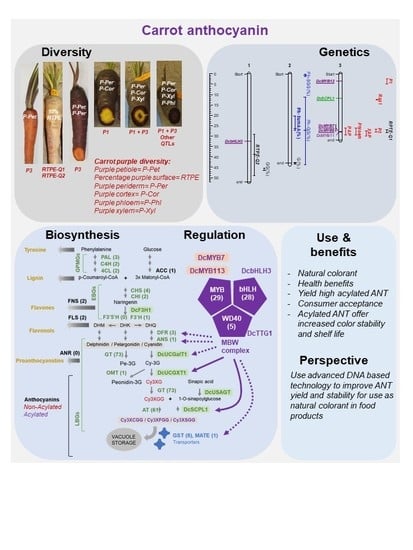
Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry
Abstract
1. Carrot as a Source of Anthocyanin for Natural Colorants
1.1. Application and Potential of Carrot as a Source of Natural Anthocyanins
1.2. Diversity for Anthocyanin Composition in the Purple Carrot Germplasm
1.3. Shelf-Life Stability of Anthocyanins Derived from Carrot (pH, Temperature and Shelf-Life)
2. Genetics and Genes Controlling Anthocyanin Pigmentation in Carrot
2.1. Anthocyanin Genetics in Carrot
2.2. Anthocyanin Structural Genes
2.3. Regulatory Anthocyanin Genes
3. External Factors Affecting Anthocyanin Accumulation and Profile in Carrots and Other Plant Species
4. Perspectives
4.1. Advancing Molecular and Biotechnology Tools to Develop Carrot Cultivars That Maximize Anthocyanin Yield in Product Performance and Stability
4.2. Exploring the Diversity of Co-Pigmentation to Enhance Anthocyanin Product Performance and Stability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Natural Food Colors Market. 2018–2022; Infiniti Research Limited: London, UK, 2018; p. 108.
- Cooperstone, J.L.; Schwartz, S. Recent Insights into Health Benefits of Carotenoids. In Handbook on Natural Pigments in Food and Beverages; Elsevier BV: London, UK, 2016; pp. 473–497. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Culver, C.A. Alternatives to those artificial FD&C food colorants. Annu. Rev. Food Sci. Technol. 2012, 3, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Amir, A. Natural Food Coloring Manufacturing in the US; IBISWorld: Melbourne, Australia, 2019; Available online: https://www.ibisworld.com (accessed on 3 July 2020).
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot ( Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Assous, M.; Abdel-Hady, M.; Medany, G.M. Evaluation of red pigment extracted from purple carrots and its utilization as antioxidant and natural food colorants. Ann. Agric. Sci. 2014, 59, 1–7. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. J. Food Sci. 2008, 73, C241–C248. [Google Scholar] [CrossRef] [PubMed]
- Buchweitz, M.; Brauch, J.; Carle, R.; Kammerer, D.R. Colour and stability assessment of blue ferric anthocyanin chelates in liquid pectin-stabilised model systems. Food Chem. 2013, 138, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Kalsec. Available online: https://www.preparedfoods.com/articles/113671-natural-color-from-carrots (accessed on 3 June 2020).
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; De Mejia, E.G. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2016, 16, 180–198. [Google Scholar] [CrossRef]
- Freeman, R. Carrot Plants With a High Anthocyanin Level. Patent WO 2015/165928 Al, 5 November 2015. [Google Scholar]
- Espin, G.B.; Luetken, H.V.; Joernsgaard, B.; Mueller, R.P.B.; Dzhanfezova, T.; Glied, S.; Madsen, B.; Okkels, F.T. A Method for Increasing Anthocyanin Content in Carrots. Patent WO 2018/055108 Al, 29 March 2018. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color Stability of Commercial Anthocyanin-Based Extracts in Relation to the Phenolic Composition. Protective Effects by Intraand Intermolecular Copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.; Clevidence, B.A.; Kurilich, A.C. Anthocyanin kinetics are dependent on anthocyanin structure. Br. J. Nutr. 2011, 107, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of Anthocyanins from Purple Carrot Juice: Effects of Acylation and Plant Matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Kurilich, A.C.; Clevidence, B.A.; Britz, S.J.; Simon, P.W.; Novotny, J.A. Plasma and Urine Responses Are Lower for Acylated vs Nonacylated Anthocyanins from Raw and Cooked Purple Carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. [Google Scholar] [CrossRef]
- Alasalvar, C.; Al-Farsi, M.; Quantick, P.C.; Shahidi, F.; Wiktorowicz, R. Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chem. 2005, 89, 69–76. [Google Scholar] [CrossRef]
- Netzel, M.E.; Netzel, M.E.; Kammerer, D.R.; Schieber, A.; Carle, R.; Simons, L.; Bitsch, I.; Bitsch, R.; Kończak, I. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins. Innov. Food Sci. Emerg. Technol. 2007, 8, 365–372. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [CrossRef]
- Shih, P.H.; Chan, Y.C.; Liao, J.W.; Wang, M.F.; Yen, G.C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. [Google Scholar] [CrossRef]
- Baranski, R.; Goldman, I.; Nothnagel, T.; Scott, J.W. Improving Color Sources by Plant Breeding and Cultivation. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2016; pp. 429–472. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Characterization of phenolic acids in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Fernandes, A.; Mateus, N.; de Freitas, V.; Esteves da Silva, J.C.G.; Casado, J. Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Comp. Anal. 2014, 33, 71–76. [Google Scholar] [CrossRef]
- Bannoud, F.; Da Peña Hamparsomián, J.; Insani, M.; Chamorro, V.; Pazos, A.; Pérez, M.B.; Yildiz, M.; Iorizzo, M.; Simon, P.W.; Cavagnaro, P.F. Assessment of genetic diversity for root anthocyanin composition and phenolic content in purple carrots. In Proceedings of the II International Symposium on Carrot and Other Apiaceae, Cracow, Poland, 19–22 September 2018. [Google Scholar]
