Homozygosity for Mobile Element Insertions Associated with WBSCR17 Could Predict Success in Assistance Dog Training Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genotyping Four Mobile Element Insertions Associated with Human-Directed Hypersociability
2.2. Canine Questionnaire Data to Identify Behavioral Types
2.3. Predictive Power of the Mobile Element Insertion’s Copy Number
2.4. Mobile Element Insertion Copy Number and Correlation with C-BARQ© behaviors
2.5. Comparing Mobile Element Insertion of Assistance and Pet Dogs
2.6. Rationale for Breed Group Categories
2.7. Comparing Mobile Element Insertions in Divergent and Recent-Radiation Breeds
3. Results
3.1. Dog Sociability Trends May Shift with Age
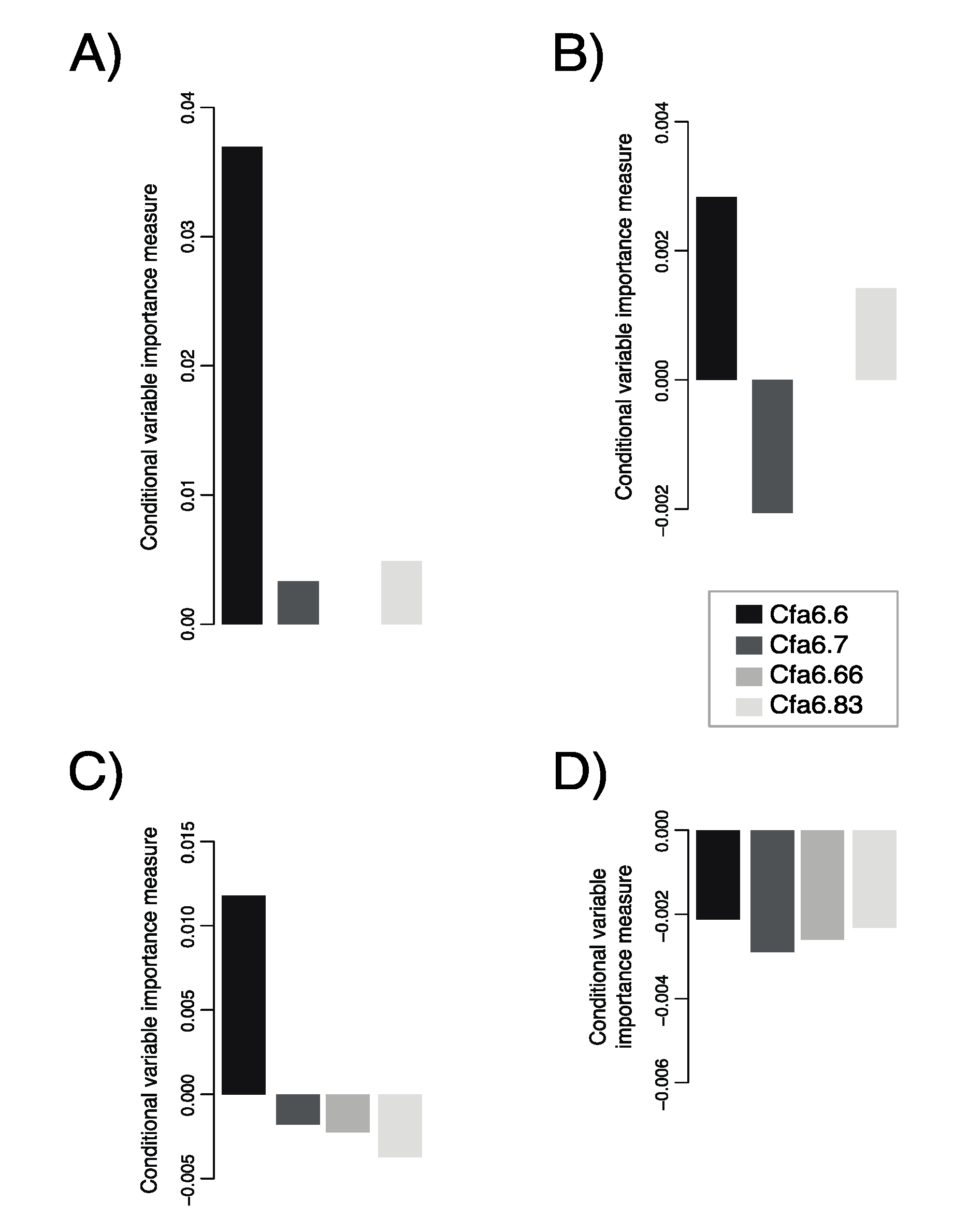
3.2. Cfa6.6 Has the Highest Predictive Power of Sociability-Related Behaviors for Dogs Aged 1–5 Years
3.3. Higher Mobile Element Copy Numbers at Cfa6.6 Co-Occur with Increased Hypersociability in Younger Dogs
3.4. Assistance Dogs Consistently Have More Mobile Element Insertions and Significant Heterozygosity Deficiency at Cfa6.6 Compared to Non-Assistance Dogs
3.5. Divergent-Breed Dogs Have Significantly More Mobile Element Insertions at Cfa6.6, Cfa6.7, and Cfa6.66
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cadieu, E.; Neff, M.W.; Quignon, P.; Walsh, K.; Chase, K.; Parker, H.G.; vonHoldt, H.G.; Rhue, A.; Boyko, A.; Byers, A.; et al. Coat Variation in the Domestic Dog Is Governed by Variants in Three Genes. Science 2009, 326, 150–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, H.G.; VonHoldt, B.M.; Quignon, P.; Margulies, E.H.; Shao, S.; Mosher, D.S.; Spady, T.C.; Elkahloun, A.; Cargill, M.; Jones, P.G.; et al. An expressed fgf4 retrogene is associated with breed-defining chondrodysplasia in domestic dogs. Science 2009, 325, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.R.; Quignon, P.; Li, L.; Schoenebeck, J.J.; Degenhardt, J.D.; Lohmueller, K.E.; Zhao, K.; Brisbin, A.; Parker, H.G.; vonHoldt, B.M.; et al. A Simple Genetic Architecture Underlies Morphological Variation in Dogs. PloS Biol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Righi, C.; Guelfi, G.; Enas, C.; Moscati, L.; Mancini, S.; Diverio, S. Multi-Operator Qualitative Behavioural Assessment for dogs entering the shelter. Appl. Anim. Behav. Sci. 2019, 213, 107–116. [Google Scholar] [CrossRef]
