Natural Root Cellular Variation in Responses to Osmotic Stress in Arabidopsis thaliana Accessions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Osmotic Potential Measurement
2.3. Pseudo-Schiff Assay
2.4. Microscopy Visualization
2.5. Kinematic Analysis
2.6. RNA Extraction and Quantitative RT-PCR
2.7. Geometric Morphometric Analysis
3. Results
3.1. Hyperosmotic Shock Stress Treatment Affects Primary Root Length, Proliferation, and Differentiation of Root Meristem Cells in the Arabidopsis Col-0 Accession
3.2. Stem Cell Niche Organization Is Refractory to Hyperosmotic Stress Conditions
3.3. Hyperosmotic Stress Conditions Result in Swelling of the Epidermis and Cortex Cells of the Col-0 Accession
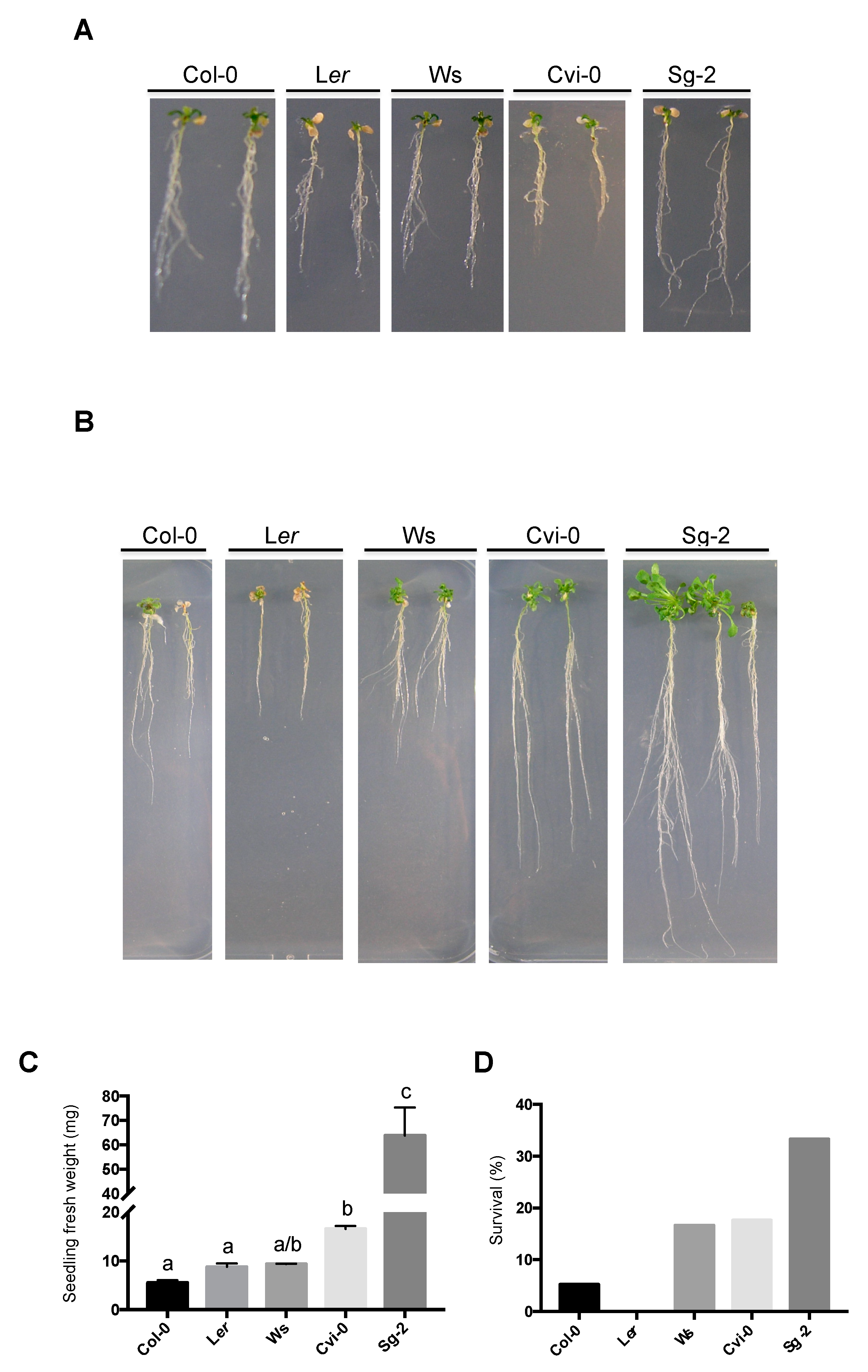
3.4. Root Growth of Arabidopsis Natural Accessions Is Differentially Affected by Hyperosmotic Stress Conditions
3.5. The Stress Zone Is Not an Adaptive Response of Plants to Hyperosmotic Stress Conditions
3.6. Resilience to Osmotic Stress: Root Growth Arrest Is Transient and Reversible
3.7. Recovery after Strong Hyperosmotic Stress Conditions Does Not Correlate with the Osmotic Effect on Root Growth
3.8. Expression of Stress-Responsive Genes Does Not Correlate with Osmotic Stress Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weigel, D. Natural variation in Arabidopsis: From molecular genetics to ecological genomics. Plant Physiol. 2012, 158, 2–22. [Google Scholar] [CrossRef]
- Alonso-Blanco, C.; Koornneef, M. Naturally occurring variation in Arabidopsis: An underexploited resource for plant genetics. Trends Plant Sci. 2000, 5, 22–29. [Google Scholar] [CrossRef]
- Buescher, E.; Achberger, T.; Amusan, I.; Giannini, A.; Ochsenfeld, C.; Rus, A.; Lahner, B.; Hoekenga, O.; Yakubova, E.; Harper, J.F.; et al. Natural genetic variation in selected populations of Arabidopsis thaliana is associated with ionomic differences. PLoS ONE 2010, 5, e11081. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Serrano, A.; Assmann, S.M. Phenotypic and genome-wide association with the local environment of Arabidopsis. Nat. Ecol. Evol. 2019, 3, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Level, A.; Korte, A.; Cooper, M.D.; Nordborg, M.; Schmitt, J.; Wilczek, A.M. A map of local adaptation in Arabidopsis thaliana. Science 2011, 334, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Hancock, A.M.; Brachi, B.; Faure, N.; Horton, M.W.; Jarymowycz, L.B.; Sperone, F.G.; Toomajian, C.; Roux, F.; Bergelson, J. Adaptation to climate across the Arabidopsis thaliana genome. Science 2011, 334, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Alonso-Blanco, C.; Vreugdenhil, D. Naturally occurring genetic variation in Arabidopsis thaliana. Annu. Rev. Plant Biol. 2004, 55, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Tabas-Madrid, D.; Mendez-Vigo, B.; Arteaga, N.; Marcer, A.; Pascual-Montano, A.; Weigel, D.; Xavier Pico, F.; Alonso-Blanco, C. Genome-wide signatures of flowering adaptation to climate temperature: Regional analyses in a highly diverse native range of Arabidopsis thaliana. Plant Cell Environ. 2018, 41, 1806–1820. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Measuring the Water Status of Plants and Soils; Academic Press: San Diego, CA, USA, 1995; p. 178. [Google Scholar]