- Leja, M.; Kaminska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant. Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, P.; Bannoud, F.; Iorizzo, M.; Senalik, D.; Ellison, S.; Simon, P. Carrot anthocyanins: Nutrition, diversity and genetics. Acta Hortic. 2019, 101–106. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Iorizzo, M.; Yildiz, M.; Senalik, D.; Parsons, J.; Ellison, S.; Simon, P.W. A gene-derived SNP-based high resolution linkage map of carrot including the location of QTL conditioning root and leaf anthocyanin pigmentation. BMC Genom. 2014, 15, 1118. [Google Scholar] [CrossRef] [PubMed]
- Bannoud, F.; Ellison, S.; Paolinelli, M.; Horejsi, T.; Senalik, D.; Fanzone, M.; Iorizzo, M.; Simon, P.W.; Cavagnaro, P. Dissecting the genetic control of root and leaf tissue-specific anthocyanin pigmentation in carrot (Daucus carota L.). Theor. Appl. Genet. 2019, 132, 2485–2507. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Iorizzo, M. Carrot Anthocyanin Diversity, Genetics, and Genomics. In The Carrot Genome; Simon, P., Iorizzo, M., Grzebelus, D., Baranski, R., Eds.; Springer International Publishing: Berlin, Germany, 2019; pp. 261–277. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Detection of peonidin and pelargonidin glycosides in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2407–2412. [Google Scholar] [CrossRef]
- Iorizzo, M.; Senalik, D.A.; Ellison, S.L.; Grzebelus, D.; Cavagnaro, P.F.; Allender, C.; Brunet, J.; Spooner, D.M.; Van Deynze, A.; Simon, P.W. Genetic structure and domestication of carrot (Daucus carota subsp. sativus) (Apiaceae). Am. J. Bot. 2013, 100, 930–938. [Google Scholar] [CrossRef]
- Ipek, A.; Turkmen, O.; Fidan, S.; Ipek, M.; Karci, H. Genetic variation within the purple carrot population grown in Ereğli District in Turkey. Turk. J. Agric. For. 2016, 40, 570–576. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Tierno, R.; Lopez-Maestresalas, A.; Riga, P.; Arazuri, S.; Jaren, C.; Benedicto, L.; De Galarreta, J.I.R. Phytochemicals determination and classification in purple and red fleshed potato tubers by analytical methods and near infrared spectroscopy. J. Sci. Food Agric. 2015, 96, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Van Camp, J.; Capanoglu, E. Black carrot polyphenols: Effect of processing, storage and digestion—An overview. Phytochem. Rev. 2017, 17, 379–395. [Google Scholar] [CrossRef]
- Turker, N.; Aksay, S.; Ekiz, H.I. Effect of Storage Temperature on the Stability of Anthocyanins of a Fermented Black Carrot (Daucus carota var. L.) Beverage: Shalgam. J. Agric. Food Chem. 2004, 52, 3807−3813. [Google Scholar] [CrossRef] [PubMed]
- Ersus, S.; Yurdagel, U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007, 80, 805–812. [Google Scholar] [CrossRef]
- Toklucu, A.K.; Özkan, M.; Cemeroğlu, B.; Cemeroǧlu, B. Storage stability of strawberry jam color enhanced with black carrot juice concentrate. J. Food Process. Preserv. 2007, 31, 531–545. [Google Scholar] [CrossRef]
- Özen, G.; Akbulut, M.; Artik, N. Stability of black carrot anthocyanins in the turkish delight (lokum) during storage. J. Food Process. Eng. 2009, 34, 1282–1297. [Google Scholar] [CrossRef]
- Türkyılmaz, M.; Özkan, M. Kinetics of anthocyanin degradation and polymeric colour formation in black carrot juice concentrates during storage. Int. J. Food Sci. Technol. 2012, 47, 2273–2281. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E. Influence of different processing and storage conditions on in vitro bioaccessibility of polyphenols in black carrot jams and marmalades. Food Chem. 2015, 186, 74–82. [Google Scholar] [CrossRef]
- Zozio, S.; Pallet, D.; Dornier, M. Evaluation of anthocyanin stability during storage of a coloured drink made from extracts of the Andean blackberry (Rubus glaucusBenth.), açai (Euterpe oleraceaMart.)and black carrot (Daucus carotaL.). Fruits 2011, 66, 203–215. [Google Scholar] [CrossRef]
- Lee, E.J.; Yoo, K.S.; Patil, B.S. Total carotenoid, anthocyanin, and sugar contents in sliced or whole purple (cv. Betasweet) and orange carrots during 4-week cold storage. Hortic. Environ. Biotechnol. 2011, 52, 402–407. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E.; Paslı, A.A. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A. Acylated anthocyanins as stable, natural food colorants—A review. Pol. J. Food Nutr. Sci. 2005, 14/55, 107–116. [Google Scholar]
- Iorizzo, M.; Cavagnaro, P.F.; Bostan, H.; Zhao, Y.; Zhang, J.; Simon, P.W. A Cluster of MYB Transcription Factors Regulates Anthocyanin Biosynthesis in Carrot (Daucus carota L.) Root and Petiole. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Curaba, J.; Bostan, H.; Cavagnaro, P.F.; Senalik, D.; Mengist, M.F.; Zhao, Y.; Simon, P.W.; Iorizzo, M. Identification of an SCPL Gene Controlling Anthocyanin Acylation in Carrot (Daucus carota L.) Root. Front. Plant. Sci 2020, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Vivek, B.S.; Simon, P.W. Linkage relationships among molecular markers and storage root traits of carrot (Daucus carota L. ssp. sativus). Theor. Appl. Genet. 1999, 99, 58–64. [Google Scholar] [CrossRef]
- Yildiz, M.; Willis, D.K.; Cavagnaro, P.F.; Iorizzo, M.; Abak, K.; Simon, P.W. Expression and mapping of anthocyanin biosynthesis genes in carrot. Theor. Appl. Genet. 2013, 126, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P.; et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef]