- Bremhorst, A.; Mongillo, P.; Howell, T.; Marinelli, L. Spotlight on Assistance Dogs-Legislation, Welfare and Research. Animals 2018, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.E.; Levy, K.M.; Kennedy, B.S.; Duffy, D.L.; Serpell, J.A.; Maclean, E.L. Predictive Models of Assistance Dog Training Outcomes Using the Canine Behavioral Assessment and Research Questionnaire and a Standardized Temperament Evaluation. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Chopik, J.W.; Weaver, J.R. Old dog, new tricks: Age differences in dog personality traits, associations with human personality traits, and links to important outcomes. J. Res. Pers. 2019, 79, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Serpell, J. The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Jones, A.C.; Gosling, S.D. Temperament and personality in dogs (Canis familiaris): A review and evaluation of past research. Appl. Anim. Behav. Sci. 2005, 95, 1–53. [Google Scholar] [CrossRef]
- Miklόsi, A. Dog Behaviour, Evolution, and Cognition, 2nd ed.; Oxford University: Oxford, UK, 2015. [Google Scholar]
- Goddard, M.E.; Beilharz, R.G. The relationship of fearfulness to, and the effects of, sex, age and experience on exploration and activity in dogs. Appl. Anim. Behav. Sci. 1984, 12, 267–278. [Google Scholar] [CrossRef]
- Murphy, J.A. Describing categories of temperament in potential guide dogs for the blind. Appl. Anim. Behav. Sci. 1998, 58, 163–178. [Google Scholar] [CrossRef]
- Maejima, M.; Inoue-Murayama, M.; Tonosaki, K.; Matsuura, N.; Kato, S.; Saito, Y.; Weiss, A.; Murayama, Y.; Ito, S. Traits and genotypes may predict the successful training of drug detection dogs. Appl. Anim. Behav. Sci. 2007, 107, 287–298. [Google Scholar] [CrossRef]
- Hsu, Y.; Serpell, J.A. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. JAVMA 2003, 223, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.; Blascovich, J. The value of service dogs for people with severe ambulatory disabilities. A randomized controlled trial. JAMA 1996, 275, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Audrestch, H.M.; Whelan, C.T.; Grice, D.; Asher, L.; England, G.C.; Freeman, S.L. Recognizing the value of assistance dogs in society. Disabil. Health J. 2015, 8, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Ericsson, N.; Hansen, N.K.; Fitzgerald, S. Benefits of assistance dogs: A review. Rehabil. Psychol. 2002, 47, 251–277. [Google Scholar] [CrossRef]
- Byrne, C.; Zuerndorfer, J.; Freil, L.; Han, X.; Sirolly, A.; Gilliland, S.; Starner, T.; Jackson, M. Predicting the Suitability of Service Animals Using Instrumented Dog Toys. IMWUT 2018, 1. [Google Scholar] [CrossRef]
- Ostrander, E.A.; National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland, USA.
