Mapping of the New Fertility Restorer Gene Rf-PET2 Close to Rf1 on Linkage Group 13 in Sunflower (Helianthus annuus L.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Field Trials
2.2. Staining for Pollen Viability
2.3. Isolation of Genomic DNA
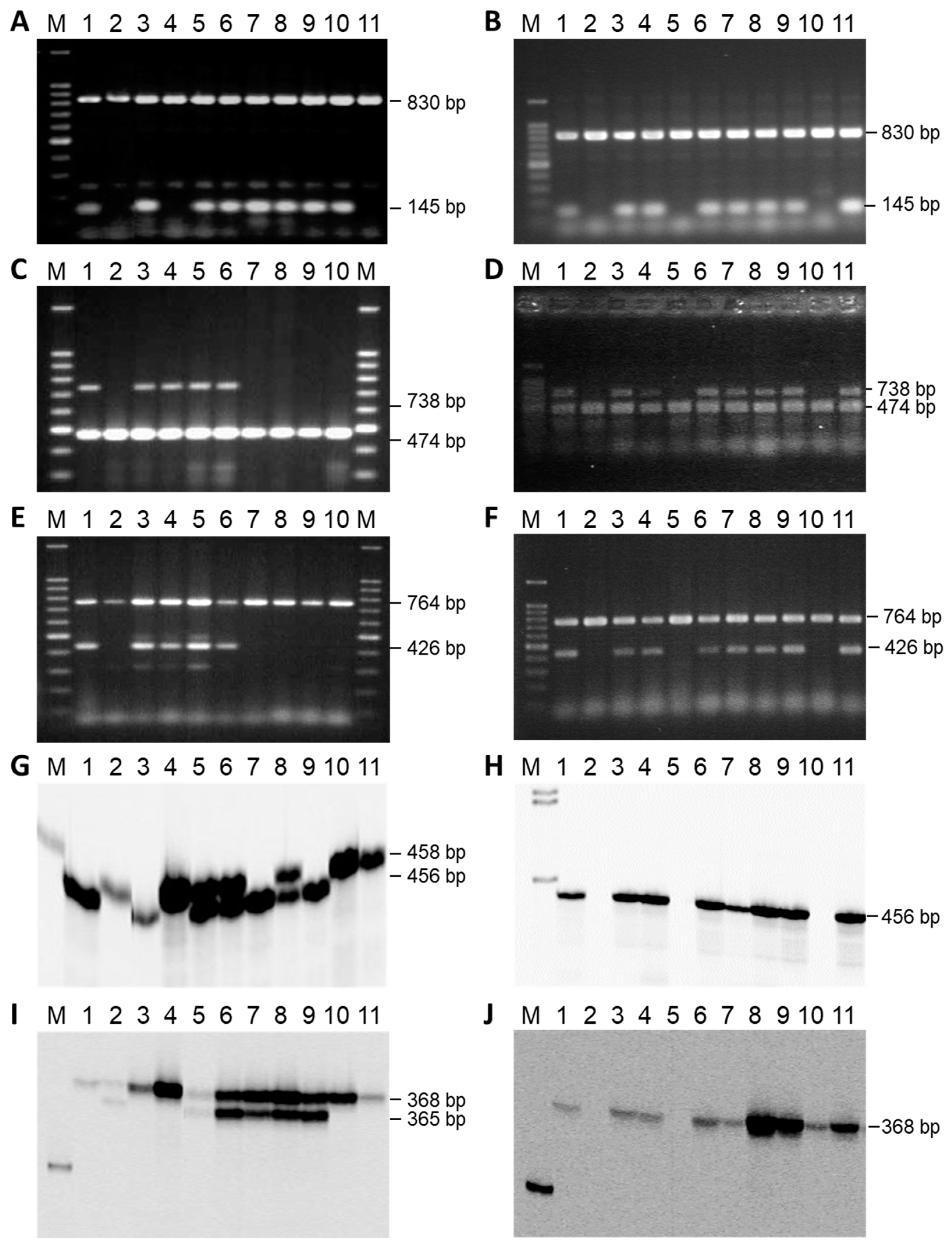
2.4. AFLP-Analyses
2.5. SSR-Analyses
2.6. Cloning and Sequencing of AFLP markers
2.7. Sequence-Tagged Site (STS) Marker Analyses
2.8. Linkage Analyses
3. Results
3.1. CMS PET2 Male Sterility and Its Fertility Restoration in the Mapping Population
3.2. Mapping the Rf-PET2 Gene to Linkage Group 13
3.3. Mapping an Additional AFLP Marker to the Rf1 Gene
3.4. Cloning and Sequencing of AFLP Markers Close to Rf-PET2 and Rf1
3.5. Comparative Mapping of the Restorer Genes Rf-PET2 and Rf1
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horn, R.; Gupta, K.J.; Colombo, N. Mitochondrion role in molecular basis of cytoplasmic male sterility. Mitochondrion 2014, 19, 19–198. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.-G. Male sterility and fertility restoration in crops. Ann. Rev. Plant. Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Bohra, A.; Jha, U.C.; Adhimoolam, P.; Bisht, D.; Singh, N.P. Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant. Cell Rep. 2016, 35, 967–993. [Google Scholar] [CrossRef]
- Vear, F. Changes in sunflower breeding over the last fifty years. OCL 2016, 23, D202. [Google Scholar] [CrossRef] [Green Version]
- Serieys, H. Identification, study and utilisation in breeding programs of new CMS sources. In FAO Subnetwork, Proceedings of the 2005 Sunflower Subnetwork Progress Report, Novi Sad, Serbia, 17–20 July 2005; FAO: Rome, Italy, 2005; pp. 47–53. [Google Scholar]
- Levings, C.S., III. Thoughts on cytoplasmic male sterility in cms-T maize. Plant. Cell 1993, 5, 1285–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, E.D.P.; Dedio, W. Registration of sunflower germplasm composite crosses CMG-1, CMG2 and CMG-3. Crop. Sci. 1980, 20, 832. [Google Scholar] [CrossRef]
- Leclercq, P. Une stérilité male cytoplasmique chez le tournesol. Ann. Amel. Plantes 1969, 19, 99–106. [Google Scholar]
- Köhler, R.H.; Horn, R.; Lössl, A.; Zetsche, K. Cytoplasmic male sterility in sunflower is correlated with the co-transcription of a new open reading frame with the atpA gene. Mol. Gen. Genet. 1991, 227, 369–376. [Google Scholar] [CrossRef]
- Horn, R.; Hustedt, J.E.G.; Horstmeyer, A.; Hahnen, J.; Zetsche, K.; Friedt, W. The CMS-associated 16 kDa protein encoded by orfH522 is also present in other male sterile cytoplasms of sunflower. Plant. Mol. Biol. 1996, 30, 523–538. [Google Scholar] [CrossRef]
- Horn, R. Molecular diversity of male sterility inducing and male-fertile cytoplasm in the genus Helianthus. Theor. Appl. Genet. 2002, 104, 562–570. [Google Scholar] [CrossRef]
- Whelan, E.D.P. Cytoplasmic male sterility in Helianthus giganteus L. × H. annuus L. interspecific hybrids. Crop. Sci. 1981, 21, 855–858. [Google Scholar] [CrossRef]
- Horn, R.; Friedt, W. CMS sources in sunflower: Different origin but same mechanism? Theor. Appl. Genet. 1999, 98, 195–201. [Google Scholar] [CrossRef]
