PARP Inhibitors in Prostate Cancer–the Preclinical Rationale and Current Clinical Development
Abstract
1. Introduction
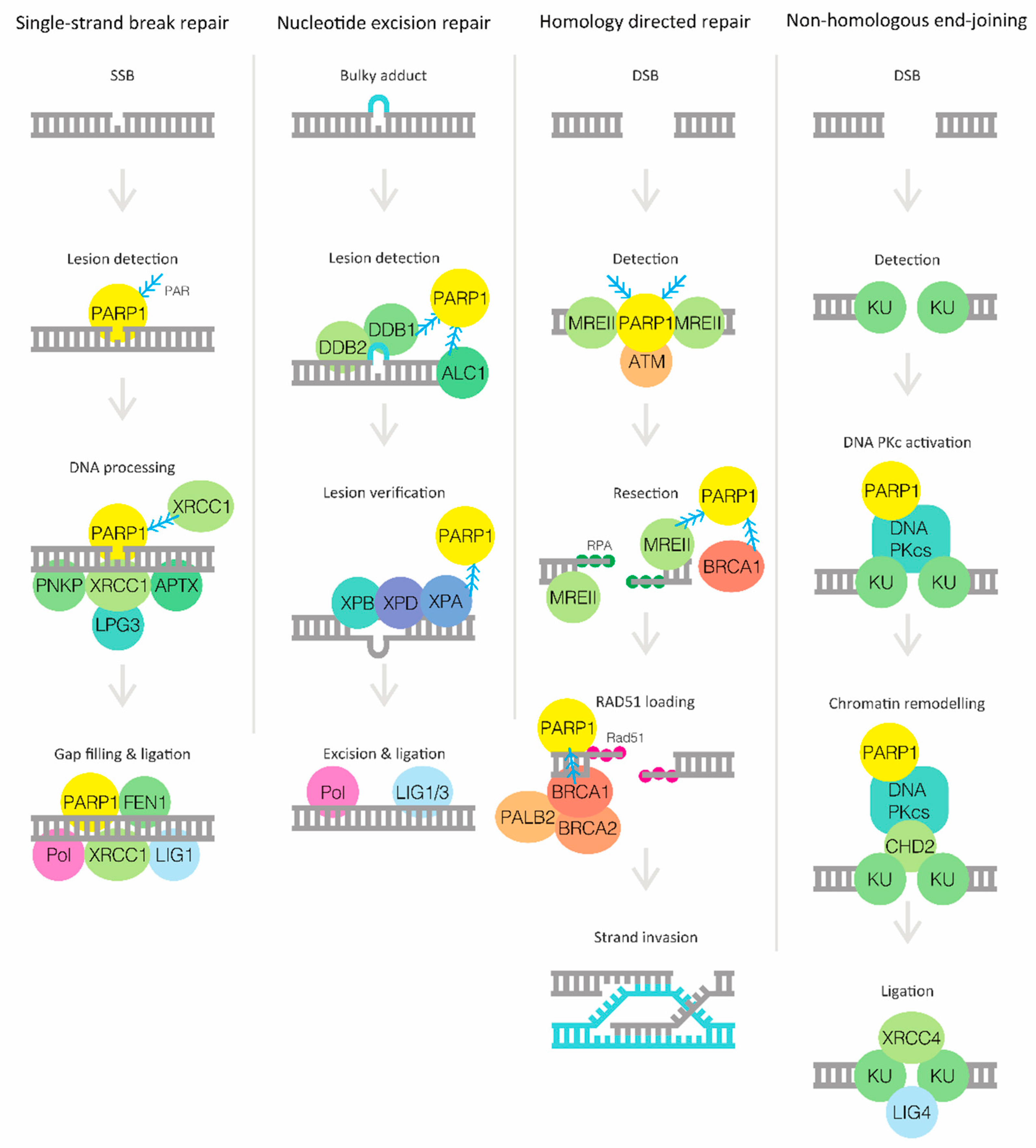
2. DNA Damage Response and PARP
3. Targeting DNA Repair Defects in Cancer with PARP Inhibitors
4. Germline Mutations in DNA Damage Repair Genes in Prostate cancer
5. Somatic Mutations in DNA Damage Repair Genes in Prostate Cancer
6. Crosstalk between AR Signaling and DNA Damage Response
7. Clinical Development of PARP Inhibitors in Prostate Cancer
Adverse Events and Tolerability
8. Predictive Markers of Response to PARP Inhibitors
9. Mechanisms of Intrinsic and Acquired Resistance to PARP Inhibitors
10. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sartor, O.; de Bono, J.S. Metastatic prostate cancer. N. Eng. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Bartkova, J.; Rezaei, N.; Liontos, M.; Karakaidos, P.; Kletsas, D.; Issaeva, N.; Vassiliou, L.F.; Kolettas, E.; Niforou, K.; Zoumpourlis, V.C.; et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006, 444, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell. 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Dantzer, F.; Ame, J.; Murcia, G.D. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Murcia, J.M.D.; Ricoul, M.; Tartier, L.; Niedergang, C.; Huber, A.; Dantzer, F.; Schreiber, V.; Amé, J.; Dierich, A.; LeMeur, M.; et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003, 22, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Stingl, L.; Morrison, C.; Jantsch, M.; Los, M.; Schulze-Osthoff, K.; Wagner, E.F. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997, 11, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Masutani, M.; Nozaki, T.; Nishiyama, E.; Shimokawa, T.; Tachi, Y.; Suzuki, H.; Nakagama, H.; Wakabayashi, K.; Sugimura, T. Function of poly(ADP-ribose) polymerase in response to DNA damage: Gene-disruption study in mice. Mol. Cell Biochem. 1999, 193, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Bürkle, A.; Virág, L. Poly(ADP-ribose): PARadigms and PARadoxes. Mol. Aspects Med. 2013, 34, 1046–1065. [Google Scholar] [CrossRef] [PubMed]
- Langelier, M.; Elsemann, T.; Riccio, A.A.; Pascal, J.M. PARP family enzymes: Regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 2018, 53, 187–198. [Google Scholar] [CrossRef]
- Caldecott, K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008, 9, 619–631. [Google Scholar] [CrossRef]
- Satoh, M.S.; Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992, 356, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.R.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Pines, A.; Vrouwe, M.; Marteijn, J.; Typas, D.; Luijsterburg, M.; Cansoy, M.; Hensbergen, P.; Deelder, A.; Groot, A.; Matsumoto, S.; et al. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol. 2012, 199, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, M.; de Krijger, I.; Wiegant, W.; Shah, G.; Shah, R.; Smeenk, G.; de Groot, A.L.; Pines, A.; Vertegaal, A.O.; Jacobs, J.L.; et al. PARP1 links CHD2-mediated chromatin expansion and H3.3 deposition to DNA repair by non-homologous end-joining. Mol. Cell 2016, 61, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Haber, J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014, 6, a016428. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Taylor, M.G.; Boulton, S. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.E.; Timinszky, G.; Arribas-bosacoma, R.; Kozlowski, M.; Hassa, P.O.; Hassler, M.; Ladurner, A.G.; Pearl, L.H.; Olive, A.W. The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat. Struct. Mol. Biol. 2012, 19, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.; Van Dang, V.D.; Lees-Miller, S.P. DNA-PK: The means to justify the ends? Adv. Immunol. 2008, 99, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Haince, J.F.; McDonald, D.; Rodrigue, A.; Déry, U.; Masson, J.Y.; Hendzel, M.J.; Poirier, G.G. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J. Biol. Chem. 2008, 283, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, X. Function of BRCA1 in the DNA damage response is mediated by ADP-ribosylation. Cancer Cell 2013, 23, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Buisson, R.; Dion-Côté, A.; Coulombe, Y.; Launay, H.; Cai, H.; Stasiak, A.Z.; Stasiak, A.; Xia, B.; Masson, J. Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination. Nat. Struct. Mol. Biol. 2010, 17, 1247. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.B.; Carreira, A.; Kowalczykowski, S.C. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 2010, 467, 678. [Google Scholar] [CrossRef] [PubMed]
- Shahid, T.; Soroka, J.; Kong, E.H.; Malivert, L.; McIlwraith, M.J.; Pape, T.; Zhang, X. Structure and mechanism of action of the BRCA2 breast cancer tumor suppressor. Nat. Struct. Mol. Biol. 2014, 21, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.S.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, T.; Lehnert, B.E.; Halbrook, J.; Le Trong, H.; Hoekstra, M.F.; Chen, D.J.; Peterson, S.R. Stimulation of the DNA-dependent protein kinase by poly(ADP-ribose) polymerase. J. Biol. Chem. 1998, 273, 14461–14467. [Google Scholar] [CrossRef]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Martin, N.M. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Ashworth, A. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Eng. J. Med. 2009, 361. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Eng. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; O’Connor, M.J.; de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016, 8, 362ps17. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, L.; Garber, J.E. PARP inhibitors in the management of breast cancer: Current data and future prospects. BMC Med. 2015, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.L.; Smith, J.Y.; Wielckens, K.; Hilz, H.; Jacobson, M.K. Cellular recovery of dividing and confluent C3H10T1/2 cells from N-methyl-N′-nitro-N-nitrosoguanidine in the presence of ADP ribosylation inhibitors. Carcinogenesis 1985, 6, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Hunting, D.; Trucco, C.; Gowans, B.; Grunwald, D.; De Murcia, G.; De Murcia, J.M. A dominant-negative mutant of human poly(ADP-ribose) polymerase affects cell recovery, apoptosis, and sister chromatid exchange following DNA damage. Proc. Natl. Acad. Sci. USA 1995, 92, 4753–4757. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.N.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Ahel, D.; Hořejší, Z.; Wiechens, N.; Polo, S.E.; Garcia-Wilson, E.; Ahel, I.; Owen-Hughes, T. Poly(ADP-ribose)–dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 2009, 325, 1240–1243. [Google Scholar] [CrossRef]
- Gottschalk, A.J.; Trivedi, R.D.; Conaway, J.W.; Conaway, R.C. Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1.PARP1.nucleosome intermediate. J. Biol. Chem. 2012, 287, 43527–43532. [Google Scholar] [CrossRef]
- Murai, J.; Huang SY, N.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Pommier, Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014, 13, 433–443. [Google Scholar] [CrossRef]