- Simon, P.W.; Hamrick, J. Inheritance and Expression of Purple and Yellow Storage Root Color in Carrot. J. Hered. 1996, 87, 63–66. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Pottorff, M.; Cavagnaro, P. Carrot Molecular Genetics and Mapping. In The Spruce Genome; Springer Science and Business Media LLC: New York, NY, USA, 2019; pp. 101–117. [Google Scholar] [CrossRef]
- Mazza, G.; Cacace, J.E.; Kay, C.D. Methods of analysis for anthocyanins in plants and biological fluids. J. AOAC Int. 2004, 87, 129–145. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Santos, A.L.D.; Chalfun-Junior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants—Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant. Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant. Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Ma, J.; Wang, F.; Ma, H.Y.; Wang, Q.X.; Xiong, A.S. Identification and characterization of DcUCGalT1, a galactosyltransferase responsible for anthocyanin galactosylation in purple carrot (Daucus carota L.) taproots. Sci. Rep. 2016, 6, 27356. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xu, Z.S.; Xiong, A.S. Identification and Characterization of DcUSAGT1, a UDP-Glucose: Sinapic Acid Glucosyltransferase from Purple Carrot Taproots. PLoS ONE 2016, 11, e0154938. [Google Scholar] [CrossRef]
- Meng, G.; Clausen, S.K.; Rasmussen, S.K. Transcriptome Analysis Reveals Candidate Genes Related to Anthocyanin Biosynthesis in Different Carrot Genotypes and Tissues. Plants 2020, 9, 344. [Google Scholar] [CrossRef]
- Fraser, C.M.; Thompson, M.G.; Shirley, A.M.; Ralph, J.; Schoenherr, J.A.; Sinlapadech, T.; Hall, M.C.; Chapple, C. Related Arabidopsis serine carboxypeptidase-like sinapoylglucose acyltransferases display distinct but overlapping substrate specificities. Plant Physiol. 2007, 144, 1986–1999. [Google Scholar] [CrossRef]
- Stehle, F.; Brandt, W.; Stubbs, M.T.; Milkowski, C.; Strack, D. Sinapoyltransferases in the light of molecular evolution. Phytochemistry 2009, 70, 1652–1662. [Google Scholar] [CrossRef]
- Xu, Z.S.; Huang, Y.; Wang, F.; Song, X.; Wang, G.L.; Xiong, A.S. Transcript profiling of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant. Biol. 2014, 14, 262. [Google Scholar] [CrossRef]
- Xu, Z.-S.; Yang, Q.-Q.; Feng, K.; Xiong, A.-S. Changing Carrot Color: Insertions in DcMYB7 Alter the Regulation of Anthocyanin Biosynthesis and Modification. Plant. Physiol. 2019, 181, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Yang, Q.Q.; Feng, K.; Yu, X.; Xiong, A.S. DcMYB113, a root-specific R2R3-MYB, conditions anthocyanin biosynthesis and modification in carrot. Plant. Biotechnol J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.W.; Rebocho, A.B.; Sagawa, J.M.; Stanley, L.E.; Bradshaw, H.D., Jr. Competition between anthocyanin and flavonol biosynthesis produces spatial pattern variation of floral pigments between Mimulus species. Proc. Natl. Acad. Sci. USA 2016, 113, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Ning, G.; Wang, Z.; Shen, Y.; Jin, H.; Li, P.; Huang, S.; Zhao, J.; Bao, M. Disequilibrium of Flavonol Synthase and Dihydroflavonol-4-Reductase Expression Associated Tightly to White vs. Red Color Flower Formation in Plants. Front. Plant. Sci 2016, 6, 1257. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.M.; Deroles, S.C.; Manson, D.G.; Lewis, D.H.; Bloor, S.J.; Bradley, J.M. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 2003, 131, 259–268. [Google Scholar] [CrossRef]
- Tan, G.F.; Ma, J.; Zhang, X.Y.; Xu, Z.S.; Xiong, A.S. AgFNS overexpression increase apigenin and decrease anthocyanins in petioles of transgenic celery. Plant. Sci 2017, 263, 31–38. [Google Scholar] [CrossRef]
- Thill, J.; Miosic, S.; Ahmed, R.; Schlangen, K.; Muster, G.; Stich, K.; Halbwirth, H. ’Le Rouge et le Noir’: A decline in flavone formation correlates with the rare color of black dahlia (Dahlia variabilis hort.) flowers. BMC Plant. Biol. 2012, 12. [Google Scholar] [CrossRef]
- LaFountain, A.M.; Chen, W.; Sun, W.; Chen, S.; Frank, H.A.; Ding, B.; Yuan, Y.W. Molecular Basis of Overdominance at a Flower Color Locus. G3 (Bethesda) 2017, 7, 3947–3954. [Google Scholar] [CrossRef]
- Wang, H.; Fan, W.; Li, H.; Yang, J.; Huang, J.; Zhang, P. Functional characterization of Dihydroflavonol-4-reductase in anthocyanin biosynthesis of purple sweet potato underlies the direct evidence of anthocyanins function against abiotic stresses. PLoS ONE 2013, 8, e78484. [Google Scholar] [CrossRef]
- Lim, S.H.; You, M.K.; Kim, D.H.; Kim, J.K.; Lee, J.Y.; Ha, S.H. RNAi-mediated suppression of dihydroflavonol 4-reductase in tobacco allows fine-tuning of flower color and flux through the flavonoid biosynthetic pathway. Plant Physiol. Biochem. 2016, 109, 482–490. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Tan, H.W.; Wang, F.; Hou, X.L.; Xiong, A.S. CarrotDB: A genomic and transcriptomic database for carrot. Database (Oxford) 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Chodacka, M.; Oleszkiewicz, T.; Lowder, L.G.; Qi, Y.; Baranski, R. Efficient CRISPR/Cas9-based genome editing in carrot cells. Plant Cell Rep. 2018, 37, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Lin-Wang, K.; Bolitho, K.; Grafton, K.; Kortstee, A.; Karunairetnam, S.; McGhie, T.K.; Espley, R.V.; Hellens, R.P.; Allan, A.C. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 2010, 10. [Google Scholar] [CrossRef]