- Horn, R.; Köhler, R.H.; Zetsche, K. A mitochondrial 16 kDa protein is associated with cytoplasmic male sterility in sunflower. Plant. Mol. Biol. 1991, 7, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Noda, D.; Kondo, M.; Terachi, T. High-level expression of a mitochondrial orf522 gene from the male-sterile sunflower is lethal to E. coli. Breed. Sci. 1995, 45, 233–236. [Google Scholar]
- Nizampatnam, R.N.; Doodhi, H.; Narasimhan, Y.K.; Mulpuri, S.; Viswanathaswamy, D.K. Expression of sunflower cytoplasmic male sterility-associated open reading frame orfH522 induces male sterility in transgenic tobacco plants. Planta 2009, 229, 987–1001. [Google Scholar] [CrossRef]
- Reddemann, A.; Horn, R. Recombination events involving the atp9 gene are associated with male sterility of CMS PET2 in sunflower. Int. J. Mol. Sci. 2018, 19, 806. [Google Scholar] [CrossRef] [Green Version]
- Makarenko, M.S.; Kornienko, I.V.; Azarin, K.V.; Usatov, A.V.; Logacheva, M.D.; Markin, N.V.; Gavrilova, V.A. Mitochondrial genomes organization in alloplasmic lines of sunflower (Helianthus annuus L.) with various types of cytoplasmic male sterility. PeerJ. 2018, 6, e5266. [Google Scholar] [CrossRef] [Green Version]
- Serieys, H.; Vincourt, P. Characterization of some new CMS sources from Helianthus genus. Helia 1987, 10, 9–13. [Google Scholar]
- De la Canal, L.; Crouzillat, D.; Quetier, F.; Ledoigt, G. A transcriptional alteration on the atp9 gene is associated with a sunflower male-sterile cytoplasm. Theor. Appl. Genet. 2001, 102, 1185–1189. [Google Scholar] [CrossRef]
- Horn, R.; Friedt, W. Fertility restoration of new CMS sources in sunflower. Plant. Breed. 1997, 116, 317–322. [Google Scholar] [CrossRef]
- Havekes, F.W.J.; Miller, J.F.; Jan, C.C. Diversity among sources of cytoplasmic male sterility in sunflower (Helianthus annuus L.). Euphytica 1991, 55, 125–129. [Google Scholar] [CrossRef]
- Kinman, M.L. New development in the USDA and state experiment station sunflower breeding programs. In Proceedings of the 4th International Sunflower Conference, Memphis, TN, USA, 23–25 June 1970; Int. Sunflower Assoc.: Paris, France, 1970; pp. 181–183. [Google Scholar]
- Korell, M.; Mösges, G.; Friedt, W. Construction of a sunflower pedigree map. Helia 1992, 15, 7–16. [Google Scholar]
- Leclercq, P. Identification de genes de restauration de fertilité sur cytoplasms stérilisants chez les tournesols. Agronomie 1984, 4, 573–576. [Google Scholar] [CrossRef]
- Jan, C.C.; Vick, B.A. Inheritance and allelic relationships of fertility restoration genes for seven new sources of male-sterile cytoplasm in sunflower. Plant. Breed. 2007, 126, 213–217. [Google Scholar] [CrossRef]
- Abratti, G.; Bazzalo, M.E.; León, A. Mapping a novel fertility restoration gene in sunflower. In Proceedings of the 17th International Sunflower Conference, Córdoba, Spain, 8–12 June 2008; Velasco, L., Ed.; International Sunflower Association: Paris, France, 2008; pp. 617–621. [Google Scholar]