- Amé, J.; Rolli, V.; Schreiber, V.; Niedergang, C.; Apiou, F.; Decker, P.; Muller, S.; Höger, T.; Ménissier-de Murcia, J.; de Murcia, G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 1999, 274, 17860–17868. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. JCO 2013, 31, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Leongamornlert, D.; Saunders, E.; Dadaev, T.; Tymrakiewicz, M.; Goh, C.; Jugurnauth-Little, S.; Kozarewa, I.; Fenwick, K.; Assiotis, I.; Barrowdale, D.; et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br. J. Cancer 2014, 110, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Romero-Laorden, N.; del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. JCO 2019, 37, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Eng. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Petrovics, G.; Price, D.K.; Lou, H.; Chen, Y.; Garland, L.; Bass, S.; Jones, K.; Kohaar, I.; Ali, A.; Ravindranath, L.; et al. Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population. Prostate Cancer Prostatic Dis. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Asangani, I.A. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.; Schultz, N.; Lonigro, R.J.; Mosquera, J.; Montgomery, B.; Taplin, M.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; Jarosz, M.; Lipson, D.; Tagawa, S.T.; Nanus, D.M.; et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 2012, 63, 920–926. [Google Scholar] [CrossRef]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis. Oncol. 2017, 2017. [Google Scholar] [CrossRef]
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.; Cai, L.; Zheng, D.; et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Mandigo, A.C.; Gordon, N.; Huang, F.; Gaur, S.; de Leeuw, R.; Zhao, S.G.; Evans, J.; Han, S.; Parsons, T.; et al. PARP-1 regulates DNA repair factor availability. EMBO Mol. Med. 2018, e8816. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Horbinski, C.; Hensley, P.J.; Matuszak, E.A.; Atkinson, T.; Kyprianou, N. PARP-1 regulates epithelial–mesenchymal transition (EMT) in prostate tumorigenesis. Carcinogenesis 2014, 35, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Karanika, S.; Karantanos, T.; Li, L.; Wang, J.; Park, S.; Yang, G.; Zuo, X.; Song, J.H.; Maity, S.N.; Manyam, G.C.; et al. Targeting DNA damage response in prostate cancer by inhibiting androgen receptor-CDC6-ATR-Chk1 signaling. Cell Rep. 2017, 18, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Schelman, W.R.; Wilding, G.; Moreno, V.; Baird, R.D.; Miranda, S.; Hylands, L.; Riisnaes, R.; Forster, M.; Omlin, A.; et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2013, 14, 882–892. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Omlin, A.; Hylands, L.; Miranda, S.; Barber, L.J.; Riisnaes, R.; Reid, A.H.; Attard, G.; Chen, L.; Kozarewa, I.; et al. Poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of advanced germline BRCA2 mutant prostate cancer. Annal. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1416–1418. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. JCO 2014, 33, 244–250. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Eng. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Carducci, M.; Slovin, S.; Cetnar, J.; Qian, J.; McKeegan, E.; Refici-Buhr, M.; Chyla, B.; Shepherd, S.; Giranda, V.; et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest. N. Drugs 2014, 32, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, S.; Appleman, L.J.; Belani, C.P.; Chen, A.; Chu, E.; Beumer, J.H.; Puhalla, S. Preliminary activity of veliparib (V) in BRCA2-mutated metastatic castration-resistant prostate cancer (mCRPC). JCO 2015, 33 (Suppl. 7), 170. [Google Scholar] [CrossRef]