- Haswell, E.S.; Verslues, P.E. The ongoing search for the molecular basis of plant osmosensing. J. Gen. Physiol. 2015, 145, 389–394. [Google Scholar] [CrossRef]
- Bouchabke, O.; Chang, F.; Simon, M.; Voisin, R.; Pelletier, G.; Durand-Tardif, M. Natural Variation in Arabidopsis thaliana as a Tool for Highlighting Differential Drought Responses. PLoS ONE 2008, 3, e1705. [Google Scholar] [CrossRef]
- Katori, T.; Ikeda, A.; Iuchi, S.; Kobayashi, M.; Shinozaki, K.; Maehashi, K.; Sakata, Y.; Tanaka, S.; Taji, T. Dissecting the genetic control of natural variation in salt tolerance of Arabidopsis thaliana accessions. J. Exp. Bot. 2010, 61, 1125–1138. [Google Scholar] [CrossRef]
- Lefebvre, V.; Kiani, S.P.; Durand-Tardif, M. A focus on natural variation for abiotic constraints response in the model species Arabidopsis thaliana. Int. J. Mol. Sci. 2009, 10, 3547–3582. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J.; Davis, D.S. Effecf of water stress and temperature on lead size and on size and number of epidarmal cells in grain sorghum. Crop Sci. 1974, 14, 751–755. [Google Scholar] [CrossRef]
- Frensch, J.; Hsiao, T.C. Transient Responses of Cell Turgor and Growth of Maize Roots as Affected by Changes in Water Potential. Plant Physiol. 1994, 104, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Emmermann, M.; Thierfelder, J.M.; zur Nieden, U.; Clericus, M.; Braun, H.P.; Nover, L.; Schmitz, U.K. HSP68—A DnaK-like heat-stress protein of plant mitochondria. Planta 1993, 190, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Couot-Gastelier, J.; Vartanian, N. Drought-induced Short Roots in Arabidopsis thaliana: Structural Characteristics. Plant Biol. 1995, 108, 407–413. [Google Scholar]
- Hsiao, T.C.; Xu, L.K. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Sharp, R.E.; Davies, W.J. Regulation of Growth and Development of Plants Growing with a Restricted Supply of Water; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Spollen, W.G.; Sharp, R.E.; Saab, I.N.; Wu, Y. Regulation of Cell Expansion in Roots and Shoots at Low Water Potentials; Bios Scientific Publishers: Oxford, UK, 1993. [Google Scholar]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Verbelen, J.P.; De Cnodder, T.; Le, J.; Vissenberg, K.; Baluska, F. The Root Apex of Arabidopsis thaliana Consists of Four Distinct Zones of Growth Activities: Meristematic Zone, Transition Zone, Fast Elongation Zone and Growth Terminating Zone. Plant Signal. Behav. 2006, 1, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Baluska, F.; Mancuso, S.; Volkmann, D.; Barlow, P.W. Root apex transition zone: A signalling-response nexus in the root. Trends Plant Sci. 2010, 15, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.B.; Dubrovsky, J.G. Longitudinal zonation pattern in plant roots: Conflicts and solutions. Trends Plant Sci. 2013, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [PubMed]