- Arata, S.; Momozawa, Y.; Takeuchi, Y.; Mori, Y. Important behavioral traits for predicting guide dog qualification. J. Vet. Med. Sci. 2010, 75, 539–545. [Google Scholar] [CrossRef]
- Duffy, D.L.; Serpell, J.A. Behavioral assessment of guide and service dogs. J. Vet. Med. Sci. 2008, 3, 186–188. [Google Scholar] [CrossRef]
- VonHoldt, B.M.; Shuldiner, E.; Janowitz, I.K.; Kartzinel, R.Y.; Hogan, A.; Brubaker, B.; Wanser, S.; Stahler, D.; Wynne, C.D.; Ostrander, E.A.; et al. Structural variants in genes associated with human Williams-Beuren Syndrome underlie stereotypical hyper-sociability in domestic dogs. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef]
- Shubert, C. The genomic basis of the Williams—Beuren syndrome. Cell Mol. Life Sci. 2009, 66, 1178–1197. [Google Scholar] [CrossRef]
- vonHoldt, B.M.; Ji, S.S.; Aardema, M.L.; Stahler, D.R.; Udell, M.A.R.; Sinsheimer, J.S. Activity of genes with functions in human William-Beuren Syndrome is impacted by mobile element insertions in the gray wolf genome. Genome Biol. Evol. 2018, 10, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Okada, N. LINEs mobilize SINEs in the eel through a shared 3′ sequence. Cell 2002, 111, 433–444. [Google Scholar] [CrossRef]
- Hothorn, T.; Buehlmann, P.; Dudoit, S.; Molinaro, A.; Laan, M.D.V. Survival Ensembles. Biostatistics 2006, 7, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Boulesteix, A.L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional Variable Importance for Random Forests. BMC Bioinform. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Cule, E.; Iorio, M.D. Ridge regression in prediction problems: Automatic choice of the ridge parameter. Genet. Epidemiol. 2013, 37, 704–714. [Google Scholar] [CrossRef]
- R: A language and environment for statistical computing. 2017 R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 10 November 2018).
- Warnes, G.; Gorjanc, G.; Leisch, F.; Man, M. Population Genetics. R package version 1.3.8.1.2. 2019. Available online: https://cran.r-project.org/web/packages/genetics/index.html (accessed on 1 November 2018).