- Gray, H.J.; Bell-McGuinn, K.; Fleming, G.F.; Cristea, M.; Xiong, H.; Sullivan, D.; Luo, Y.; McKee, M.D.; Munasinghe, W.; Martin, L.P. Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecol. Oncol. 2018, 148, 507–514. [Google Scholar] [CrossRef]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.; Robinson, D.; Lonigro, R.J.; et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: Results from NCI 9012. J. Clin. Oncol. 2017. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; McGovern, U.B.; Elliott, T.; Jones, R.J.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.A.; Parikh, O.; et al. TOPARP-B: A phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. JCO 2019, 37 (Suppl. 15), 5005. [Google Scholar] [CrossRef]
- De Bono, J.S.; Hussain, M.; Thiery-Vuillemin, A.; Mateo, J.; Sartor, A.O.; Chi, K.N.; Fizazi, K.; Twardowski, P.; Agarwal, N.; Sandhu, S.K.; et al. PROfound: A randomized phase III trial evaluating olaparib in patients with metastatic castration-resistant prostate cancer and a deleterious homologous recombination DNA repair aberration. JCO 2017, 35 (Suppl. 15), TPS5091. [Google Scholar] [CrossRef]
- Ryan, C.J.; Abida, W.; Bryce, A.H.; Balar, A.V.; Dumbadze, I.; Given, R.W.; Morris, D.; Petrylak, D.P.; Redfern, C.H.; Scher, H.I.; et al. TRITON3: An international, randomized, open-label, phase III study of the PARP inhibitor rucaparib vs. physician’s choice of therapy for patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD). JCO 2018, 36 (Suppl. 6), TPS389. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Li, L.; Corn, P.G.; Thompson, T.C. DNA damage response and prostate cancer: Defects, regulation and therapeutic implications. Oncogene 2015, 34, 2815. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Ye, D.; Mateo, J.; Goessl, C.D.; Kang, J.; Liu, S.; Saad, F. PROPEL: A randomized, phase III trial evaluating the efficacy and safety of olaparib combined with abiraterone as first-line therapy in patients with metastatic castration-resistant prostate cancer (mCRPC). JCO 2019, 37 (Suppl. 7), TPS340. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Fay, A.; Carles, J.; Shore, N.D.; Nordquist, L.T.; Karsh, L.I.; Dunshee, C.; Ponnathapura Nandakumar, S.; Sullivan, B.; et al. Talapro-2: A 2-part, placebo-controlled phase 3 study of talazoparib (TALA) with background enzalutamide (ENZA) in metastatic castration-resistant prostate cancer (mCRPC) with DNA damage repair deficiencies. JCO 2018, 36 (Suppl. 15), TPS5091. [Google Scholar] [CrossRef]
- Janssen Research & Development, LLC. A Study of Niraparib in Combination with Abiraterone Acetate and Prednisone Versus Abiraterone Acetate and Prednisone for Treatment of Participants with Metastatic Prostate Cancer (MAGNITUDE). Available online: https://clinicaltrials.gov/ct2/show/NCT03748641 (accessed on 30 May 2019).
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef] [PubMed]
- Merck Sharp & Dohme Corp. Study of Pembrolizumab (MK-3475) Plus Olaparib Versus Abiraterone Acetate or Enzalutamide in Metastatic Castration-Resistant Prostate Cancer (mCRPC) (MK-7339-010/KEYLYNK-010). Available online: https://clinicaltrials.gov/ct2/show/NCT03834519 (accessed on 30 May 2019).