- Pi, L.; Aichinger, E.; van der Graaff, E.; Llavata-Peris, C.I.; Weijers, D.; Hennig, L.; Groot, E.; Laux, T. Organizer-Derived WOX5 Signal Maintains Root Columella Stem Cells through Chromatin-Mediated Repression of CDF4 Expression. Dev. Cell 2015, 33, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.; Willemsen, V.; Hage, W.; Weisbeek, P.; Scheres, B. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 1995, 378, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Burssens, S.; de Almeida Engler, J.; Beeckman, T.; Richard, C.; Shaul, O.; Ferreira, P.; Van Montagu, M.; Inze, D. Developmental expression of the Arabidopsis thaliana CycA2;1 gene. Planta 2000, 211, 623–631. [Google Scholar] [CrossRef]
- Rymen, B.; Coppens, F.; Dhondt, S.; Fiorani, F.; Beemster, G.T. Kinematic analysis of cell division and expansion. Methods Mol. Biol. 2010, 655, 203–227. [Google Scholar] [CrossRef]
- Truernit, E.; Haseloff, J. A simple way to identify non-viable cells within living plant tissue using confocal microscopy. Plant Methods 2008, 4, 15. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Garay-Arroyo, A.; Ortiz-Moreno, E.; de la Paz Sanchez, M.; Murphy, A.S.; Garcia-Ponce, B.; Marsch-Martinez, N.; de Folter, S.; Corvera-Poire, A.; Jaimes-Miranda, F.; Pacheco-Escobedo, M.A.; et al. The MADS transcription factor XAL2/AGL14 modulates auxin transport during Arabidopsis root development by regulating PIN expression. EMBO J. 2013, 32, 2884–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Escobedo, M.A.; Ivanov, V.B.; Ransom-Rodriguez, I.; Arriaga-Mejia, G.; Avila, H.; Baklanov, I.A.; Pimentel, A.; Corkidi, G.; Doerner, P.; Dubrovsky, J.G.; et al. Longitudinal zonation pattern in Arabidopsis root tip defined by a multiple structural change algorithm. Ann. Bot. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlade, N.B.; Feng, X.; Dransfield, T.; Copsey, L.; Hanna, A.I.; Thebaud, C.; Bangham, A.; Hudson, A.; Coen, E. Evolution through genetically controlled allometry space. Proc. Natl. Acad. Sci. USA 2005, 102, 10221–10226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristova, D.; Rosas, U.; Krouk, G.; Ruffel, S.; Birnbaum, K.D.; Coruzzi, G.M. RootScape: A landmark-based system for rapid screening of root architecture in Arabidopsis. Plant Physiol. 2013, 161, 1086–1096. [Google Scholar] [CrossRef] [Green Version]
- Van der Weele, C.M.; Spollen, W.G.; Sharp, R.E.; Baskin, T.I. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 2000, 51, 1555–1562. [Google Scholar] [CrossRef] [Green Version]
- Deak, K.I.; Malamy, J. Osmotic regulation of root system architecture. Plant J. 2005, 43, 17–28. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Baskin, T.I. Patterns of root growth acclimation: Constant processes, changing boundaries. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 65–73. [Google Scholar] [CrossRef]
- Colon-Carmona, A.; You, R.; Haimovitch-Gal, T.; Doerner, P. Technical advance: Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999, 20, 503–508. [Google Scholar] [CrossRef]
- Cajero Sanchez, W.; Garcia-Ponce, B.; Sanchez, M.P.; Alvarez-Buylla, E.R.; Garay-Arroyo, A. Identifying the transition to the maturation zone in three ecotypes of Arabidopsis thaliana roots. Commun. Integr. Biol. 2018, 11, e1395993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, Y.; Tamaki, T.; Fukuda, H. Regulation of xylem cell fate. Front. Plant Sci. 2014, 5, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef]
