Cryopreservation and the Freeze–Thaw Stress Response in Yeast
Abstract
:1. Introduction
2. Reagents and Methods for Yeast Cryopreservation
2.1. Overview of Cryopreservation
2.2. Cryopreserving Yeast Grown on Plates
2.3. Cryopreserving Yeast Grown in Medium Broth
2.4. Reviving Yeast Cells after Freezing
3. Freeze–Thaw Stress Response in Yeast
3.1. Freezing and Thawing as a Form of Stress and a Cause of Damage
3.2. Molecules That Minimize Freeze–Thaw Stress Damage during Cryopreservation
3.3. Investigation of Freeze–Thaw Stress Induction and Tolerance
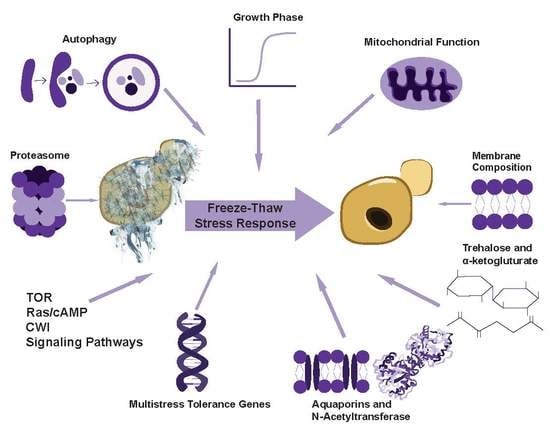
4. Cellular Factors That Can Influence Freeze–Thaw Stress Tolerance of S. cerevisiae
4.1. Overview
4.2. Growth Phase
4.3. Mitochondrial Function and Cellular Respiration
4.4. Membrane Composition
4.5. Trehalose Accumulation
4.6. Alpha-ketoglutarate
4.7. N-Acetyltransferase
4.8. Aquaporins
4.9. Cold-Induced Stress Changes in Gene Expression
4.10. Multistress Tolerance Genes and the Intersection between Freeze–Thaw Stress and Other Cellular Stress Coping Mechanisms
4.11. Ras/cAMP Signaling Pathway
4.12. Proteasome
4.13. Autophagy
5. Emerging Lessons from Antarctic Yeasts
5.1. Overview
5.2. Stress Resistance and Metabolism
5.3. Trehalose Production
5.4. Membrane Composition
5.5. Antifreeze Proteins
5.6. Complex Adaptation of Antarctic Yeast to Cold Environments
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, Z. Protectants used in the cryopreservation of microorganisms. Cryobiology 2003, 46, 205–229. [Google Scholar] [CrossRef]
- Linde, G.A.; Luciani, A.; Lopes, A.D.; Valle, J.S.D.; Colauto, N.B. Long-term cryopreservation of basidiomycetes. Braz. J. Microbiol. 2018, 49, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Amberg, D.C.; Strathern, J.N. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual; CSHL Press: New York, NY, USA, 2005. [Google Scholar]
- Coutinho, C.; Bernardes, E.; Félix, D.; Panek, A.D. Trehalose as cryoprotectant for preservation of yeast strains. J. Biotechnol. 1988, 7, 23–32. [Google Scholar] [CrossRef]
- Dumont, F.; Marechal, P.A.; Gervais, P. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl. Environ. Microbiol. 2004, 70, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Mazur, P.; Leibo, S.P.; Chu, E.H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp. Cell Res. 1972, 71, 345–355. [Google Scholar] [CrossRef]
- Mazur, P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology 1977, 14, 251–272. [Google Scholar] [CrossRef]
- Park, J.-I.; Grant, C.M.; Attfield, P.V.; Dawes, I.W. The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal transduction pathway. Appl. Environ. Microbiol. 1997, 63, 3818–3824. [Google Scholar] [CrossRef] [Green Version]
- Suga, M.; Isobe, M.; Hatakeyama, T. Cryopreservation of competent intact yeast cells for efficient electroporation. Yeast 2000, 16, 889–896. [Google Scholar] [CrossRef]
- Diniz-Mendes, L.; Bernardes, E.; De Araujo, P.; Panek, A.; Paschoalin, V. Preservation of frozen yeast cells by trehalose. Biotechnol. Bioeng. 1999, 65, 572–578. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Hrynkiv, O.V.; Knyhynytska, R.V.; Lushchak, V.I. Alpha-ketoglutarate enhances freeze–thaw tolerance and prevents carbohydrate-induced cell death of the yeast Saccharomyces cerevisiae. Arch. Microbiol. 2018, 200, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vargas, S.; Estruch, F.; Randez-Gil, F. Gene expression analysis of cold and freeze stress in Baker’s yeast. Appl. Environ. Microbiol. 2002, 68, 3024–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, T. Viability assays to monitor yeast autophagy. Methods Enzymol. 2008, 451, 27–32. [Google Scholar] [PubMed]