- Juvekar, A.; Burga, L.N.; Hu, H.; Lunsford, E.P.; Ibrahim, Y.H.; Balmañà, J.; Rajendran, A.; Papa, A.; Spencer, K.; Lyssiotis, C.A.; et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012, 2, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.H.; García-García, C.; Serra, V.; He, L.; Torres-Lockhart, K.; Prat, A.; Anton, P.; Cozar, P.; Guzmán, M.; Grueso, J.; et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012, 2, 1036–1047. [Google Scholar] [CrossRef]
- González-Billalabeitia, E.; Seitzer, N.; Song, S.J.; Song, M.S.; Patnaik, A.; Liu, X.; Epping, M.T.; Papa, A.; Hobbs, R.M.; Chen, M.; et al. Vulnerabilities of PTEN–TP53-deficient prostate cancers to compound PARP–PI3K inhibition. Cancer Discov. 2014, 4, 896–904. [Google Scholar] [CrossRef]
- Bryant, H.E.; Helleday, T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006, 34, 1685–1691. [Google Scholar] [CrossRef]
- Peng, Y.; Liao, Q.; Tan, W.; Peng, C.; Hu, Z.; Chen, Y.; Li, Z.; Li, J.; Zhen, B.; Zhu, W.; et al. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat. Commun. 2019, 10, 1224. [Google Scholar] [CrossRef]
- McCabe, N.; Turner, N.C.; Lord, C.J.; Kluzek, K.; Bialkowska, A.; Swift, S.; Giavara, S.; OConnor, M.J.; Tutt, A.N.; Zdzienicka, M.Z.; et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006, 66, 8109–8115. [Google Scholar] [CrossRef] [PubMed]
- Kalev, P.; Simicek, M.; Vazquez, I.; Munck, S.; Chen, L.; Soin, T.; Danda, N.; Chen, W.; Sablina, A. Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition. Cancer Res. 2012, 72, 6414–6424. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Lord, C.J.; Iorns, E.; Brough, R.; Swift, S.; Elliott, R.; Rayter, S.; Tutt, A.N.; Ashworth, A. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008, 27, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.H.; Sokolova, A.O.; McNatty, A.L.; Cheng, H.H.; Eisenberger, M.A.; Bryce, A.H.; Schweizer, M.T.; Antonarakis, E.S. Differential response to olaparib treatment among men with metastatic castration-resistant prostate cancer harboring BRCA1 or BRCA2 versus ATM mutations. Eur.Urol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef]
- Naipal, K.A.T.; Verkaik, N.S.; Ameziane, N.; van Deurzen, C.H.; ter Brugge, P.; Meijers, M.; Sieuwerts, A.M.; Martens, J.W.; O’Connor, M.J.; Vrieling, H.; et al. Functional ex vivo assay to select homologous Recombination–Deficient breast tumors for PARP inhibitor treatment. Clin. Cancer Res. 2014, 20, 4816–4826. [Google Scholar] [CrossRef]
- Chan, N.; Pires, I.M.; Bencokova, Z.; Coackley, C.; Luoto, K.R.; Bhogal, N.; Lakshman, M.; Gottipati, P.; Oliver, F.J.; Helleday, T.; et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. 2010, 70, 8045–8054. [Google Scholar] [CrossRef]
- Pettitt, S.J.; Lord, C.J. Dissecting PARP inhibitor resistance with functional genomics. Curr. Opin. Genet. Dev. 2019, 54, 55–63. [Google Scholar] [CrossRef]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.; et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef]
- Ding, X.; Chaudhuri, A.R.; Callen, E.; Pang, Y.; Biswas, K.; Klarmann, K.D.; Martin, B.K.; Burkett, S.; Cleveland, L.; Stauffer, S.; et al. Synthetic viability by BRCA2 and PARP1/ARTD1 deficiencies. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Feng, W.; Jasin, M. Homologous recombination and replication fork protection: BRCA2 and more! Cold Spring Harb. Symp. Quant. Biol. 2017, 82, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.; Alumkal, J.J.; Wyatt, A.W.; Kothari, V.; Foye, A.; Lloyd, P.; Aggarwal, R.; Kim, W.; Lu, E.; Schwartzman, J.; et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017, 7, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Oplustilova, L.; Wolanin, K.; Mistrik, M.; Korinkova, G.; Simkova, D.; Bouchal, J.; Lenobel, R.; Bartkova, J.; Lau, A.; OO’Connor, M.J.; et al. Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment. Cell Cycle 2012, 11, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, J.E.; Kersbergen, A.; Boon, U.; Sol, W.; van Deemter, L.; Zander, S.A.; Drost, R.; Wientjens, E.; Ji, J.; Aly, A.; et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013, 3, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R.; Barral, P.; Vannier, J.; Borel, V.; Steger, M.; Tomas-Loba, A.; Sartori, A.A.; Adams, I.R.; Batista, F.D.; Boulton, S.J. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 2013, 49, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Chapman, J.R.; Brandsma, I.; Yuan, J.; Mistrik, M.; Bouwman, P.; Bartkova, J.; Gogola, E.; Warmerdam, D.; Barazas, M.; et al. REV7 counteracts DNA double-strand break resection and affects PARP inhibition. Nature 2015, 521, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Barazas, M.; Annunziato, S.; Pettitt, S.J.; Krijger, I.d.; Ghezraoui, H.; Roobol, S.J.; Lutz, C.; Frankum, J.; Song, F.F.; Brough, R.; et al. The CST complex mediates end protection at double-strand breaks and promotes PARP inhibitor sensitivity in BRCA1-deficient cells. Cell Rep. 2018, 23, 2107–2118. [Google Scholar] [CrossRef]
- Gogola, E.; Duarte, A.A.; de Ruiter, J.R.; Wiegant, W.W.; Schmid, J.A.; de Bruijn, R.; James, D.I.; Guerrero Llobet, S.; Vis, D.J.; Annunziato, S.; et al. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell 2018, 33, 1078–1093.e12. [Google Scholar] [CrossRef]
- Dréan, A.; Williamson, C.T.; Brough, R.; Brandsma, I.; Menon, M.; Konde, A.; Garcia-Murillas, I.; Pemberton, H.N.; Frankum, J.; Rafiq, R.; et al. Modeling therapy resistance in BRCA1/2-mutant cancers. Mol. Cancer Ther. 2017, 16, 2022–2034. [Google Scholar] [CrossRef]
- Gourley, C.; Balmaña, J.; Ledermann, J.A.; Serra, V.; Dent, R.; Loibl, S.; Pujade-Lauraine, E.; Boulton, S.J. Moving from poly (ADP-ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. JCO 2019, 18, 02050. [Google Scholar] [CrossRef]
- Dylgjeri, E.; McNair, C.; Goodwin, J.F.; Raymon, H.K.; McCue, P.A.; Shafi, A.A.; Leiby, B.; de Leeuw, R.; Kothari, V.; McCann, J.; et al. Pleiotropic impact of DNA-PK in cancer and implications for therapeutic strategies. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kothari, V.; Goodwin, J.F.; Zhao, S.G.; Drake, J.M.; Yin, Y.; Chang, S.L.; Evans, J.R.; Wilder-Romans, K.; Gabbara, K.; Dylgjeri, E.; et al. DNA-dependent protein kinase drives prostate cancer progression through transcriptional regulation of the wnt signaling pathway. Clin. Cancer Res. 2019, 2387, 2018. [Google Scholar] [CrossRef] [PubMed]

| Compound | Company | Indications | Date of Approval | Stage of Development for PCa |
|---|---|---|---|---|
| Olaparib (Lynparza ®) | AstraZeneca Pharmaceuticals LP (Cambridge, UK) | gBRCA-mutated advanced ovarian cancer | December 2014 | III |
| Maintenance treatment of recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer | August 2017 | |||
| gBRCA-mutated HER2-negative metastatic breast cancer | January 2018 | |||
| Maintenance treatment of gBRCA- or sBRCA-mutated advanced epithelial ovarian, fallopian tube or primary peritoneal cancer | December 2018 | |||
| Rucaparib (Rubraca ®) | Clovis Oncology, Inc. (Boulder, CO, USA) | gBRCA- or sBRCA-mutated advanced ovarian cancer | December 2016 | III |