- Aceves-Garcia, P.; Alvarez-Buylla, E.R.; Garay-Arroyo, A.; Garcia-Ponce, B.; Munoz, R.; Sanchez Mde, L. Root Architecture Diversity and Meristem Dynamics in Different Populations of Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 858. [Google Scholar] [CrossRef] [Green Version]
- Rosas, U.; Cibrian-Jaramillo, A.; Ristova, D.; Banta, J.A.; Gifford, M.L.; Fan, A.H.; Zhou, R.W.; Kim, G.J.; Krouk, G.; Birnbaum, K.D.; et al. Integration of responses within and across Arabidopsis natural accessions uncovers loci controlling root systems architecture. Proc. Natl. Acad. Sci. USA 2013, 110, 15133–15138. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef] [Green Version]
- Kornet, N.; Scheres, B. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis. Plant Cell 2009, 21, 1070–1079. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Lopez, R.; Garcia-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Perez-Ruiz, R.V.; Kim, S.H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol. 2008, 146, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, R.; Girke, T.; Bray, E.A.; Bailey-Serres, J. Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana. Plant J. 2004, 38, 823–839. [Google Scholar] [CrossRef]
- Kreps, J.A.; Wu, Y.; Chang, H.S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002, 130, 2129–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, M.; Ishida, J.; Narusaka, M.; Fujita, M.; Nanjo, T.; Umezawa, T.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression pattern of around 7000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct. Integr. Genom. 2002, 2, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Xiong, L.; Li, W.; Zhu, J.K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1971–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Sawada, Y.; Takahashi, H.; Okamoto, M.; Ikegami, K.; Koiwai, H.; Seo, M.; Toyomasu, T.; Mitsuhashi, W.; Shinozaki, K.; et al. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008, 147, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, A.; Yamauchi, A. Root Osmotic Adjustment under Osmotic Stress in Maize Seedlings 1. Transient Change of Growth and Water Relations in Roots inResponse to Osmotic Stress. Plant Prod. Sci. 2006, 9, 12. [Google Scholar]
- Julkowska, M.M.; Hoefsloot, H.C.; Mol, S.; Feron, R.; de Boer, G.J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef] [Green Version]
- Bizet, F.; Hummel, I.; Bogeat-Triboulot, M.B. Length and activity of the root apical meristem revealed in vivo by infrared imaging. J. Exp. Bot. 2015, 66, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Royer, M.; Cohen, D.; Aubry, N.; Vendramin, V.; Scalabrin, S.; Cattonaro, F.; Bogeat-Triboulot, M.B.; Hummel, I. The build-up of osmotic stress responses within the growing root apex using kinematics and RNA-sequencing. J. Exp. Bot. 2016, 67, 5961–5973. [Google Scholar] [CrossRef] [Green Version]
- Sacks, M.M.; Silk, W.K.; Burman, P. Effect of Water Stress on Cortical Cell Division Rates within the Apical Meristem of Primary Roots of Maize. Plant Physiol. 1997, 114, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Samarajeewa, P.K.; Barrero, R.A.; Umeda-Hara, C.; Kawai, M.; Uchimiya, H. Cortical cell death, cell proliferation, macromolecular movements and rTip1 expression pattern in roots of rice (Oryza sativa L.) under NaCl stress. Planta 1999, 207, 354–361. [Google Scholar] [CrossRef]
