Purification and Characterization of Double-Stranded Nucleic Acid-Dependent ATPase Activities of Tagged Dicer-Related Helicase 1 and its Short Isoform in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid Construction
2.3. Preparation of the Tagged Proteins
2.4. Protein Analysis
2.5. Nucleic Acid-Dependent ATPase Assay
2.6. Sequence Analysis
3. Results and Discussion
3.1. Domains and Predicted Secondary Structures in C. elegans DRHs
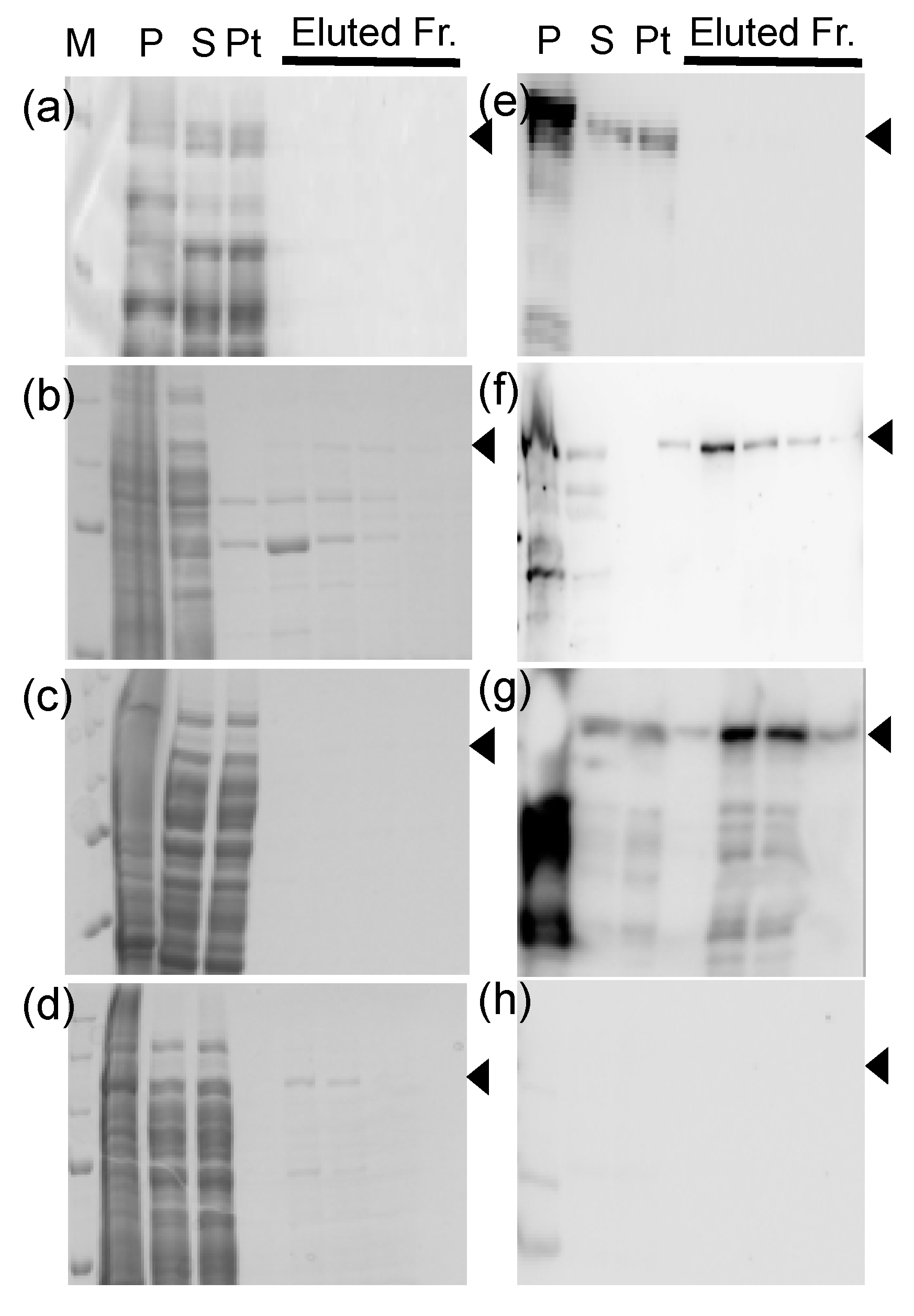
3.2. Investigation of Tags Suitable for Expression and Purification
3.3. Preparations of His-SUMO-Tagged DRH-1 and its Short Isoform
3.4. Nucleic Acid-Dependent ATPases of the Purified DRH-1 Proteins
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Bruns, A.M.; Horvath, C.M. Activation of RIG-I-like receptor signal transduction. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Hur, S. Helicases in antiviral immunity: Dual properties as sensors and effectors. Trends Biochem. Sci. 2015, 40, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, M.; Fujita, T. RIG-I family RNA helicases: Cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007, 18, 545–551. [Google Scholar] [CrossRef]
- Myong, S.; Cui, S.; Cornish, P.V.; Kirchhofer, A.; Gack, M.U.; Jung, J.U.; Hopfner, K.P.; Ha, T. Cytosolic viral sensor RIG-I is a 5’-triphosphate-dependent translocase on double-stranded RNA. Science 2009, 323, 1070–1074. [Google Scholar] [CrossRef] [Green Version]
- Peisley, A.; Lin, C.; Wu, B.; Orme-Johnson, M.; Liu, M.; Walz, T.; Hur, S. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc. Natl. Acad. Sci. USA 2011, 108, 21010–21015. [Google Scholar] [CrossRef] [Green Version]
- Pippig, D.A.; Hellmuth, J.C.; Cui, S.; Kirchhofer, A.; Lammens, K.; Lammens, A.; Schmidt, A.; Rothenfusser, S.; Hopfner, K.P. The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA. Nucleic Acids Res. 2009, 37, 2014–2025. [Google Scholar] [CrossRef] [Green Version]
- Eki, T.; Ishihara, T.; Katsura, I.; Hanaoka, F. A genome-wide survey and systematic RNAi-based characterization of helicase-like genes in Caenorhabditis elegans. DNA Res. 2007, 14, 183–199. [Google Scholar] [CrossRef]
- Nakamura, M.; Ando, R.; Nakazawa, T.; Yudazono, T.; Tsutsumi, N.; Hatanaka, N.; Ohgake, T.; Hanaoka, F.; Eki, T. Dicer-related drh-3 gene functions in germ-line development by maintenance of chromosomal integrity in Caenorhabditis elegans. Genes Cells 2007, 12, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Yigit, E.; Li, W.X.; Ding, S.W. An RIG-I-like RNA helicase mediates antiviral RNAi downstream of viral siRNA biogenesis in Caenorhabditis elegans. PloS Pathog. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Ashe, A.; Belicard, T.; Le Pen, J.; Sarkies, P.; Frezal, L.; Lehrbach, N.J.; Felix, M.A.; Miska, E.A. A deletion polymorphism in the Caenorhabditis elegans RIG-I homolog disables viral RNA dicing and antiviral immunity. Elife 2013, 2, e00994. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Zhang, R.; Wang, J.; Ding, S.W.; Lu, R. Homologous RIG-I-like helicase proteins direct RNAi-mediated antiviral immunity in C. elegans by distinct mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 16085–16090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffman, S.R.; Lu, J.; Guo, X.; Zhong, J.; Jiang, H.; Broitman-Maduro, G.; Li, W.X.; Lu, R.; Maduro, M.; Ding, S.W. Caenorhabditis elegans RIG-I homolog mediates antiviral RNA interference downstream of Dicer-dependent biogenesis of viral small interfering RNAs. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammon, D.B.; Ishidate, T.; Li, L.; Gu, W.; Silverman, N.; Mello, C.C. The antiviral RNA interference response provides resistance to lethal arbovirus infection and vertical transmission in Caenorhabditis elegans. Curr. Biol. 2017, 27, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Sowa, J.N.; Jiang, H.; Somasundaram, L.; Tecle, E.; Xu, G.; Wang, D.; Troemel, E.R. The Caenorhabditis elegans RIG-I homolog DRH-1 mediates the intracellular pathogen response upon viral infection. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Duchaine, T.F.; Wohlschlegel, J.A.; Kennedy, S.; Bei, Y.; Conte, D., Jr.; Pang, K.; Brownell, D.R.; Harding, S.; Mitani, S.; Ruvkun, G.; et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell 2006, 124, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Dalzell, J.J.; McVeigh, P.; Warnock, N.D.; Mitreva, M.; Bird, D.M.; Abad, P.; Fleming, C.C.; Day, T.A.; Mousley, A.; Marks, N.J.; et al. RNAi effector diversity in nematodes. PloS Negl. Trop. Dis. 2011, 5, e1176. [Google Scholar] [CrossRef] [Green Version]
- Matranga, C.; Pyle, A.M. Double-stranded RNA-dependent ATPase DRH-3: Insight into its role in RNAsilencing in Caenorhabditis elegans. J. Biol. Chem. 2010, 285, 25363–25371. [Google Scholar] [CrossRef] [Green Version]
- Dumon-Seignovert, L.; Cariot, G.; Vuillard, L. The toxicity of recombinant proteins in Escherichia coli: A comparison of overexpression in BL21(DE3), C41(DE3), and C43(DE3). Protein Expr. Purif. 2004, 37, 203–206. [Google Scholar] [CrossRef]
- Drozdetskiy, A.; Cole, C.; Procter, J.; Barton, G.J. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015, 43, W389–W394. [Google Scholar] [CrossRef]
