, J.; Tanguay, R.; Greenwood, J.A. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem. Biophys. Res. Commun. 2018, 506, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Pudelko, L.; Edwards, S.; Balan, M.; Nyqvist, D.; Al-Saadi, J.; Dittmer, J.; Almlöf, I.; Helleday, T.; Bräutigam, L. An orthotopic glioblastoma animal model suitable for high-throughput screenings. Neuro Oncol. 2018, 20, 1475–1484. [Google Scholar] [CrossRef]
- Eden, C.J.; Ju, B.; Murugesan, M.; Phoenix, T.N.; Nimmervoll, B.; Tong, Y.; Ellison, D.W.; Finkelstein, D.; Wright, K.; Boulos, N.; et al. Orthotopic models of pediatric brain tumors in zebrafish. Oncogene 2015, 34, 1736–1742. [Google Scholar] [CrossRef]
- Zeng, A.; Ye, T.; Cao, D.; Huang, X.; Yang, Y.; Chen, X.; Xie, Y.; Yao, S.; Zhao, C. Identify a Blood-Brain Barrier Penetrating Drug-TNB using Zebrafish Orthotopic Glioblastoma Xenograft Model. Sci. Rep. 2017, 7, 14372. [Google Scholar] [CrossRef]
- Casey, M.J.; Modzelewska, K.; Anderson, D.; Goodman, J.; Boer, E.F.; Jimenez, L.; Grossman, D.; Stewart, R.A. Transplantation of Zebrafish Pediatric Brain Tumors into Immune-competent Hosts for Long-term Study of Tumor Cell Behavior and Drug Response. J. Vis. Exp. 2017, 55712. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Dunkel, I.J. Retinoblastoma. J. Child Neurol. 2016, 31, 227–236. [Google Scholar] [CrossRef]
- Asnaghi, L.; White, D.T.; Key, N.; Choi, J.; Mahale, A.; Alkatan, H.; Edward, D.P.; Elkhamary, S.M.; Al-Mesfer, S.; Maktabi, A.; et al. ACVR1C/SMAD2 signaling promotes invasion and growth in retinoblastoma. Oncogene 2019, 38, 2056–2075. [Google Scholar] [CrossRef]
- Asnaghi, L.; White, D.T.; Yoon, L.; Price, A.; Lee, G.Y.; Sahoo, A.; Mumm, J.S.; Eberhart, C.G. Downregulation of Nodal inhibits metastatic progression in retinoblastoma. Acta Neuropathol. Commun. 2019, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; van Eeden, F.; Wilkinson, R. The power of zebrafish in personalised medicine. In Personalised Medicine; Bodiroga-Vukobrat, N., Rukavina, D., Pavelić, K., Sander, G.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 179–197. [Google Scholar]
- Wang, L.; Chen, H.; Fei, F.; He, X.; Sun, S.; Lv, K.; Yu, B.; Long, J.; Wang, X. Patient-derived Heterogeneous Xenograft Model of Pancreatic Cancer Using Zebrafish Larvae as Hosts for Comparative Drug Assessment. J. Vis. Exp. 2019, 59507. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, G.; Mercatali, L.; Liverani, C.; De Vita, A.; Spadazzi, C.; Pieri, F.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. Management and potentialities of primary cancer cultures in preclinical and translational studies. J. Transl. Med. 2017, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Zhai, J.; Li, C.-Y.; Tan, A.-M.; Wei, P.; Shen, L.-Z.; He, M.-F. Patient-derived xenograft in zebrafish embryos: A new platform for translational research in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 160. [Google Scholar] [CrossRef]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.-D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Zhao, J.J.; Kwart, A.; Yang, C.; Ma, D.; Ren, X.; Tai, Y.T.; Anderson, K.; Handin, R.; et al. A clinically relevant in vivo zebrafish model of human multiple myeloma (MM) to study preclinical therapeutic efficacy. Blood 2016, 128. [Google Scholar] [CrossRef]