- Odani, M.; Komatsu, Y.; Oka, S.; Iwahashi, H. Screening of genes that respond to cryopreservation stress using yeast DNA microarray. Cryobiology 2003, 47, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Nakamura, T.; Murata, Y.; Takagi, H.; Shima, J. Identification and classification of genes required for tolerance to freeze–thaw stress revealed by genome-wide screening of Saccharomyces cerevisiae deletion strains. FEMS Yeast Res. 2007, 7, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Kitagaki, H.; Takagi, H. Mitochondrial metabolism and stress response of yeast: Applications in fermentation technologies. J. Biosci. Bioeng. 2014, 117, 383–393. [Google Scholar] [CrossRef]
- Kruuv, J.; Lepock, J.; Keith, A. The effect of fluidity of membrane lipids on freeze-thaw survival of yeast. Cryobiology 1978, 15, 73–79. [Google Scholar] [CrossRef]
- Villarreal, P.; Carrasco, M.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Antarctic yeasts: Analysis of their freeze-thaw tolerance and production of antifreeze proteins, fatty acids and ergosterol. BMC Microbiol. 2018, 18, 66. [Google Scholar] [CrossRef]
- Hirasawa, R.; Yokoigawa, K.; Isobe, Y.; Kawai, H. Improving the freeze tolerance of bakers’ yeast by loading with trehalose. Biosci. Biotechnol. Biochem. 2001, 65, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Attfield, P.V.; Raman, A.; Northcott, C.J. Construction of Saccharomyces cerevisiae strains that accumulate relatively low concentrations of trehalose, and their application in testing the contribution of the disaccharide to stress tolerance. FEMS Microbiol. Lett. 1992, 94, 271–276. [Google Scholar] [CrossRef]
- Shima, J.; Hino, A.; Yamada-Iyo, C.; Suzuki, Y.; Nakajima, R.; Watanabe, H.; Mori, K.; Takano, H. Stress tolerance in doughs of Saccharomyces cerevisiae trehalase mutants derived from commercial baker’s yeast. Appl. Environ. Microbiol. 1999, 65, 2841–2846. [Google Scholar] [CrossRef] [Green Version]
- Soto, T.; Fernández, J.; Vicente-Soler, J.; Cansado, J.; Gacto, M. Accumulation of Trehalose by Overexpression oftps1, Coding for Trehalose-6-Phosphate Synthase, Causes Increased Resistance to Multiple Stresses in the Fission Yeast Schizosaccharomyces pombe. Appl. Environ. Microbiol. 1999, 65, 2020–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; He, L.; Yao, K. The antioxidative function of alpha-ketoglutarate and its applications. Biomed Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nasuno, R.; Hirano, Y.; Itoh, T.; Hakoshima, T.; Hibi, T.; Takagi, H. Structural and functional analysis of the yeast N-acetyltransferase Mpr1 involved in oxidative stress tolerance via proline metabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 11821–11826. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Takagi, H. N-Acetyltransferase Mpr1 confers ethanol tolerance on Saccharomyces cerevisiae by reducing reactive oxygen species. Appl. Microbiol. Biotechnol. 2007, 75, 1343–1351. [Google Scholar] [CrossRef]