| Maintenance treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer | April 2018 | |||
| Niraparib (Zejula ®) | Tesaro, Inc., (Waltham, MA, USA) | Maintenance treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer. | March 2017 | III |
| Talazoparib (Talzenna ®) | Pfizer, Inc. (New York, NY, USA) | gBRCA-mutated HER2-negative locally advanced or metastatic breast cancer | October 2018 | III |
| Treatment Regimen | Phase | Number of Pca Patients | Biomarkers | CR (%) | PR (%) | SD (%) | PD (%) | OS (Months) | PFS (Months) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Niraparib | I | 21 | BRCAm 5 % (n = 1) | 0 | 43 | [58] | ||||
| Olaparib | I | 3 | BRCA2m 33 % (n = 1) | [30] | ||||||
| Olaparib or niraparib | I | 4 | BRCA2m 100 % (n = 4) | 0 | 25 | 25 | 50 | [59] | ||
| Olaparib | II | 8 | gBRCA2m 87.5% (n = 7), gBRCA1m 12.5 % (n = 1) | 0 | 50 | 25 | 25 | 18.4 | 7.2 | [60] |
| Olaparib (TOPARP-A) | II | 50 | Overall 33 % (n = 16), BRCA2m (n = 7), ATMm (n = 5), BRCA1m or CHEK2m with FANCAm (n = 3), PALB2m (n =1), HDAC2m (n = 1) | 0 | 19 | 6 | 13.8 vs 7.5 * | 9.8 vs 2.7 * | [61] | |
| Abiraterone and prednisolone with or without olaparib | II | 71 vs 71 † | HRR mutation 15 vs 14 % † | 0 | 27 vs 32 †, ns | 48 vs 21 † | 21vs 47 † | 22.7 vs 20.9 †, ns | 13.8 vs 8.2 † | [62] |
| Olaparib and durvalumab | II | 17 | DDRm 77% of responders‡, gBRCA2m 33%, sBRCA2m 22%, gNBNm 11%, PMS2m 11% | 24 | 16.1 | [63] | ||||
| Talazoparib | I | 4 | [64] | |||||||
| Veliparib and temozolomide | I | 26 | 0 | 0§ | 9.2 | 2.1 | [65] | |||
| Veliparib | I | 3 | BRCA2m 100% | 0 | 66 | 33 | [66] | |||
| Veliparib, carboplatin and gemcitabine | I | 1 | BRCAm 100 % (n = 1) | 100 | [67] | |||||
| Abiraterone and prednisolone with or without veliparib | II | 79 vs 74 † | Overall DDRm 31% (n = 25) of 80 analyzed, BRCA2m (n = 15), ATMm (n = 4), BRCA1m (n = 4), RAD51Bm (n = 1), RAD51Cm (n = 1), PALB2m (n = 1), FANCAm (n =1) | 0 vs 3 † | 52 vs 45 † | 26 vs 35 † | 17 vs 20 † | 32.3 vs 30.6 † | 11 vs 10.1 † | [68] |
| Treatment Regimen | Status | Allocation | HRD Selection | Estimated Enrollment | Phase | CTID |
|---|---|---|---|---|---|---|
| PARPi monotherapies | ||||||
| Niraparib | Recruiting | Yes | 301 | II | NCT02854436 | |
| Olaparib | Recruiting | No | 89 | II | NCT01682772 | |
| Olaparib | Active, not recruiting | Randomized | Yes | 340 | III | NCT02987543 |
| Olaparib | Recruiting | Yes * | 50 | II | NCT03047135 | |
| Olaparib | Recruiting | Randomized | No | 96 | II | NCT03263650 |
| Olaparib | Recruiting | Yes | 27 | II | NCT03434158 | |
| Pamiparib | Recruiting | Yes | 100 | II | NCT03712930 | |
| Rucaparib | Recruiting | Yes | 360 | II | NCT02952534 | |
| Rucaparib | Recruiting | Yes | 30 | II | NCT03413995 | |
| Rucaparib | Recruiting | Yes | 29 | II | NCT03533946 | |
| Talazoparib | Recruiting | Yes | 100 | II | NCT03148795 | |
| PARPi + AR signaling inhibitors | ||||||
| Niraparib and Abiraterone and Prednisolone | Recruiting | Randomized | Yes | 1000 | III | NCT03748641 |
| Olaparib or Olaparib and Abiraterone and Prednisone | Recruiting | Randomized | Yes | 70 | II | NCT03012321 |
| Olaparib and Abiraterone and Prednisolone | Recruiting | Randomized | No | 720 | III | NCT03732820 |
| Rucaparib and Abiraterone, Enzalutamide or Docetaxel | Recruiting | Randomized | Yes | 400 | III | NCT02975934 |
| Niraparib and Apalutamide or Abiraterone and Prednisolone | Active, not recruiting | No | 34 | I | NCT02924766 | |
| Niraparib and Enzalutamide | Terminated (Suspended by funder) | No | 2 | I | NCT02500901 | |