- Lotkowska, M.E.; Tohge, T.; Fernie, A.R.; Xue, G.P.; Balazadeh, S.; Mueller-Roeber, B. The Arabidopsis Transcription Factor MYB112 Promotes Anthocyanin Formation during Salinity and under High Light Stress. Plant Physiol. 2015, 169, 1862–1880. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB transcription factors in transcriptional regulation of anthocyanin biosynthesis in horticultural plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.N.; Arun, M.; Naing, A.H.; Lim, S.-H.; Kil Kim, C. Combinatorial expression of transcription factor genes B—Peru and mPAP1 enhances anthocyanin accumulation in transgenic Petunia hybrid. Sci. Hortic. 2016, 200, 186–196. [Google Scholar] [CrossRef]
- Campanella, J.; Smalley, J.V.; E Dempsey, M. A phylogenetic examination of the primary anthocyanin production pathway of the Plantae. Bot. Stud. 2014, 55, 10. [Google Scholar] [CrossRef]
- Lloyd, A.; Brockman, A.; Aguirre, L.; Campbell, A.; Bean, A.; Cantero, A.; Gonzalez, A. Advances in the MYB-bHLH-WD Repeat (MBW) Pigment Regulatory Model: Addition of a WRKY Factor and Co-option of an Anthocyanin MYB for Betalain Regulation. Plant Cell Physiol. 2017, 58, 1431–1441. [Google Scholar] [CrossRef]
- Xiong, A.-S.; Feng, K.; Que, F.; Wang, F.; Xiong, A.-S. A MYB transcription factor, DcMYB6, is involved in regulating anthocyanin biosynthesis in purple carrot taproots. Sci. Rep. 2017, 7, 45324. [Google Scholar] [CrossRef]
- Shan, X.; Li, Y.; Yang, S.; Gao, R.; Zhou, L.; Bao, T.; Han, T.; Wang, S.; Gao, X.; Wang, L. A functional homologue of Arabidopsis TTG1 from Freesia interacts with bHLH proteins to regulate anthocyanin and proanthocyanidin biosynthesis in both Freesia hybrida and Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef]
- Li, W.; Ding, Z.; Ruan, M.; Yu, X.; Peng, M.; Liu, Y. Kiwifruit R2R3-MYB transcription factors and contribution of the novel AcMYB75 to red kiwifruit anthocyanin biosynthesis. Sci. Rep. 2017, 7, 16861. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; De Vos, R.C.H.; Wein, M.; Sun, Z.; Greco, R.; Kroon, A.; Mol, J.N.; O’Connell, A.P. The strawberry FaMYB1 transcription factor suppresses anthocyanin and ¯avonol accumulation in transgenic tobacco. Plant J. 2001, 28, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Brinch-Pedersen, H.; Sharma, S.; Holme, I.B.; Joernsgaard, B.; Dzhanfezova, T.; Amby, D.B.; Vieira, F.G.; Liu, S.; Gilbert, M.T.P. Identification of transcription factor genes involved in anthocyanin biosynthesis in carrot (Daucus carota L.) using RNA-Seq. BMC Genom. 2018, 19, 811. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Davies, K.M.; Lewis, D.H.; Zhang, H.; Montefiori, M.; Brendolise, C.; Boase, M.R.; Ngo, H.; Jameson, P.E.; Schwinn, K.E. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014, 26, 962–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, B.; Qin, Y.; Hu, G.; Zhao, J. Advance of the negative regulation of anthocyanin biosynthesis by MYB transcription factors. Plant Physiol. Biochem. 2019, 136, 178–187. [Google Scholar] [CrossRef]
- Xu, Z.S.; Feng, K.; Xiong, A.S. CRISPR/Cas9-Mediated Multiply Targeted Mutagenesis in Orange and Purple Carrot Plants. Mol. Biotechnol. 2019, 61, 191–199. [Google Scholar] [CrossRef]
- Walker, A.R. The TRANSPARENT TESTA GLABRA1 Locus, Which Regulates Trichome Differentiation and Anthocyanin Biosynthesis in Arabidopsis, Encodes a WD40 Repeat Protein. Plant Cell 1999, 11, 1337–1349. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Long, Y.; Schiefelbein, J. Novel TTG1 Mutants Modify Root-Hair Pattern Formation in Arabidopsis. Front. Plant Sci. 2020, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids--biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre-Gonon, E.; Law, S.R.; Schwartz, M.; Robe, K.; Keech, O.; Didierjean, C.; Dubos, C.; Rouhier, N.; Hecker, A. Functional, Structural and Biochemical Features of Plant Serinyl-Glutathione Transferases. Front. Plant Sci. 2019, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, M.; He, N.; Chen, X.; Wang, N.; Sun, Q.; Zhang, T.; Xu, H.; Fang, H.; Wang, Y.; et al. MdGSTF6, activated by MdMYB1, plays an essential role in anthocyanin accumulation in apple. Hortic. Res. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Nishiyama, Y.; Hirai, M.Y.; Yano, M.; Nakajima, J.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.B.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005, 42, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance. In Plant Signaling Molecules; Elsevier BV: London, UK, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An Alternative Use of Horticultural Crops: Stressed Plants as Biofactories of Bioactive Phenolic Compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal. Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef]
- Peng, M.; Hudson, D.; Schofield, A.; Tsao, R.; Yang, R.; Gu, H.; Bi, Y.M.; Rothstein, S.J. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J. Exp. Bot. 2008, 59, 2933–2944. [Google Scholar] [CrossRef]
- Allan, A.C.; Espley, R.V. MYBs Drive Novel Consumer Traits in Fruits and Vegetables. Trends Plant Sci. 2018, 23, 693–705. [Google Scholar] [CrossRef]