- Liu, Z.; Mulpuri, S.; Feng, J.; Vick, B.A.; Jan, C.C. Molecular mapping of the Rf3 fertility restoration gene to facilitate its utilization in breeding confection sunflower. Mol. Breed. 2012, 29, 275–284. [Google Scholar] [CrossRef]
- Qi, L.L.; Seiler, G.J.; Vick, B.A.; Gulya, T.J. Genetics and mapping of the R11 gene conferring resistance to recently emerged rust races, tightly linked to male fertility restoration, in sunflower (Helianthus annuus L.). Theor. Appl. Genet. 2012, 125, 921–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentzbittel, L.; Vear, F.; Zhang, Y.X.; Berville, A. Development of a consensus linkage RFLP map of cultivated sunflower (Helianthus annuus L.). Theor. Appl. Genet. 1995, 90, 1079–1086. [Google Scholar] [CrossRef]
- Gentzbittel, L.; Mestries, E.; Mouzeyar, S.; Mazeyrat, F.; Badaoui, S.; Vear, F.; Tourvieille de Labrouhe, D.; Nicolas, P. A composite map of expressed sequences and phenotypic traits of the sunflower (Helianthus annuus L.). Theor. Appl. Genet. 1999, 99, 218–234. [Google Scholar] [CrossRef]
- Jan, C.C.; Vick, B.A.; Miller, J.F.; Kahler, A.L.; Butler, E.T. Construction of an RFLP linkage map for the cultivated sunflower. Theor. Appl. Genet. 1998, 96, 15–22. [Google Scholar] [CrossRef]
- Tang, S.X.; Kishore, V.K.; Knapp, S.J. PCR-multiplexes for a genome-wide framework of simple sequence repeat marker loci in cultivated sunflower. Theor. Appl. Genet. 2003, 107, 6–19. [Google Scholar] [CrossRef]
- Kusterer, B.; Horn, R.; Friedt, W. Molecular mapping of the fertility restoration locus Rf1 in sunflower and development of diagnostic markers for the restorer gene. Euphytica 2005, 143, 35–42. [Google Scholar] [CrossRef]
- Badouin, H.; Gouzy, J.; Grassa, C.; Murat, F.; Staton, S.E.; Cottret, L.; Lelandais-Brière, C.; Owens, G.L.; Carrère, S.; Mayjonade, B.; et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature 2017, 546, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Horn, R.; Radanovic, A.; Fuhrmann, L.; Sprycha, Y.; Hamrit, S.; Jockovic, M.; Miladinovic, D.; Jansen, C. Development and validation of markers for the fertility restorer gene Rf1 in sunflower. Int. J. Mol. Sci. 2019, 20, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goryunov, D.V.; Anisimova, I.A.; Gavrilova, V.A.; Chernova, A.I.; Sotnikova, E.A.; Martynova, E.U.; Boldyrev, S.V.; Ayupova, A.F.; Gubaev, R.F.; Mazin, P.V.; et al. Association mapping of fertility restorer gene for CMS PET1 in sunflower. Agronomy 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Hübner, S.; Bercovich, N.; Todesco, M.; Mandel, J.R.; Odenheimer, J.; Ziegler, E.; Lee, J.S.; Baute, G.J.; Owens, G.L.; Grassa, C.J.; et al. Sunflower pan-genome analysis shows that hybridization altered gene content and disease resistance. Nature Plant. 2019, 5, 54–62. [Google Scholar] [CrossRef]