- Al-Samadi, A.; Tuomainen, K.; Kivimaki, A.; Salem, A.; Al-Kubati, S.; Hyytiainen, A.; Parikka, M.; Mesimaki, K.; Wilkman, T.; Makitie, A.; et al. PCR-based zebrafish model for personalised medicine in head and neck cancer. J. Transl. Med. 2019, 17, 235. [Google Scholar] [CrossRef]
- Sun, D.Y.; Wu, J.Q.; He, Z.H.; He, M.F.; Sun, H.B. Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-beta signaling pathway. Life Sci. 2019, 235, 116791. [Google Scholar] [CrossRef]
- Ren, J.; Smid, M.; Iaria, J.; Salvatori, D.C.F.; van Dam, H.; Zhu, H.J.; Martens, J.W.M.; Ten Dijke, P. Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Res. BCR 2019, 21, 109. [Google Scholar] [CrossRef]
- Gacha-Garay, M.J.; Niño-Joya, A.F.; Bolaños, N.I.; Abenoza, L.; Quintero, G.; Ibarra, H.; Gonzalez, J.M.; Akle, V.; Garavito-Aguilar, Z.V. Pilot Study of an Integrative New Tool for Studying Clinical Outcome Discrimination in Acute Leukemia. Front. Oncol. 2019, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Huang, Y.-L.; Wang, Y.-R.S.; Hsiao, E.; Hsu, T.-A.; Shiao, H.-Y.; Jiaang, W.-T.; Sampurna, B.P.; Lin, K.-H.; Wu, M.-S.; et al. Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index than Sorafenib via Zebrafish Drug Screening Platform. Cancers 2019, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914.e1914. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Choudhury, S.; Wangsa, D.; Lescott, C.J.; Wilkins, D.J.; Sripadhan, P.; Liu, X.; Wangsa, D.; Ried, T.; Moskaluk, C.; et al. A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci. Rep. 2017, 7, 11410. [Google Scholar] [CrossRef] [PubMed]
- Liverani, C.; La Manna, F.; Groenewoud, A.; Mercatali, L.; Van Der Pluijm, G.; Pieri, F.; Cavaliere, D.; De Vita, A.; Spadazzi, C.; Miserocchi, G.; et al. Innovative approaches to establish and characterize primary cultures: An ex vivo 3D system and the zebrafish model. Biol. Open 2017, 6, 133–140. [Google Scholar] [CrossRef]
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Meregalli, M.; Belicchi, M.; Vaira, V.; Corbetta, S.; Verdelli, C.; Verrua, E.; Serban, A.L.; et al. Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int. J. Cancer 2017, 140, 1870–1880. [Google Scholar] [CrossRef]
- Gaudenzi, G.; Albertelli, M.; Dicitore, A.; Würth, R.; Gatto, F.; Barbieri, F.; Cotelli, F.; Florio, T.; Ferone, D.; Persani, L.; et al. Patient-derived xenograft in zebrafish embryos: A new platform for translational research in neuroendocrine tumors. Endocrine 2017, 57, 214–219. [Google Scholar] [CrossRef]
- Astone, M.; Dankert, E.N.; Alam, S.K.; Hoeppner, L.H. Fishing for cures: The alLURE of using zebrafish to develop precision oncology therapies. NPJ Precis. Oncol. 2017, 1, 39. [Google Scholar] [CrossRef]
- Hason, M.; Bartůněk, P. Zebrafish Models of Cancer-New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Martinez-Ordonez, A.; Seoane, S.; Cabezas, P.; Eiro, N.; Sendon-Lago, J.; Macia, M.; Garcia-Caballero, T.; Gonzalez, L.O.; Sanchez, L.; Vizoso, F. Breast cancer metastasis to liver and lung is facilitated by Pit-1-CXCL12-CXCR4 axis. Oncogene 2018, 37, 1430–1444. [Google Scholar] [CrossRef]
- Chen, L.; Groenewoud, A.; Tulotta, C.; Zoni, E.; Kruithof-de Julio, M.; Van Der Horst, G.; Van Der Pluijm, G.; Snaar-Jagalska, B.E. A zebrafish xenograft model for studying human cancer stem cells in distant metastasis and therapy response. In Methods in Cell Biology; Detrich, H.W., III, Westerfield, M., Zon, L.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 138, pp. 471–496. [Google Scholar]