- Li, Y.; Lin-Wang, K.; Liu, Z.; Allan, A.C.; Qin, S.; Liu, Y.; Zhang, J. Genome-wide analysis and expression profiles of the StR2R3-MYB transcription factor superfamily in potato (Solanum tuberosum L.). Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Glied, S.; Crocoll, C.; Dzhanfezova, T.; Joernsgaard, B.; Okkels, F.; Lutken, H.; Muller, R. Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. sativus var. atrorubens Alef.). BMC Plant Biol. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Ribera-Fonseca, A.; Jimenez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Diaz, M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Yıldız, K.; Ozkan, Y. Effects of Pre-Harvest Methyl Jasmonate Treatments on Bioactive Compounds and Peel Color Development of “Fuji” Apples. Int. J. Food Prop. 2015, 18, 954–962. [Google Scholar] [CrossRef]
- Yi, T.G.; Park, Y.; Park, J.-E.; Park, N.I. Enhancement of Phenolic Compounds and Antioxidative Activities by the Combination of Culture Medium and Methyl Jasmonate Elicitation in Hairy Root Cultures of Lactuca indica L. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Das, P.K.; Shin, D.H.; Choi, S.B.; Park, Y.I. Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol. Cells 2012, 34, 501–507. [Google Scholar] [CrossRef]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tan, H.; Ma, Z.; Huang, J. DELLA Proteins Promote Anthocyanin Biosynthesis via Sequestering MYBL2 and JAZ Suppressors of the MYB/bHLH/WD40 Complex in Arabidopsis thaliana. Mol. Plant 2016, 9, 711–721. [Google Scholar] [CrossRef]
- Jeong, S.W.; Das, P.K.; Jeoung, S.C.; Song, J.Y.; Lee, H.K.; Kim, Y.K.; Kim, W.J.; Park, Y.I.; Yoo, S.D.; Choi, S.B.; et al. Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiol. 2010, 154, 1514–1531. [Google Scholar] [CrossRef]
- Cormier, F.; CREVIER, H.A.; Do, C.B. Effects of sucrose concentration on the accumulation of anthocyanins in grape (Vitis vinifera) cell suspension. Can. J. Bot. 1990, 68, 1822–1825. [Google Scholar] [CrossRef]
- Hara, M.R.; Oki, K.; Hoshino, K.; Kuboi, T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci. 2003, 164, 259–265. [Google Scholar] [CrossRef]
- Weiss, D. Regulation of flower pigmentation and growth: Multiple signaling pathways control anthocyanin synthesis in expanding petals. Physiol. Plant. 2000, 110, 152–157. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.N.; Naing, A.H.; Arun, M.; Lim, S.H.; Kim, C.K. Sucrose-induced anthocyanin accumulation in vegetative tissue of Petunia plants requires anthocyanin regulatory transcription factors. Plant Sci. 2016, 252, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Ravishankar, G.A.; Venkataraman, L.V.; Prathiba, K.R. Anthocyanin production in callus cultures of daucus carota as influenced by nutrient stress and osmoticum. Biotechnol. Lett. 1992, 14, 707–712. [Google Scholar] [CrossRef]
- Narayan, M.S.; Venkataraman, L.V. Effect of Sugar and Nitrogen on the Production of Anthocyanin in Cultured Carrot (Daucus carota) cells. J. Food Sci. 2002, 67. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef]
- Lea, U.S.; Slimestad, R.; Smedvig, P.; Lillo, C. Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 2007, 225, 1245–1253. [Google Scholar] [CrossRef]
- Wang, X.F.; An, J.P.; Liu, X.; Su, L.; You, C.X.; Hao, Y.J. The Nitrate-Responsive Protein MdBT2 Regulates Anthocyanin Biosynthesis by Interacting with the MdMYB1 Transcription Factor. Plant Physiol. 2018, 178, 890–906. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2009, 51, 242–249. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant. Sci 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velazquez, D.A.; Martinez-Hernandez, G.B.; Del, C.R.S.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velazquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Du, W.X.; Avena-Bustillos, R.J.; Breksa, A.P., 3rd; McHugh, T.H. Effect of UV-B light and different cutting styles on antioxidant enhancement of commercial fresh-cut carrot products. Food Chem. 2012, 134, 1862–1869. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Plants as biofactories: Glyphosate-induced production of shikimic acid and phenolic antioxidants in wounded carrot tissue. J. Agric. Food Chem. 2012, 60, 11378–11386. [Google Scholar] [CrossRef]
- Alegria, C.; Pinheiro, J.; Duthoit, M.; Gonçalves, E.M.; Moldão-Martins, M.; Abreu, M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT Food Sci. Technol. 2012, 48, 197–203. [Google Scholar] [CrossRef]
- Simões, A.D.N.; Allende, A.; Tudela, J.A.; Puschmann, R.; Gil, M.I. Optimum controlled atmospheres minimise respiration rate and quality losses while increase phenolic compounds of baby carrots. LWT Food Sci. Technol. 2011, 44, 277–283. [Google Scholar] [CrossRef]