- Tanghe, A.; Van Dijck, P.; Colavizza, D.; Thevelein, J.M. Aquaporin-mediated improvement of freeze tolerance of Saccharomyces cerevisiae is restricted to rapid freezing conditions. Appl. Environ. Microbiol. 2004, 70, 3377–3382. [Google Scholar] [CrossRef] [Green Version]
- Sabir, F.; Loureiro-Dias, M.C.; Soveral, G.; Prista, C. Functional relevance of water and glycerol channels in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2017, 364, fnx0802017. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhang, C.-Y.; Bai, X.-W.; Feng, B.; Xiao, D.-G. Improvement of stress tolerance and leavening ability under multiple baking-associated stress conditions by overexpression of the SNR84 gene in baker’s yeast. Int. J. Food Microbiol. 2015, 197, 15–21. [Google Scholar] [CrossRef]
- Tanghe, A.; Van Dijck, P.; Dumortier, F.; Teunissen, A.; Hohmann, S.; Thevelein, J.M. Aquaporin expression correlates with freeze tolerance in baker’s yeast, and overexpression improves freeze tolerance in industrial strains. Appl. Environ. Microbiol. 2002, 68, 5981–5989. [Google Scholar] [CrossRef] [Green Version]
- Bonhivers, M.; Carbrey, J.M.; Gould, S.J.; Agre, P. Aquaporins in Saccharomyces genetic and functional distinctions between laboratory and wild-type strains. J. Biol. Chem. 1998, 273, 27565–27572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersson, N.; Filipsson, C.; Becit, E.; Brive, L.; Hohmann, S. Aquaporins in yeasts and filamentous fungi. Biol. Cell 2005, 97, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Carbrey, J.M.; Bonhivers, M.; Boeke, J.D.; Agre, P. Aquaporins in Saccharomyces: Characterization of a second functional water channel protein. Proc. Natl. Acad. Sci. USA 2001, 98, 1000–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schade, B.; Jansen, G.; Whiteway, M.; Entian, K.D.; Thomas, D.Y. Cold adaptation in budding yeast. Mol. Biol. Cell 2004, 15, 5492–5502. [Google Scholar] [CrossRef] [Green Version]
- Estruch, F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 2000, 24, 469–486. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Seita, J.; Komiyama, S.; Yamamura, H.; Hayakawa, M.; Iimura, Y. A new simple method for isolating multistress-tolerant semidominant mutants of Saccharomyces cerevisiae by one-step selection under lethal hydrogen peroxide stress condition. Biosci. Biotechnol. Biochem. 2013, 77, 224–228. [Google Scholar] [CrossRef]
- Görner, W.; Durchschlag, E.; Martinez-Pastor, M.T.; Estruch, F.; Ammerer, G.; Hamilton, B.; Ruis, H.; Schüller, C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998, 12, 586–597. [Google Scholar] [CrossRef]
- De Wever, V.; Reiter, W.; Ballarini, A.; Ammerer, G.; Brocard, C. A dual role for PP1 in shaping the Msn2-dependent transcriptional response to glucose starvation. EMBO J. 2005, 24, 4115–4123. [Google Scholar] [CrossRef] [Green Version]
- Angeles de la Torre-Ruiz, M.; Pujol, N.; Sundaran, V. Coping with oxidative stress. Yeast Model. Curr. Drug Targets 2015, 16, 2–12. [Google Scholar] [CrossRef]
- Budovskaya, Y.V.; Stephan, J.S.; Reggiori, F.; Klionsky, D.J.; Herman, P.K. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 20663–20671. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Fujita, R.; Yamamura, H.; Hayakawa, M. Identification of CDC25-P1306L, a novel mutant allele of CDC25, conferring tolerance to multiple stresses associated with food production on Saccharomyces cerevisiae. Biotechnol. Biotechnol. Equip. 2019, 33, 162–169. [Google Scholar] [CrossRef]