- West, G.; Inze, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, G.; Liu, J.; Ding, Z. The Root Transition Zone: A Hot Spot for Signal Crosstalk. Trends Plant Sci. 2018, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Spollen, W.G.; Sharp, R.E. Spatial Distribution of Turgor and Root Growth at Low Water Potentials. Plant Physiol. 1991, 96, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beemster, G.T.; De Vusser, K.; De Tavernier, E.; De Bock, K.; Inze, D. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol. 2002, 129, 854–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Liu, L.; Li, K.; Xie, Q.; Wang, Z.; Zhao, X.; Li, X. PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat. J. Exp. Bot. 2014, 65, 4863–4872. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Li, X. ABA mediates PEG-mediated premature differentiation of root apical meristem in plants. Plant Signal. Behav. 2014, 9, e977720. [Google Scholar] [CrossRef] [Green Version]
- Dinneny, J.R.; Long, T.A.; Wang, J.Y.; Jung, J.W.; Mace, D.; Pointer, S.; Barron, C.; Brady, S.M.; Schiefelbein, J.; Benfey, P.N. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 2008, 320, 942–945. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Wu, R.; Wee, C.W.; Xie, F.; Wei, X.; Chan, P.M.; Tham, C.; Duan, L.; Dinneny, J.R. A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 2013, 25, 2132–2154. [Google Scholar] [CrossRef] [Green Version]
- Schindelman, G.; Morikami, A.; Jung, J.; Baskin, T.I.; Carpita, N.C.; Derbyshire, P.; McCann, M.C.; Benfey, P.N. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001, 15, 1115–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Kost, B.; Chua, N.H. Reduced expression of alpha-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 2001, 28, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Y.; Wang, Z.; Zhou, J.; Fan, B.; Chen, Z. A Critical Role of Lyst-Interacting Protein5, a Positive Regulator of Multivesicular Body Biogenesis, in Plant Responses to Heat and Salt Stresses. Plant Physiol. 2015, 169, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meychik, N.R.; Yermakov, I.P. Swelling of root cell walls as an indicator of their functional state. Biochemistry 2001, 66, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Savina, M.; Du, J.; Devendran, A.; Kannivadi Ramakanth, K.; Tian, X.; Sim, W.S.; Mironova, V.V.; Xu, J. A Sacrifice-for-Survival Mechanism Protects Root Stem Cell Niche from Chilling Stress. Cell 2017, 170, 102–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulcher, N.; Sablowski, R. Hypersensitivity to DNA damage in plant stem cell niches. Proc. Natl. Acad. Sci. USA 2009, 106, 20984–20988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, L.; Zheng, Z.; Grumet, R.; Loescher, W.; Zhu, J.K.; Yang, P.; Hu, Y.; Chan, Z. Transcriptomic and physiological variations of three Arabidopsis ecotypes in response to salt stress. PLoS ONE 2013, 8, e69036. [Google Scholar] [CrossRef] [Green Version]
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. Plant J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef]