- Parker, H.G.; Kim, L.V.; Sutter, N.B.; Carlson, S.; Lorentzen, T.D.; Malek, T.B.; Johnson, G.S.; DeFrance, H.B.; Ostrander, E.A.; Kruglyak, L. Genetic structure of the purebred domestic dog. Science 2004, 304, 1160–1164. [Google Scholar] [CrossRef]
- VonHoldt, B.M.; Pollinger, J.P.; Lohmueller, K.E.; Han, E.; Parker, H.G.; Quignon, P.; Degenhardt, J.D.; Boyko, A.R.; Earl, D.A.; Auton, A.; et al. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 2010, 464, 898–902. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.G.; Dreger, D.L.; Rimbault, M.; Davis, B.W.; Mullen, A.B.; Carpintero-Ramirez, G.; Ostrander, E.A. Genomic Analyses Reveal the Influence of Geographic Origin, Migration, and Hybridization on Modern Dog Breed Development. Cell Rep. 2017, 19, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, B.; Walkowicz, C. The Atlas of Dog Breeds of the World, 5th ed.; TFH Publications: Neptune, NJ, USA, 1995. [Google Scholar]
- Bruce, F. The New Encyclopedia of the Dog, 2nd ed.; Dorling Kindersly: London, UK, 2000. [Google Scholar]
- American Kennel Club. The Complete Dog Book, 20th ed.; Ballentine Books: New York, NY, USA, 2006. [Google Scholar]
- Nakamura, N.; Toba, S.; Hirai, M.; Morishita, S.; Mikami, T.; Konishi, M.; Itoh, N.; Kurosaka, A. Cloning and expression of a brain-specific putative UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase gene. Biol. Pharm. Bull. 2005, 28, 429–433. [Google Scholar] [CrossRef]
- Nakayama, Y.; Nakamura, N.; Oki, S.; Wakabayashi, M.; Ishihana, Y.; Miyake, A.; Itoh, N.; Kurosaka, A. A putative polypeptide N-acetylgalactosaminyltransferase/Williams-Beuren syndrome chromosome region 17 (WBSCR17) regulates lamellipodium formation and macropinocytosis. J. Biol. Chem. 2012, 287, 32222–32235. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, G.C.; Harvey, N.D.; England, G.C.W.; Aher, L. Using the incidence and impact of behavioural conditions in guide dogs to investigate patterns in undesirable behaviour in dogs. Sci. Rep. UK 2016, 6. [Google Scholar] [CrossRef]



| Dataset Locus Name | Social Interest in Strangers * | Hypersociability | Attention Bias to Stimuli * |
|---|---|---|---|
| Assistance and pet dogs of 1–5 years of age (n = 117) | |||
| Cfa6.6 | 0.071 (0.479) | 0.609 (0.137) | 0.093 (0.841) |
| Cfa6.7 | 0.029 (0.777) | 0.257 (0.530) | 0.001 (0.999) |
| Cfa6.66 | 0.238 (0.024) | −0.398 (0.345) | 1.239 (0.009) |
| Cfa6.83 | 0.031 (0.762) | −0.257 (0.540) | 0.085 (0.858) |
| Non-assistance dogs of 1–5 years of age (n = 69) | |||
| Cfa6.6 | 0.328 (0.479) | 0.057 (0.242) | 0.495 (0.127) |
| Cfa6.7 | 0.260 (0.777) | −0.005 (0.924) | −0.097 (0.768) |
| Cfa6.66 | 0.771 (0.053) | −0.039 (0.442) | 0.363 (0.319) |
| Cfa6.83 | 0.059 (0.882) | −0.082 (0.110) | −0.085 (0.816) |
| Non-assistance dogs >5 years of age (n = 95) | |||
| Cfa6.6 | −0.148 (0.708) | −1.167 (0.002) | −0.526 (0.217) |
| Cfa6.7 | 0.367 (0.353) | 0.209 (0.592) | 0.077 (0.856) |
| Cfa6.66 | −0.392 (0.342) | −0.268 (0.500) | −0.273 (0.545) |
| Cfa6.83 | 0.186 (0.654) | 0.137 (0.731) | 0.006 (0.990) |
| Assistance and pet dogs owned for 1–5 years (n = 115) | |||
| Cfa6.6 | 0.198 (0.562) | 0.521 (0.206) | −0.210 (0.474) |