- Ceoldo, S.; Toffali, K.; Mantovani, S.; Baldan, G.; Levi, M.; Guzzo, F. Metabolomics of Daucus carota cultured cell lines under stressing conditions reveals interactions between phenolic compounds. Plant Sci. 2009, 176, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Eiro, M.J.; Heinonen, M. Anthocyanin Color Behavior and Stability during Storage: Effect of Intermolecular Copigmentation. J. Agric. Food Chem. 2002, 50, 7461−7466. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, M.; Zhang, G.; Li, P.; Ma, F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. Int. J. Mol. Sci. 2019, 20, 6060. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, D.; Zhou, L.; Zhang, X.; Liao, J.; Duan, Y.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; et al. Transcriptomic and metabolomic profiling of Camellia sinensis L. cv. ’Suchazao’ exposed to temperature stresses reveals modification in protein synthesis and photosynthetic and anthocyanin biosynthetic pathways. Tree Physiol. 2019, 39, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Luscher, J.; Sanchez-Diaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomes, E. Ultraviolet-B radiation and water deficit interact to alter flavonol and anthocyanin profiles in grapevine berries through transcriptomic regulation. Plant Cell Physiol. 2014, 55, 1925–1936. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Sharkhuu, A.; Batelli, G.; Bressan, R.A.; Maggio, A. The Arabidopsis thaliana mutant air1 implicates SOS3 in the regulation of anthocyanins under salt stress. Plant Mol. Biol. 2013, 83, 405–415. [Google Scholar] [CrossRef]
- Schmidt, L.; Sorg, S.; Tittmann, S.; Max, J.; Zinkernagel, J. Do Extended Cultivation Periods and Reduced Nitrogen Supply Increase Root Yield and Anthocyanin Content of Purple Carrots? Horticulturae 2018, 4, 7. [Google Scholar] [CrossRef]
- Simon, P.W. Classical and Molecular Carrot Breeding. In The Spruce Genome; Springer Science and Business Media LLC: New York, NY, USA, 2019; pp. 137–147. [Google Scholar] [CrossRef]
- Grassmann, J.; Schnitzler, W.H.; Habegger, R. Evaluation of different coloured carrot cultivars on antioxidative capacity based on their carotenoid and phenolic contents. Int. J. Food Sci Nutr. 2007, 58, 603–611. [Google Scholar] [CrossRef]
- Ribaut, J.M.; de Vicente, M.C.; Delannay, X. Molecular breeding in developing countries: Challenges and perspectives. Curr. Opin. Plant. Biol. 2010, 13, 213–218. [Google Scholar] [CrossRef]
- Ellison, S.; Senalik, D.; Bostan, H.; Iorizzo, M.; Simon, P. Fine Mapping, Transcriptome Analysis, and Marker Development for Y2, the Gene That Conditions beta-Carotene Accumulation in Carrot (Daucus carota L.). G3 (Bethesda) 2017, 7, 2665–2675. [Google Scholar] [CrossRef]
- Platten, J.D.; Cobb, J.N.; Zantua, R.E. Criteria for evaluating molecular markers: Comprehensive quality metrics to improve marker-assisted selection. PLoS ONE 2019, 14, e0210529. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-Z.; Xie, D. Biosynthesis and Metabolic Engineering of Anthocyanins in Arabidopsis thaliana. Recent Patents Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Lin-Wang, K.; McGhie, T.K.; Wang, M.; Liu, Y.; Warren, B.; Storey, R.; Espley, R.V.; Allan, A.C. Engineering the anthocyanin regulatory complex of strawberry (Fragaria vesca). Front. Plant. Sci. 2014, 5, 651. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Abe, Y.; Kakizaki, Y.; Yamamura, S.; Nishihara, M. Production of red-flowered plants by genetic engineering of multiple flavonoid biosynthetic genes. Plant Cell Rep. 2007, 26, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Mishiba, K.; Kubota, A.; Abe, Y.; Yamamura, S.; Nakamura, N.; Tanaka, Y.; Nishihara, M. Genetic engineering of novel flower colour by suppression of anthocyanin modification genes in gentian. J. Plant Physiol. 2010, 167, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Aida, R.; Kishimoto, S.; Ishiguro, K.; Fukuchi-Mizutani, M.; Tanaka, Y.; Ohmiya, A. Genetic engineering of novel bluer-colored chrysanthemums produced by accumulation of delphinidin-based anthocyanins. Plant Cell Physiol. 2013, 54, 1684–1695. [Google Scholar] [CrossRef]
- Mezzetti, B.; Smagghe, G.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Kostov, K.; Sabbadini, S.; Opsahl-Sorteberg, H.-G.; Ventura, V.; et al. RNAi: What is its position in agriculture? J. Pest. Sci. 2020. [Google Scholar] [CrossRef]
- Morita, Y.; Takagi, K.; Fukuchi-Mizutani, M.; Ishiguro, K.; Tanaka, Y.; Nitasaka, E.; Nakayama, M.; Saito, N.; Kagami, T.; Hoshino, A.; et al. A chalcone isomerase-like protein enhances flavonoid production and flower pigmentation. Plant J. 2014, 78, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Imani, J.; Mahler, V.; Foetisch, K.; Kaul, S.; Paulus, K.E.; Scheurer, S.; Vieths, S.; Kogel, K.H. Dau c 1.01 and Dau c 1.02-silenced transgenic carrot plants show reduced allergenicity to patients with carrot allergy. Transgenic Res. 2011, 20, 547–556. [Google Scholar] [CrossRef]
- Flores-Ortiz, C.; Alvarez, L.M.; Undurraga, A.; Arias, D.; Duran, F.; Wegener, G.; Stange, C. Differential role of the two zeta-carotene desaturase paralogs in carrot (Daucus carota): ZDS1 is a functional gene essential for plant development and carotenoid synthesis. Plant Sci. 2020, 291, 110327. [Google Scholar] [CrossRef]
- Gläßgen, W.E.; Rose, A.; Madlung, J.; Koch, W.; Gleitz, J.; Seitz, H.U. Regulation of enzymes involved in anthocyanin biosynthesis in carrot cell cultures in response to treatment with ultraviolet light and fungal elicitors. Planta 1998, 204, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.S.; Venkataraman, L.V. Characterisation of anthocyanins derived from carrot (Daucus carota) cell culture. Food Chem. 2000, 70, 361–363. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Asen, S.; Stewart, R.N.; Norris, K.H. Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Darias-Martin, J.; Carrillo, M.; Diaz, E.; Boulton, R.B. Enhancement of red wine colour by pre-fermentation addition of copigments. Food Chem. 2001, 73, 217–220. [Google Scholar] [CrossRef]