- Owens, G.L.; Baute, G.J.; Hübner, S.; Rieseberg, L.H. Genomic sequence and copy number evolution during hybrid crop development in sunflowers. Evol. Appl. 2019, 12, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Talukder, Z.I.; Ma, G.; Hulke, B.S.; Jan, C.C.; Qi, L. Linkage mapping and genome-wide association studies of the Rf Gene cluster in sunflower (Helianthus annuus L.) and their distribution in world sunflower collections. Front. Genet. 2019, 10, 216. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Jan, C.C. Introgression and molecular tagging of Rf4, a new male fertility restoration gene from wild sunflower Helianthus maximiliani L. Theor. Appl. Genet. 2008, 117, 241–249. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.M.; Feng, J.; Seiler, G.J.; Cai, X.; Jan, C.C. Diversifying sunflower germplasm by integration and mapping of a novel male fertility restoration gene. Genetics 2013, 193, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Schnabel, U.; Engelmann, U.; Horn, R. Development of markers for the use of the PEF1-cytoplasm in sunflower hybrid breeding. Plant. Breed. 2008, 127, 587–591. [Google Scholar] [CrossRef]
- Horn, R.; Kusterer, B.; Lazarescu, E.; Prüfe, M.; Friedt, W. Molecular mapping of the Rf1 gene restoring pollen fertility in PET1-based F1 hybrids in sunflower (Helianthus annuus L.). Theor. Appl. Genet. 2003, 106, 599–606. [Google Scholar] [CrossRef]
- Alexander, P. Differential staining of aborted and non-aborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Doyle, J.L.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucl. Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [Green Version]
- Oetting, W.S.; Lee, H.K.; Flanders, D.J.; Wiesner, G.L.; Sellers, T.A.; King, R.A. Linkage analysis with multiplexed short tandem repeat polymorphisms using infrared fluorescence and M13 tailed primers. Genomics 1995, 30, 450–458. [Google Scholar] [CrossRef]
- Sajer, O.; Scorza, R.; Dardick, C.; Zhebentyayeva, T.; Abbott, A.G.; Horn, R. Development of sequence-tagged site markers linked to the pillar growth type in peach [Prunus persica L. (Batsch)]. Plant. Breed. 2012, 131, 186–192. [Google Scholar] [CrossRef]
- Van Ooijen, J.W. Multipoint maximum likelihood mapping in a full-sib family of an outbreeding species. Genetics Res. 2011, 93, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. The combination of linkage values and the calculation of the distance between the loci of linked factors. J. Genet. 1919, 8, 299–309. [Google Scholar]
- Akagi, H.; Yokozeki, Y.; Inagaki, A.; Nakamura, A.; Fujimura, T. A codominant DNA marker closely linked to the rice nuclear restorer, Rf-1, identified with inter-SSR fingerprinting. Genome 1996, 39, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Y.; Li, X.; Zhang, Q.; Chen, L.; Wu, H.; Su, D.; Chen, Y.; Guo, J.; Luo, D.; et al. Cytoplasmic male sterility of rice with Boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant. Cell 2006, 18, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.Y.; Xu, C.G.; Yu, S.B.; Li, J.X.; Gac, Y.J.; Li, X.H.; Zhang, Q. Mapping and genetic analysis of two fertility restorer loci in the wild-abortive cytoplasmic male sterility system of rice (Oryza sativa L.). Euphytica 1997, 98, 183–187. [Google Scholar] [CrossRef]