| Cfa6.7 | 0.064 (0.848) | 0.349 (0.395) | −0.020 (0.944) |
| Cfa6.66 | −0.592 (0.105) | −0.385 (0.359) | 0.656 (0.038) |
| Cfa6.83 | 0.127 (0.723) | −0.248 (0.553) | 0.152 (0.629) |
| Pet dogs owned for 1–5 years (n = 65) | |||
| Cfa6.6 | 0.458 (0.208) | 0.521 (0.206) | 0.492 (0.131) |
| Cfa6.7 | 0.312 (0.391) | 0.349 (0.395) | 0.006 (0.984) |
| Cfa6.66 | 0.392 (0.335) | −0.385 (0.359) | 0.092 (0.803) |
| Cfa6.83 | −0.043 (0.914) | −0.248 (0.553) | −0.001 (0.997) |
| Non-assistance dogs owned for >5 years (n = 96) | |||
| Cfa6.6 | −0.025 (0.770) | −1.215 (0.003) | 0.058 (0.804) |
| Cfa6.7 | 0.037 (0.668) | 0.094 (0.821) | −0.071 (0.761) |
| Cfa6.66 | −0.132 (0.137) | −0.571 (0.921) | −0.197 (0.415) |
| Cfa6.83 | −0.060 (0.504) | 0.042 (0.921) | 0.192 (0.431) |
| Locus | Assistance Dogs (n = 196) | Pet Dogs (n = 126) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | p-Value * | 0 | 1 | 2 | p-Value * | p-Value ** | |
| Assistance (n = 49) and pet dogs (n = 69) of 1–5 years of age with C-BARQ© data | |||||||||
| Cfa6.6 | 0.04 | 0.00 | 0.96 | 3.3 × 10−4 | 0.22 | 0.34 | 0.44 | 0.021 | 2.3 × 10−10 |
| Cfa6.7 | 0.10 | 0.49 | 0.41 | 0.75 | 0.34 | 0.40 | 0.27 | 0.094 | 0.02 |
| Cfa6.66 | 1.00 | 0.00 | 0.00 | NP | 0.75 | 0.13 | 0.12 | 3.4 × 10−5 | 9.7 × 10−7 |
| Cfa6.83 | 0.63 | 0.33 | 0.04 | 1.00 | 0.57 | 0.28 | 0.16 | 0.007 | 0.53 |
| Assistance (n = 147) and pet dogs (n = 58) of the Retriever clade | |||||||||
| Cfa6.6 | 0.16 | 0.19 | 0.65 | 1.1 × 10−8 | 0.19 | 0.36 | 0.45 | 0.094 | 2.1 × 10−12 |
| Cfa6.7 | 0.48 | 0.41 | 0.11 | 0.566 | 0.03 | 0.47 | 0.03 | 0.309 | 1 |
| Cfa6.66 | 0.69 | 0.27 | 0.04 | 0.400 | 0.93 | 0.05 | 0.02 | 0.085 | 8.5 × 10−4 |
| Cfa6.83 | 0.79 | 0.13 | 0.08 | 5.4 × 10−7 | 0.40 | 0.29 | 0.40 | 0.002 | 1.1 × 10−14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandon, D.; Ressler, K.; Petticord, D.; Papa, A.; Jiranek, J.; Wilkinson, R.; Kartzinel, R.Y.; Ostrander, E.A.; Burney, N.; Borden, C.; et al. Homozygosity for Mobile Element Insertions Associated with WBSCR17 Could Predict Success in Assistance Dog Training Programs. Genes 2019, 10, 439. https://doi.org/10.3390/genes10060439
Tandon D, Ressler K, Petticord D, Papa A, Jiranek J, Wilkinson R, Kartzinel RY, Ostrander EA, Burney N, Borden C, et al. Homozygosity for Mobile Element Insertions Associated with WBSCR17 Could Predict Success in Assistance Dog Training Programs. Genes. 2019; 10(6):439. https://doi.org/10.3390/genes10060439
Chicago/Turabian StyleTandon, Dhriti, Kyra Ressler, Daniel Petticord, Andrea Papa, Juliana Jiranek, Riley Wilkinson, Rebecca Y. Kartzinel, Elaine A. Ostrander, Nathaniel Burney, Carol Borden, and et al. 2019. "Homozygosity for Mobile Element Insertions Associated with WBSCR17 Could Predict Success in Assistance Dog Training Programs" Genes 10, no. 6: 439. https://doi.org/10.3390/genes10060439
APA StyleTandon, D., Ressler, K., Petticord, D., Papa, A., Jiranek, J., Wilkinson, R., Kartzinel, R. Y., Ostrander, E. A., Burney, N., Borden, C., Udell, M. A. R., & VonHoldt, B. M. (2019). Homozygosity for Mobile Element Insertions Associated with WBSCR17 Could Predict Success in Assistance Dog Training Programs. Genes, 10(6), 439. https://doi.org/10.3390/genes10060439