- Rein, M.J.; Heinonen, M. Stability and Enhancement of Berry Juice Color. J. Agric. Food Chem. 2004, 52, 3106–3114. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-Garcia, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Mazza, G.; Brouillard, R. The mechanism of co-pigmentation of anthocyanins in aqueous solutions. Phytochemistry 1990, 29, 1097–1102. [Google Scholar] [CrossRef]
- Gras, C.C.; Nemetz, N.; Carle, R.; Schweiggert, R.M. Anthocyanins from purple sweet potato (Ipomoea batatas (L.) Lam.) and their color modulation by the addition of phenolic acids and food-grade phenolic plant extracts. Food Chem. 2017, 235, 265–274. [Google Scholar] [CrossRef]
- Plazas, M.; Andújar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herráiz, F.J.; Prohens, J. Breeding for Chlorogenic Acid Content in Eggplant: Interest and Prospects. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef]
- Kaushik, P.; Gramazio, P.; Vilanova, S.; Raigón, M.D.; Prohens, J.; Plazas, M. Phenolics content, fruit flesh colour and browning in cultivated eggplant, wild relatives and interspecific hybrids and implications for fruit quality breeding. Food Res. Int. 2017, 102, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Gras, C.C.; Bogner, H.; Carle, R.; Schweiggert, R.M. Effect of genuine non-anthocyanin phenolics and chlorogenic acid on color and stability of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) anthocyanins. Food Res. Int. 2016, 85, 291–300. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]





| Compound | Abbreviation | RT | MW |
|---|---|---|---|
| Cyanidin 3-xylosylglucosylgalactoside | Cy3XGG | 14.0 | 743 |
| Cyanidin 3-xylosylgalactoside | Cy3XG | 15.1 | 581 |
| Cyanidin 3-xylosyl(sinapoylglucosyl)galactoside | Cy3XSGG | 15.4 | 949 |
| Cyanidin 3-xylosyl(feruloylglucosyl)galactoside | Cy3XFGG | 16.0 | 919 |
| Cyanidin 3-xylosyl(coumuroylglucosyl)galactoside | Cy3XCGG | 16.4 | 889 |
| Pelargonidin 3-xylosyl(feruloylglucosyl)galactoside | - | 21.8 | 903 |
| Peonidin 3-xylosylgalactoside | - | 22.3 | 595 |
| Peonidin 3-xylosyl(sinapoylglucosyl)galactoside | - | 22.7 | 963 |
| Peonidin 3-xylosyl(feruloylglucosyl)galactoside | - | 23.3 | 933 |
| Sample | Storage Parameters | Key Findings | Citation |
|---|---|---|---|
| Fermented black carrot beverage (Shalgam) | T = 4, 25, and 40 °C ST = 90 days | Anthocyanin degradation rate was significantly higher at 40 °C storage temperature; Anthocyanin degradation rate at 4 °C and 25 °C was not significantly different AA were significantly more stable than NAA | [42] |
| Black carrot concentrate | T = 4, 20 and 37 °C ST = based on t1/2 Brix = 30, 45 and 64 pH = 2.5, 3, 4, 5, 6, 7 | Degradation of anthocyanin stored at 37 °C was much faster compared to storage at 4 °C (t1/2 = 4.0–4.5 and 71.8–215 weeks, respectively) Significant decrease in anthocyanin stability was observed at pH values above 5.0 | [44] |
| Black carrot juice concentrate | T1 = −23, 5 and 20 °C ST1 = 319 days T2 = 30 °C ST2 = 53 days | AA exhibited higher stability during storage; under sub-freezing conditions, anthocyanin degradation was minimal | [46] |
| Turkish delight colored with black carrot juice concentrate | T = 12, 20 and 30 °C ST = 5 months | At higher temperatures anthocyanin degradation rate was higher | [45] |
| Soft drink colored with black carrot extract | T = 4, 20, 30 and 50 °C ST = 60 days | At 4 °C no degradation was detected Anthocyanin from black carrot degraded more slowly than blackberry and aҫai anthocyanin | [48] |
| Sliced purple carrots | T = 2, 4 °C ST = 4 weeks | No significant difference in anthocyanin content were detected between 2 and 4 °C storage temperatures | [49] |
| Black carrot jams and marmalades | T = 4 and 25 °C ST = 20 weeks | The reduction of anthocyanin content in samples stored at 4 °C was lower than that of samples stored at 25 °C AA were significantly more stable than NAA | [47,50] |
| Black carrot concentrate | pH = 3, 4, 5 | Cy3SGG was found to exhibit a lower visual detection threshold and a higher pH than Cy3FGG and Cy3XCG | [6] |
| Black carrot concentrate | pH = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 ST = 30 min | Degradation rates of anthocyanin increased with pH | [8] |
| Function | Name | Locus ID | Overlapping Anthocyanin QTLsn | Expression in Purple Tissue | |
|---|---|---|---|---|---|
| Type | Genotype | ||||
| Regulatory Genes | DcMYB5 | DCAR_024737 | XSGG(1,1); Ph-SumNAA(1,1) | DW(R) | PP68 |
| DcMYB6 | DCAR_000385 | P2(1,1); P3(1,2); RPTE(1,1); TotalANT(1,1); SumAA(2,1); SumNAA(1,1); G(1,1); GG(2,1); SGG(3,2); FGG(3,2); CGG(2,2); Ph-TotalANT(2,1); Ph-SumAA(2,1); Ph-SumNAA(3,1); Ph-G(2,1); Ph-GG(3,1); Ph-CGG(3,1); Ph-SGG(2,1); Ph-FGG(1,1)Xy-TotalANT(1,1); Xy-SumAA(1,1); Xy-SumNAA(1,1); Xy-G(1,1); Xy-CGG(1,1); Xy-SGG(1,1); Xy-FGG(1,1); PAP(1,2); Phloem(1,1); XAP(1,1) | UP(R) | 5394, 3242, DPP, PP68, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7 | |
| DW(P) | 5723, 95710 | ||||
| DcMYB7 | DCAR_010745 | UP(R) | 7280, 5394, 95710, 3242, DPP, PP68, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7 | ||
| UP(P) | 5723, 95710, 35SMyb7 | ||||
| DcMYB10 | DCAR_010749 | UP(P) | 5723 | ||
| DcMYB11 | DCAR_010751 | UP(P) | 5723, 95710 | ||