- Spaink, H.P.; Cui, C.; Wiweger, M.I.; Jansen, H.J.; Veneman, W.J.; Marín-Juez, R.; de Sonneville, J.; Ordas, A.; Torraca, V.; van der Ent, W.; et al. Robotic injection of zebrafish embryos for high-throughput screening in disease models. Methods 2013, 62, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Sertori, R.; Trengove, M.; Basheer, F.; Ward, A.C.; Liongue, C. Genome editing in zebrafish: A practical overview. Brief. Funct. Genom. 2016, 15, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, M.; Mione, M. The toolbox for conditional zebrafish cancer models. In Cancer and Zebrafish; Langenau, M.D., Ed.; Springer: New York, NY, USA, 2016; pp. 21–59. [Google Scholar]

| Temperature | Incubation Time | Injection Site | Time of Injection | Cell Line | Number of Cells Injected | Reference |
|---|---|---|---|---|---|---|
| 28 °C | 1–2 days | Perivitelline space | 48 hpf | FGF2-T-MAE, Tet-FGF2, A2780, MDA-MB-435 and B16-BL16 | 4–10 nL | [29] |
| 28 °C | 4 days | Perivitelline space | 48 hpf | BT549 | n/a | [62] |
| 28.5 °C | 3 days | Yolk sac | 48 hpf | ES2 and OV90 | 100–200 | [63] |
| 32 °C | 3 days | Yolk sac | 48 hpf | K562 | 100–200 | [64] |
| 32 °C | 2 days | Perivitelline space | 48 hpf | A549 | 100 | [65] |
| 33 °C | 6 days | Blastodisc/Duct of Cuvier | Early blastula/48 hpf | hPSC-derived ECs | 100/400 | [66] |
| 33 °C | 3 days | Duct of Cuvier | 48 hpf | HT29 | 200 | [67] |
| 34 °C | 4 days | Duct of Cuvier | 48 hpf | FGF-T-MAE, 4T1, MDA-MB-231, PC3, MAE and ZF4/PAC2 | 50–400 | [39] |
| 34 °C | 7 days | Yolk sac | 48 hpf | BT-474, MCF7 and MDA-MB-435 | 500 | [52] |
| 34 °C | 6 days | Yolk sac | 48 hpf | PC3, LNCAP, MCF7, BT474, A549, H460, H1299, HT29, SW620, MV3 and HT1080 | 100 | [53] |
| 34 °C | 3 days | Yolk sac | 48 hpf | TC252 and A673 | 500 | [54] |
| 34 °C | 6 days | Yolk sac | 48 hpf | Primary tumors (92.1 and Mel270) and UM metastases (OMM1, OMM2.3, OMM2.5). | 400–500 | [55] |
| 34 °C | 4 days | Duct of Cuvier | 48 hpf | MDA-MB-231 | 300 | [56] |
| 35 °C | 3 days | Yolk sac | 48 hpf | MDA-MB-231 | n/a | [59] |
| 35 °C | 1 day | Duct of Cuvier | 48 hpf | Nalm-6 cells and CAR T cells | 50–300 | [60] |
| 35 °C | 3 days | Yolk sac | 48 hpf | HCT 116, Mia Paca-2 and cancer tissue | n/a | [61] |
| 35° C | 3 days | Yolk sac | 48 hpf | K562 and NB-4 | 25–50 | [68] |
| 35° C | 2–4 days | Yolk sac | 48 hpf | Jurkat, Karpas45, and TALL1 and patient samples | 50–100/500 | [69] |
| 34 °C and 36 °C | 3 days | Yolk sac | 48 hpf | HCT116 | 100–200 or 400–500 | [58] |
| Tumor Type | Nº of Patients | Sample Collection | Zebrafish Line | Nº of Cells | Site of Injection | Stage | T | Remarkable Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Colon, gastric, and pancreatic ductal adenocarcinoma | 6 (2 of each) | Surgery | WT | Tissue | Yolk sac | Larvae- 48 hpf | 35 °C | Cancer tissue survives and invade the yolk sac | [61] |
| Pancreatic, colon and gastric cancers | 24 (12,8,4 respectively) | Surgery | WT | Tissue | Yolk sac | Larvae- 48 hpf | 35 °C | Chemo sensitivity assays in agreement with clinical studies | [61] |
| Pancreatic cancer | 3 | Surgery | WT/Casper | 50-80 | Yolk sac | Larvae- 48 hpf | 32 °C | Lentiviral use to prolong the observation window | [141] |