| Talazoparib and Enzalutamide | Recruiting | Randomized | Yes† | 872 | III | NCT03395197 |
| PARPi + immune checkpoint inhibitors | ||||||
| Talazoparib and Avelumab | Recruiting | Non-Randomized | No | 242 | Ib/II | NCT03330405 |
| Olaparib and Durvalumab | Recruiting | Yes | 32 | II | NCT03810105 | |
| Niraparib and JNJ-63723283 or Abiraterone and Prednisolone | Recruiting | Non-Randomized | Yes | 150 | Ib–II | NCT03431350 |
| Rucaparib and Nivolumab | Recruiting | Non-Randomized | No | 330 | II | NCT03338790 |
| Rucaparib or Rucaparib and Nivolumab | Recruiting | Randomized | No | 60 | Ib/Iia | NCT03572478 |
| Olaparib and Pembrolizumab | Recruiting | Non-Randomized | No | 400 | I | NCT02861573 |
| Olaparib and Pembrolizumab | Not yet recruiting | Randomized | No | 780 | III | NCT03834519 |
| PARPi + chemotherapy agents | ||||||
| Rucaparib, Docetaxel and Carboplatin | Recruiting | Yes | 20 | II | NCT03442556 | |
| Pamiparib and Temozolomide | Recruiting | Non-Randomized | Yes | 150 | I | NCT03150810 |
| PARPi + radionuclide therapies | ||||||
| Niraparib and Radium Ra 223 Dichloride | Recruiting | No | 6 | I | NCT03076203 | |
| Olaparib and Radium Ra 223 Dichloride | Recruiting | Randomized | No | 112 | II | NCT03317392 |
| Olaparib and 177Lu-PSMA | Not yet recruiting | No | 52 | I | NCT03874884 | |
| PARPi + surgical procedures | ||||||
| Olaparib and RP | Recruiting | Yes | 13 | II | NCT03432897 | |
| Olaparib and RP | Recruiting | Yes | 15 | II | NCT03570476 | |
| PARPi + VEGF RTK inhibitors | ||||||
| Olaparib and Cediranib | Active, not recruiting | Randomized | No | 90 | II | NCT02893917 |
| PARPi + AKT inhibitors | ||||||
| Rucaparib and Ipatasertib | Not yet recruiting | Non-Randomized | No | 54 | Ib | NCT03840200 |
| PARPi + androgens | ||||||
| Olaparib and Testosterone Enanthate or Cypionate | Recruiting | Yes | 30 | II | NCT03516812 | |
| PARPi + ATR inhibitors | ||||||
| Olaparib and AZD6738 | Not yet recruiting | Non-Randomized | No | 47 | II | NCT03787680 |
| PARPi + GnRH antagonists | ||||||
| Olaparib and Degarelix | Recruiting | Randomized | No | 20 | I | NCT02324998 |
| PARPi + nanoparticle conjugate | ||||||
| Olaparib and CRLX101 | Recruiting | Non-Randomized | No | 123 | I/II | NCT02769962 |
| Personalized medicine approach | ||||||
| SMMART therapy | Not yet recruiting | No | 52 | I | NCT03878524 | |
| PARPi + radiation treatment | ||||||
| Olaparib and RT | Recruiting | Randomized | No | 112 | I/II | NCT03317392 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtanen, V.; Paunu, K.; Ahlskog, J.K.; Varnai, R.; Sipeky, C.; Sundvall, M. PARP Inhibitors in Prostate Cancer–the Preclinical Rationale and Current Clinical Development. Genes 2019, 10, 565. https://doi.org/10.3390/genes10080565
Virtanen V, Paunu K, Ahlskog JK, Varnai R, Sipeky C, Sundvall M. PARP Inhibitors in Prostate Cancer–the Preclinical Rationale and Current Clinical Development. Genes. 2019; 10(8):565. https://doi.org/10.3390/genes10080565
Chicago/Turabian StyleVirtanen, Verneri, Kreetta Paunu, Johanna K. Ahlskog, Reka Varnai, Csilla Sipeky, and Maria Sundvall. 2019. "PARP Inhibitors in Prostate Cancer–the Preclinical Rationale and Current Clinical Development" Genes 10, no. 8: 565. https://doi.org/10.3390/genes10080565
APA StyleVirtanen, V., Paunu, K., Ahlskog, J. K., Varnai, R., Sipeky, C., & Sundvall, M. (2019). PARP Inhibitors in Prostate Cancer–the Preclinical Rationale and Current Clinical Development. Genes, 10(8), 565. https://doi.org/10.3390/genes10080565