- Zhang, G.; Bharaj, T.S.; Lu, Y.; Virmani, S.S.; Huang, N. Mapping of the Rf-3 nuclear fertility-restoring gene for WA cytoplasmic male sterility in rice using RAPD and RFLP markers. Theor. Appl. Genet. 1997, 94, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Liu, Y.G.; Mei, M.T. Molecular mapping of the fertility restorer gene Rf4 for WA cytoplasmic male sterility. Acta Genet. Sin. 2002, 29, 1001–1004. [Google Scholar] [PubMed]
- Börner, A.; Korzun, V.; Polley, A.; Maleyshev, S.; Melz, G. Genetics and molecular mapping of a male fertility restoration locus (Rfg1) in rye (Secale cereale L). Theor Appl. Genet. 1998, 97, 99–102. [Google Scholar] [CrossRef]
- Miedaner, T.; Glass, C.; Dreyer, F.; Wilde, P.; Wortmann, H.; Geiger, H.H. Mapping of genes for male-fertility restoration in Pampa CMS winter rye (Secale cereale L.). Theor. Appl. Genet. 2000, 101, 1226–1233. [Google Scholar] [CrossRef]
- Stracke, S.; Schilling, A.G.; Förster, J.; Weiss, C.; Glass, C.; Miedaner, T.; Geiger, H.H. Development of PCR-based markers linked to dominant genes for male-fertility restoration in Pampa CMS of rye (Secale cereale L.). Theor. Appl. Genet. 2003, 106, 1184–1190. [Google Scholar] [CrossRef]
- Cui, X.; Wise, R.P.; Schnable, P.S. The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 1996, 272, 1334–1335. [Google Scholar] [CrossRef]
- Liu, F.; Cui, X.; Horner, H.T.; Weiner, H.; Schnable, P. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant. Cell 2001, 13, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Auborg, S.; Boudet, N.; Kreis, M.; Lecharny, A. In Arabidopsis thaliana, 1% of the genome codes a novel protein family unique to plants. Plant Mol. Biol. 2000, 42, 603–613. [Google Scholar] [CrossRef]
- Lurin, C.; Andres, C.; Aubourg, S.; Bellaoui, M.; Bitton, F.; Bruyere, C.; Caboche, M.; Debast, C.; Gualberto, J.; Hoffmann, B.; et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant. Cell 2004, 16, 2089–2103. [Google Scholar] [CrossRef] [Green Version]
- Bentolila, S.; Alfonso, A.A.; Hanson, M.R. A pentatricopeptide-repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10887–10892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, R.; Koizuka, N.; Fujimoto, H.; Hayakawa, T.; Sakai, T.; Imamura, J. Delimitation of the fertility restorer locus Rfk1 to a 43-kb contig in Kosena radish (Raphanus sativus L.). Mol. Gen. Genomics 2003, 269, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.R.; Klein, P.E.; Mullet, J.E.; Minx, P.; Rooney, W.L.; Schertz, K.F. Fertility restorer locus Rf1 of sorghum (Sorghum bicolor L.) encodes a pentatricopeptide repeat protein not present in the collinear region of rice chromosome 12. Theor. Appl. Genet. 2005, 111, 994–1012. [Google Scholar] [CrossRef] [PubMed]
- Koizuka, N.; Imai, R.; Fujimoto, H.; Hayakawa, T.; Kimura, Y.; Kohno-Murase, J.; Sakai, T.; Kawasaki, S.; Imamura, J. Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant. J. 2003, 34, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Ohta, S.; Murai, N.; Takakura, Y.; Kuraya, Y.; Suzuki, S.; Hiei, Y.; Imaseki, H.; Nitta, N. 2004: Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant. J. 2004, 37, 315–325. [Google Scholar] [CrossRef]
- Brown, G.G.; Formanova, N.; Jin, H.; Wargachuk, R.; Dendy, C.; Patil, P.; Laforest, M.; Zhang, J.; Cheung, W.Y.; Landry, B.S. The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant. J. 2003, 35, 262–272. [Google Scholar] [CrossRef]