| Acute Leukemia | 7 | Bone marrow | WT | 200–500 | Pericardial space | Larvae- 48 hpf | 32.5 °C | Correlation between differential cell dissemination and clinical outcome | [150] |
| Head and neck cancer | 1 | Surgery | WT | 1000 | Perivitelline space | Larvae- 48 hpf | 34 °C | Tumor response evaluation through PCR | [147] |
| Hepatocellular carcinoma | 13 | Surgery | WT | 200 | Yolk sac | Larvae- 48 hpf | 28–37 °C gradient | Tumor response to established drugs | [151] |
| Glioblastoma, Rhabdomyosarcoma, metastatic melanoma, breast cancer | 6 | Surgery/ Blood | Casper, prkdc-/-,il2rga-/- | 5 × 105 | Intraperitoneal | Adult | 37 °C | PDX engraftment/Zebrafish reared at 37º | [152] |
| Gastric cancer | 14 (5 failed) | Surgery | Tg(fli-1:EGFP) | 600–800 | Yolk sac | Larvae- 48 hpf | 32 °C | Angiogenesis, metastasis/Correlation between zPDX and clinic result | [144] |
| Adenoid cystic carcinoma of the salivary gland | 2 | Surgery | Tg(kdrl:grcfp) | 100–200/ Tissue | Yolk sac/Precardiac sinus | Larvae- 48 hpf | CR PDX-cell cultures preserve tumor biology, metastatic behavior, and drug response | [153] | |
| Colorectal cancer | 10 | Surgery | WT/Tg(fli:EGFP) | Cell suspension (5) Tissue (5) | Perivitelline space | Larvae- 48 hpf | 34 °C | Proliferation, angiogenesis, and histological and treatment correlation with relapse and mutational status | [34] |
| Abdominal liposarcoma | 1 | Surgery | Tg(kdrl:mCherry) | 50–400 | Heart cavity | Larvae- 48 hpf | 34 °C | Cell ability to survive and migrate | [154] |
| Pituitary tumor | 2 | Surgery | Tg(fli1a:EGFP) | 100 (derived from spheres) | Sub-epidermal space (close to the SIV plexus) | Larvae- 48 hpf | 32 °C | Isolation of progenitor/stem cells from patient-derived spheres/Invasive and angiogenic behavior | [155] |
| Multiple myeloma | 6 | Plasma | Casper | 50–200 | Perivitelline space | Larvae- 48 hpf | Cell growth/Response-Resistance correlation | [146] | |
| Pituitary adenoma and NET | 8 (2 failed) | Surgery | Tg(fli1a:EGFP) | 100 | Perivitelline space | Larvae- 48 hpf | 32 °C | Angiogenesis, invasive behavior, and migration | [156] |
| Bone metastasis from breast cancer | 1 | Surgery | Tg(fli1a:GFP) | 50–400 | Duct of Cuvier | Larvae- 48 hpf | 34 °C | Patient and primary cells behavioral correlation but not with a cell line | [37] |
| T-cell acute lymphoblastic leukemia | 2 | Bone marrow | Casper | 500 | Yolk sac | Larvae- 48 hpf | 35 °C | Differential response to treatment in correlation to mutational status | [69] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabezas-Sáinz, P.; Pensado-López, A.; Sáinz, B., Jr.; Sánchez, L. Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment. Cells 2020, 9, 1978. https://doi.org/10.3390/cells9091978
Cabezas-Sáinz P, Pensado-López A, Sáinz B Jr., Sánchez L. Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment. Cells. 2020; 9(9):1978. https://doi.org/10.3390/cells9091978
Chicago/Turabian StyleCabezas-Sáinz, Pablo, Alba Pensado-López, Bruno Sáinz, Jr., and Laura Sánchez. 2020. "Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment" Cells 9, no. 9: 1978. https://doi.org/10.3390/cells9091978
APA StyleCabezas-Sáinz, P., Pensado-López, A., Sáinz, B., Jr., & Sánchez, L. (2020). Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment. Cells, 9(9), 1978. https://doi.org/10.3390/cells9091978