- Satoh, T.; Kato, H.; Kumagai, Y.; Yoneyama, M.; Sato, S.; Matsushita, K.; Tsujimura, T.; Fujita, T.; Akira, S.; Takeuchi, O. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. USA 2010, 107, 1512–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, A.; Li, X.; Ranjith-Kumar, C.T.; Bhardwaj, K.; Holzenburg, A.; Li, P.; Kao, C.C. Structure and function of LGP2, a DEX(D/H) helicase that regulates the innate immunity response. J. Biol. Chem. 2008, 283, 15825–15833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabara, H.; Yigit, E.; Siomi, H.; Mello, C.C. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 2002, 109, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Bamming, D.; Horvath, C.M. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J. Biol. Chem. 2009, 284, 9700–9712. [Google Scholar] [CrossRef] [Green Version]





| Constructs | Primers | Nucleotide Sequence (5’–3’) |
|---|---|---|
| pET-SUMO/drh-1op | drh1op-pET-SUMO15-IFF | GAACAGATTGGTGGTATGCGTAAAAAACAGTGTAGCAGC |
| drh1op-pET-SUMO15-IFR | TACCTAAGCTTGTCTTTATGCTTCACGAATCAGGTTCAC | |
| pET-SUMO-5’-invR | ACCACCAATCTGTTCTCTGTGAGCC | |
| pET-SUMO-3’-invF | AGACAAGCTTAGGTATTTATTCGG | |
| pET-Strep-SUMO/drh-1op | pET-SUMO_invR_half Strep tail | ctgcgggtggctccaGCTGCTGCCCATATGTATATC |
| pET-SUMO_invF_half Strep tail | gacgatgacaaaCTGGTGCCGCGCGGCAGCGCT | |
| pET-FLAG-SUMO/drh-1op | pET-SUMO_invR_half FLAG tail | gacgatgacaaaCTGGTGCCGCGCGGCAGCGCT |
| pET-SUMO_invF_half FLAG tail | ttcgaaaaaggcgcgCTGGTGCCGCGCGGCAGCGCT | |
| pET-SUMO/drh-1op isoform b | pET-SUMO-5’-invR | ACCACCAATCTGTTCTCTGTGAGCC |
| drh1op_259-invF | ATGATTAACATCCGCGTGGATAAC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Murakami, T.; Hirose, Y.; Eki, T. Purification and Characterization of Double-Stranded Nucleic Acid-Dependent ATPase Activities of Tagged Dicer-Related Helicase 1 and its Short Isoform in Caenorhabditis elegans. Genes 2020, 11, 734. https://doi.org/10.3390/genes11070734
Kobayashi T, Murakami T, Hirose Y, Eki T. Purification and Characterization of Double-Stranded Nucleic Acid-Dependent ATPase Activities of Tagged Dicer-Related Helicase 1 and its Short Isoform in Caenorhabditis elegans. Genes. 2020; 11(7):734. https://doi.org/10.3390/genes11070734
Chicago/Turabian StyleKobayashi, Taishi, Takuro Murakami, Yuu Hirose, and Toshihiko Eki. 2020. "Purification and Characterization of Double-Stranded Nucleic Acid-Dependent ATPase Activities of Tagged Dicer-Related Helicase 1 and its Short Isoform in Caenorhabditis elegans" Genes 11, no. 7: 734. https://doi.org/10.3390/genes11070734