- Kim, J.; Alizadeh, P.; Harding, T.; Hefner-Gravink, A.; Klionsky, D.J. Disruption of the yeast ATH1 gene confers better survival after dehydration, freezing, and ethanol shock: Potential commercial applications. Appl. Environ. Microbiol. 1996, 62, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Virgilio, C.; Hottiger, T.; Dominguez, J.; Boller, T.; Wiemken, A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 1994, 219, 179–186. [Google Scholar] [CrossRef]
- Watanabe, D.; Sekiguchi, H.; Sugimoto, Y.; Nagasawa, A.; Kida, N.; Takagi, H. Importance of proteasome gene expression during model dough fermentation after preservation of baker’s yeast cells by freezing. Appl. Environ. Microbiol. 2018, 84, e00406–e00418. [Google Scholar] [CrossRef] [Green Version]
- Teets, N.M.; Denlinger, D.L. Autophagy in Antarctica: Combating dehydration stress in the world’s southernmost insect. Autophagy 2013, 9, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Teets, N.M.; Peyton, J.T.; Colinet, H.; Renault, D.; Kelley, J.L.; Kawarasaki, Y.; Lee, R.E.; Denlinger, D.L. Gene expression changes governing extreme dehydration tolerance in an Antarctic insect. Proc. Natl. Acad. Sci. USA 2012, 109, 20744–20749. [Google Scholar] [CrossRef] [Green Version]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeasts from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef]
- Troncoso, E.; Barahona, S.; Carrasco, M.; Villarreal, P.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Identification and characterization of yeasts isolated from the South Shetland Islands and the Antarctic Peninsula. Polar Biol. 2017, 40, 649–658. [Google Scholar] [CrossRef]
- Ballester-Tomás, L.; Prieto, J.A.; Gil, J.V.; Baeza, M.; Randez-Gil, F. The Antarctic yeast Candida sake: Understanding cold metabolism impact on wine. Int. J. Food Microbiol. 2017, 245, 59–65. [Google Scholar] [CrossRef]
- Amato, P.; Doyle, S.; Christner, B.C. Macromolecular synthesis by yeasts under frozen conditions. Environ. Microbiol. 2009, 11, 589–596. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Y.; He, Y.; Xing, L.; Zhang, X.; Wang, S.; Qi, X.; Zheng, Z.; Lu, J.; Miao, J. Cloning and expression analysis of tps, and cryopreservation research of trehalose from Antarctic strain Pseudozyma sp. 3 Biotech 2017, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Do, H.; Jung, W. Production of antifreeze proteins by cold-adapted yeasts. In Cold-Adapted Yeasts; Springer: Berlin/Heidelberg, Germany, 2014; pp. 259–280. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, E.; Welch, L.C.; Robinson, M.R.; Sturgeon, C.M.; Crow, M.M.; Segarra, V.A. Cryopreservation and the Freeze–Thaw Stress Response in Yeast. Genes 2020, 11, 835. https://doi.org/10.3390/genes11080835
Cabrera E, Welch LC, Robinson MR, Sturgeon CM, Crow MM, Segarra VA. Cryopreservation and the Freeze–Thaw Stress Response in Yeast. Genes. 2020; 11(8):835. https://doi.org/10.3390/genes11080835
Chicago/Turabian StyleCabrera, Elizabeth, Laylah C. Welch, Meaghan R. Robinson, Candyce M. Sturgeon, Mackenzie M. Crow, and Verónica A. Segarra. 2020. "Cryopreservation and the Freeze–Thaw Stress Response in Yeast" Genes 11, no. 8: 835. https://doi.org/10.3390/genes11080835
APA StyleCabrera, E., Welch, L. C., Robinson, M. R., Sturgeon, C. M., Crow, M. M., & Segarra, V. A. (2020). Cryopreservation and the Freeze–Thaw Stress Response in Yeast. Genes, 11(8), 835. https://doi.org/10.3390/genes11080835