- Huang, Z.; Footitt, S.; Finch-Savage, W.E. The effect of temperature on reproduction in the summer and winter annual Arabidopsis thaliana ecotypes Bur and Cvi. Ann. Bot. 2014, 113, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Abarca, D.; Roldan, M.; Martin, M.; Sabater, B. Arabidopsis thaliana ecotype Cvi shows an increased tolerance to photo-oxidative stress and contains a new chloroplastic copper/zinc superoxide dismutase isoenzyme. J. Exp. Bot. 2001, 52, 1417–1425. [Google Scholar] [CrossRef] [Green Version]
- Monda, K.; Negi, J.; Iio, A.; Kusumi, K.; Kojima, M.; Hashimoto, M.; Sakakibara, H.; Iba, K. Environmental regulation of stomatal response in the Arabidopsis Cvi-0 ecotype. Planta 2011, 234, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.; Tatematsu, K.; Kanno, Y.; Hobo, T.; Kimura, M.; Jikumaru, Y.; Yano, R.; Kamiya, Y.; Nambara, E. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: A comparative study on dormant and non-dormant accessions. Plant Cell Physiol. 2009, 50, 1786–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koornneef, M.; Jorna, M.L.; Brinkhorst-van der Swan, D.L.C.; Karssen, C.M. The isolation of abscisic acid (ABA)-deficient mutants by selection of induced revertants in non-germinating gibberellins sensitive lines of Arabidopsis thaliana (L.). Heynh. Theor. Appl. Genet. 1992, 61, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Reuling, G.; Karssen, C. The isolation and characterization of abscisic acid-insensitive mutants of Arabid- opsis thaliana. Physiol. Plant. 1984, 61, 377–383. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef] [Green Version]
- Barrio, R.A.; Romero-Arias, J.R.; Noguez, M.A.; Azpeitia, E.; Ortiz-Gutierrez, E.; Hernandez-Hernandez, V.; Cortes-Poza, Y.; Alvarez-Buylla, E.R. Cell patterns emerge from coupled chemical and physical fields with cell proliferation dynamics: The Arabidopsis thaliana root as a study system. PLoS Comput. Biol. 2013, 9, e1003026. [Google Scholar] [CrossRef] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cajero-Sanchez, W.; Aceves-Garcia, P.; Fernández-Marcos, M.; Gutiérrez, C.; Rosas, U.; García-Ponce, B.; Álvarez-Buylla, E.R.; Sánchez, M.d.l.P.; Garay-Arroyo, A. Natural Root Cellular Variation in Responses to Osmotic Stress in Arabidopsis thaliana Accessions. Genes 2019, 10, 983. https://doi.org/10.3390/genes10120983
Cajero-Sanchez W, Aceves-Garcia P, Fernández-Marcos M, Gutiérrez C, Rosas U, García-Ponce B, Álvarez-Buylla ER, Sánchez MdlP, Garay-Arroyo A. Natural Root Cellular Variation in Responses to Osmotic Stress in Arabidopsis thaliana Accessions. Genes. 2019; 10(12):983. https://doi.org/10.3390/genes10120983
Chicago/Turabian StyleCajero-Sanchez, Wendy, Pamela Aceves-Garcia, María Fernández-Marcos, Crisanto Gutiérrez, Ulises Rosas, Berenice García-Ponce, Elena R. Álvarez-Buylla, Maria de la Paz Sánchez, and Adriana Garay-Arroyo. 2019. "Natural Root Cellular Variation in Responses to Osmotic Stress in Arabidopsis thaliana Accessions" Genes 10, no. 12: 983. https://doi.org/10.3390/genes10120983
APA StyleCajero-Sanchez, W., Aceves-Garcia, P., Fernández-Marcos, M., Gutiérrez, C., Rosas, U., García-Ponce, B., Álvarez-Buylla, E. R., Sánchez, M. d. l. P., & Garay-Arroyo, A. (2019). Natural Root Cellular Variation in Responses to Osmotic Stress in Arabidopsis thaliana Accessions. Genes, 10(12), 983. https://doi.org/10.3390/genes10120983