- Desloire, S.; Gherbi, H.; Laloui, W.; Marhadour, S.; Clouet, V.; Cattolico, L.; Falentin, C.; Giancola, S.; Renard, M.; Budar, F.; et al. Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep. 2003, 4, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Fujii, S.; Bond, C.S.; Small, I.D. Selection patterns on restorer-like genes reveal a conflict between nuclear and mitochondrial genomes throughout angiosperm evolution. Proc. Natl. Acad. Sci. USA 2011, 108, 1723–1728. [Google Scholar] [CrossRef] [Green Version]
- Gaborieau, L.; Brown, G.G.; Mireau, H. The propensity of pentatricopeptide repeat genes to evolve into restorers of cytoplasmic male sterility. Front. Plant. Sci. 2016, 7, 1816. [Google Scholar] [CrossRef] [Green Version]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant. Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; Horn, R. Sunflower hybrid breeding: From markers to genomic selection. Front. Plant. Sci. 2018, 8, 2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Marker | Size bp | Forward Primer | Reverse Primer | TA | Genbank Accession |
|---|---|---|---|---|---|
| ORS317 * | 200 | TTTCCCAGTCACGACGTT CGTATGCTTAATTCTTTCTCT | TTTGGCAGTTTGGTGGCTTA | 55 | BV006839 |
| ORS1030 * | 426 | TTTCCCAGTCACGACGTT TGATGTAGTTAAGGAAGTTGTG | CGATCAATTTATATGACCGAATTACC | 55 | BV006414 |
| ORS630 * | 344 | TTTCCCAGTCACGACGTT CGACCCGGATATGTAAC | TGTGCTGAGGATGATATGCAG | 55 | BV006710.1 |
| STSY10_740 | 738 | AAACGTGGGAGAGAGGTGG | AAACGTGGGCTGAAGAACTA | 65 | [44] |
| STSK13_426 | 426 | TATGCATAATTAGTTATACCC | ACATAAGGATTATGTACGGG | 60 | [44] |
| STS3948_145 | 145 | GTTTTTGGGACATCGCCATTTT | GCGGGTGGAAATCCATATATGAG | 52 | This study |
| STS67N4 | 471 | TTTGAGGGCTCATCTCCAGTCA | TGCAATGAGCTTAGCCCATCG | This study | |
| coxII | 764 | CGAGAAATAGATGCTCAGCCTG | GATAATGCGCAGTGGAAAGG | 60 | X62341 |
| atp9 | 474 | GGTGCAAAATCAATAGGGGCCG | ACCGAATGAATGCGTCACAAGG | 65 | X51895 |
| rbcL | 830 | CGTCTGGAAGATTTGCGAATC | GGGTGCCCTAAAGTTCCTCC | 52 | AF097517 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajer, O.; Schirmak, U.; Hamrit, S.; Horn, R. Mapping of the New Fertility Restorer Gene Rf-PET2 Close to Rf1 on Linkage Group 13 in Sunflower (Helianthus annuus L.). Genes 2020, 11, 269. https://doi.org/10.3390/genes11030269
Sajer O, Schirmak U, Hamrit S, Horn R. Mapping of the New Fertility Restorer Gene Rf-PET2 Close to Rf1 on Linkage Group 13 in Sunflower (Helianthus annuus L.). Genes. 2020; 11(3):269. https://doi.org/10.3390/genes11030269
Chicago/Turabian StyleSajer, Osama, Uta Schirmak, Sonia Hamrit, and Renate Horn. 2020. "Mapping of the New Fertility Restorer Gene Rf-PET2 Close to Rf1 on Linkage Group 13 in Sunflower (Helianthus annuus L.)" Genes 11, no. 3: 269. https://doi.org/10.3390/genes11030269
APA StyleSajer, O., Schirmak, U., Hamrit, S., & Horn, R. (2020). Mapping of the New Fertility Restorer Gene Rf-PET2 Close to Rf1 on Linkage Group 13 in Sunflower (Helianthus annuus L.). Genes, 11(3), 269. https://doi.org/10.3390/genes11030269