| DcMYB113 | DCAR_008994 | P1(1,1); Ph-TotalANT(2,1);Ph-SumAA(2,1); Ph-SumNAA(2,1); Ph-G(2,1); Ph-GG(2,1); Ph-CGG(3,1); Ph-SGG(2,1); Xy-CGG(1,1) | UP(R) | PPHZ, 35SMyb113 | |
| DcMYB17 | DCAR_007287 | Ph-GG(1,1) | UP(R) | PP68 | |
| DcMYB19 | DCAR_015602 | Ph-FGG(2,1) | UP(R) | PP68 | |
| DcMYB22 | DCAR_018882 | Ph-TotalANT(1,1) | DW(R) | PP68 | |
| DcMYB1R1-1 | DCAR_026095 | - | DW(R) | PP68 | |
| DcMYB1R1-2 | DCAR_024503 | SGG(1,1); Ph-SumNAA(1,1) | DW(R) | PP68 | |
| DcbHLH3 | DCAR_002739 | RTPE(1,1) | UP(R) | 5394, 95710, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| DcTTG1 | DCAR_020377 | - | X(R) | 5394, 95710 | |
| X(P) | 5723, 95710 | ||||
| DcGST1 | DCAR_003401 | RTPE(1,1) | UP(R) | DPP, PP68, PPHZ, CPP, 35SMyb113 | |
| Structural Genes | DcPAL4 | DCAR_017697 | - | UP(R) | 5394, 95710, DPP, PP68, TZ2H |
| DcC4H1 | DCAR_018641 | - | UP(R) | 5394, 95710, DPP, PP68, TZ2H | |
| Dc4CL3-1 | DCAR_021385 | - | UP(R) | 95710, DPP, PP68, TZ2H | |
| DW(P) | 95710 | ||||
| Dc4CL3-2 | DCAR_025617 | - | UP(R) | 95710 | |
| DcCHS1 | DCAR_030786 | - | UP(R) | B9547, B7262, 5394, 95710, 3242, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| UP(P) | 5723 | ||||
| DcCHI1 | DCAR_027694 | - | UP(R) | 5394, 95710, 3242, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| UP(P) | 5723 | ||||
| DcF3H1 | DCAR_009483 | - | UP(R) | B9547, B7262, 95710, 3242, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| UP(P) | 5723 | ||||
| DcF3’H1 | DCAR_014032 | Ph-TotalANT(2,1); Ph-SumNAA(3,1); Ph-G(3,1); Ph-GG(4,1) | UP(R) | 95710, 3242, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| DcDFR1 | DCAR_021485 | - | UP(R) | B9547, B7262, 5394, 95710, 3242, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| UP(P) | 5723, 9571 | ||||
| DcUSAGT | DCAR_029082 | - | UP(R) | 7280, 5394, 95710 | |
| DcLDOX1 | DCAR_006772 | - | UP(P) | DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 5723, 95710 | |
| DcUCGXT1 | DCAR_021269 | SGG(1,1) | UP(R) | 7280, 5394, 95710, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| DcUCGalT1 | DCAR_009912 | TotalANT(1,1); SumAA(1,1); XFGG(1,1); Ph-CGG(2,1) | UP(R) | 3242, 7280, 5394, 95710, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| DcSCPL1 | LOC108214129 | Raa1(1,1); XGG-(1,1); SGG(1,1); FGG(1,1); Ph-CGG(2,1) | UP(R) | 7280, 5394, 95710, DPP, PP68, PPHZ, TZ2H, ZBC-S, ZBC-P, CPP, 35SMyb7, 35SMyb113 | |
| DcSCPL12 | LOC108227197 | Ph-SGG(2,1) | UP(R) | 95710 | |
| DcSCPL13 | LOC108227196 | UP(R) | 5394, 95710 | ||
| DcSCPL14 | LOC108227198 | UP(R) | 7280 | ||
| DcSCPL15 | LOC108192824 | X(R) | 5394, 95710 | ||
| DcBAHD39 | LOC108196041 | SumAA(1,1); SumNAA(1,1); G(1,1); GG(1,1); SGG(1,1); FGG(1,1); CGG(1,1); Ph-TotalANT(2,1); Ph-SumNAA(1,1); Ph-G(1,1); Ph-GG(%)(2,1); Ph-SGG(1,1); Ph-FGG(1,1) | UP(R) | 95710 | |
| External Factor | Phenolic Quantified ** | Species | Tissue | Reference |
|---|---|---|---|---|
| Ethephon | TA65%; TP25% | Black Carrot | root | [112] |
| Sucrose | TA756% | Carrot | Callus | [126] |
| TA225% | Carrot | Callus | [127] | |
| TA600% | Arabidopsis | Seedling | [117] | |
| TA>600% | Arabidopsis | Seedling | [124] | |
| TA570%; A5>2000%; A8>600%; A9>4000%; A11>300% | Arabidopsis | Seedling | [123] | |
| TA300% | Grape | Cell culture | [120] | |
| TA1500% | Radish | Hypocotyl | [121] | |
| TA>60% | Petunia | Seedling | [125] | |
| Mannitol+SUC | TA156% | Carrot | Callus | [126] |
| Mannitol | TA60% | Arabidopsis | Seedling | [131] |
| N limitation | TA160% | Carrot | Callus | [126] |
| TA4400% | Arabidopsis | Seedling | [129] | |
| TA750%; quercetin700%; kaempferol200%; cyanidin>3000% | Arabidopsis | Seedling | [109] | |
| Pi limitation | TA120% | Carrot | Callus | [126] |
| TA500% | Arabidopsis | Seedling | [109] | |
| Wounding | TP750%; CHA500%; FA165%; IC290% | Carrot | Root * | [132] |
| TP800%; CHA500%; IC1300% | Carrot | Root * | [133] | |
| TP252%; CHA1000%; 3,5-diCQA80%; FA>1000%; IC>1000% | Carrot | Root * | [134] | |
| TP287%; 3-CQA700%; 3,5-diCQA>3500%; 4,5-diCQA150%; FA140% | Carrot | Root * | [135] | |
| ET+W | TP65%; CHA90%; IC1860% | Carrot | Root * | [132] |
| UV+W | TP143%; CHA600%; FA100%; IC60% | Carrot | Root * | [136] |
| TP250%; CHA750% | Carrot | Root * | [137] | |
| Hyperoxia+W | TP30%; 3-CQA75%; 3,5-diCQA75%; 4,5-diCQA100%; FA70% | Carrot | Root * | [135] |
| High Temp+W | TP150% | Carrot | Root * | [138] |
| Glyphosate+W | SA938%; CHA1988%; FA938% | Carrot | Root * | [139] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorizzo, M.; Curaba, J.; Pottorff, M.; Ferruzzi, M.G.; Simon, P.; Cavagnaro, P.F. Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry. Genes 2020, 11, 906. https://doi.org/10.3390/genes11080906
Iorizzo M, Curaba J, Pottorff M, Ferruzzi MG, Simon P, Cavagnaro PF. Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry. Genes. 2020; 11(8):906. https://doi.org/10.3390/genes11080906
Chicago/Turabian StyleIorizzo, Massimo, Julien Curaba, Marti Pottorff, Mario G. Ferruzzi, Philipp Simon, and Pablo F. Cavagnaro. 2020. "Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry" Genes 11, no. 8: 906. https://doi.org/10.3390/genes11080906
APA StyleIorizzo, M., Curaba, J., Pottorff, M., Ferruzzi, M. G., Simon, P., & Cavagnaro, P. F. (2020). Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry. Genes, 11(8), 906. https://doi.org/10.3390/genes11080906