Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Nissl Staining
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Methylation Template Purification and DNA Modification
2.5. PCR Amplification, Cloning, and Sequencing of Modified DNA
2.6. Illumina Sequencing and Data Analysis
2.7. Data Deposition
2.8. GO, KEGG Enrichment, and PPI Analysis of DEGs
2.9. Quantitative Real-Time PCR (qRT-PCR) Validation
2.10. Immunohistochemistry
2.11. Statistical Analyses
3. Results
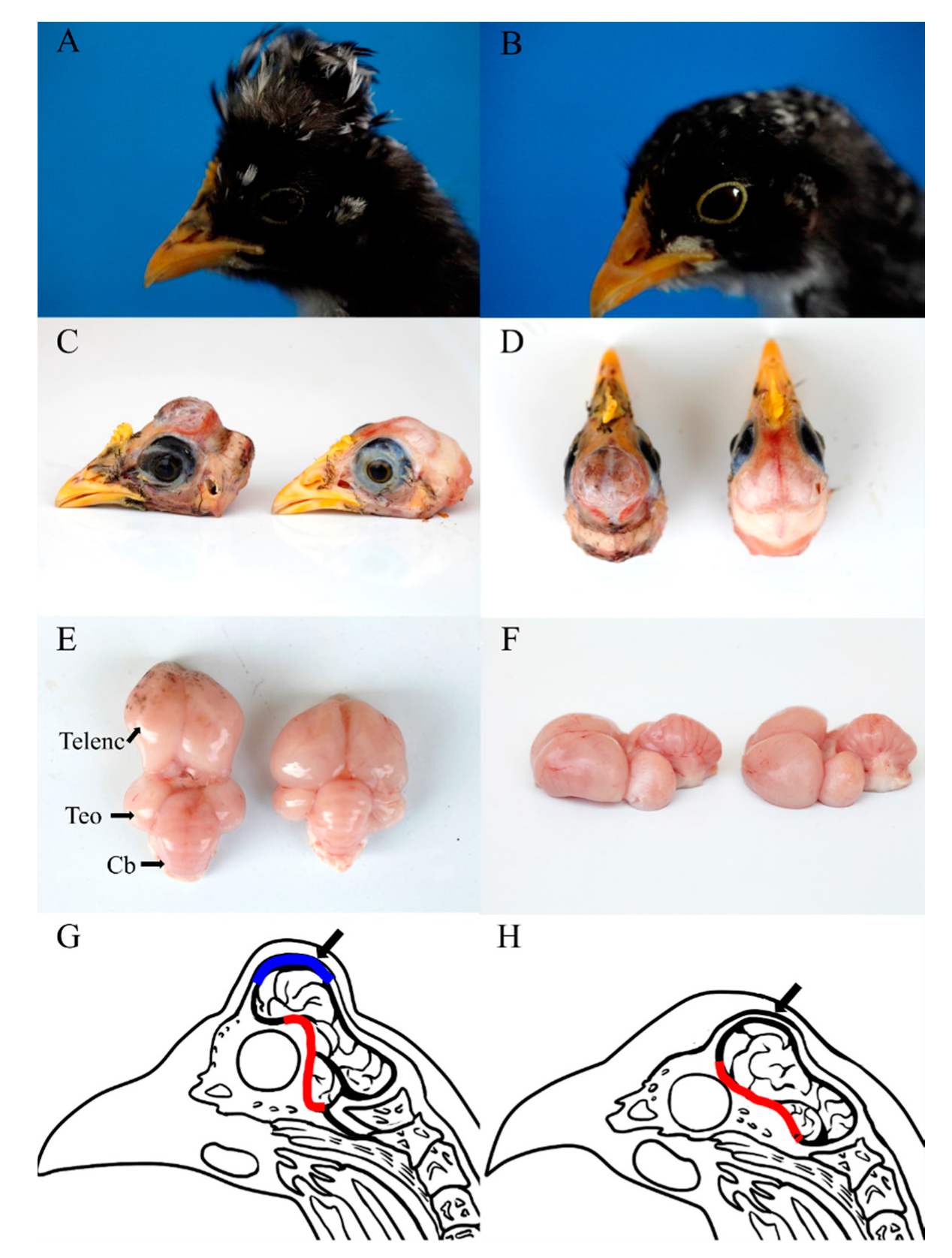
3.1. Morphological Characterization of Cerebral Hernia in Crested Chickens
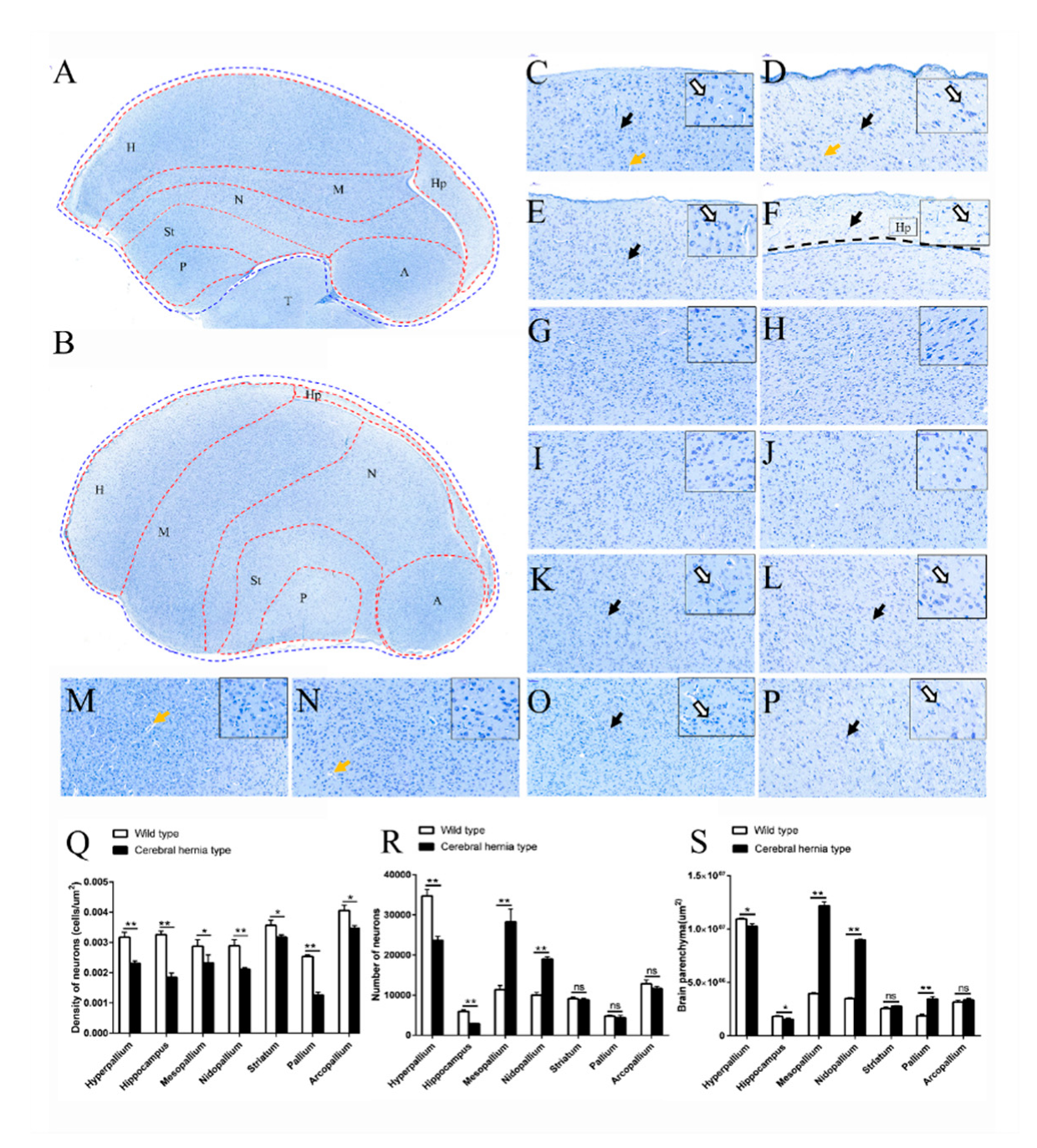
3.2. Telencephalon Deformation Accompanied with Ectopic Neurons
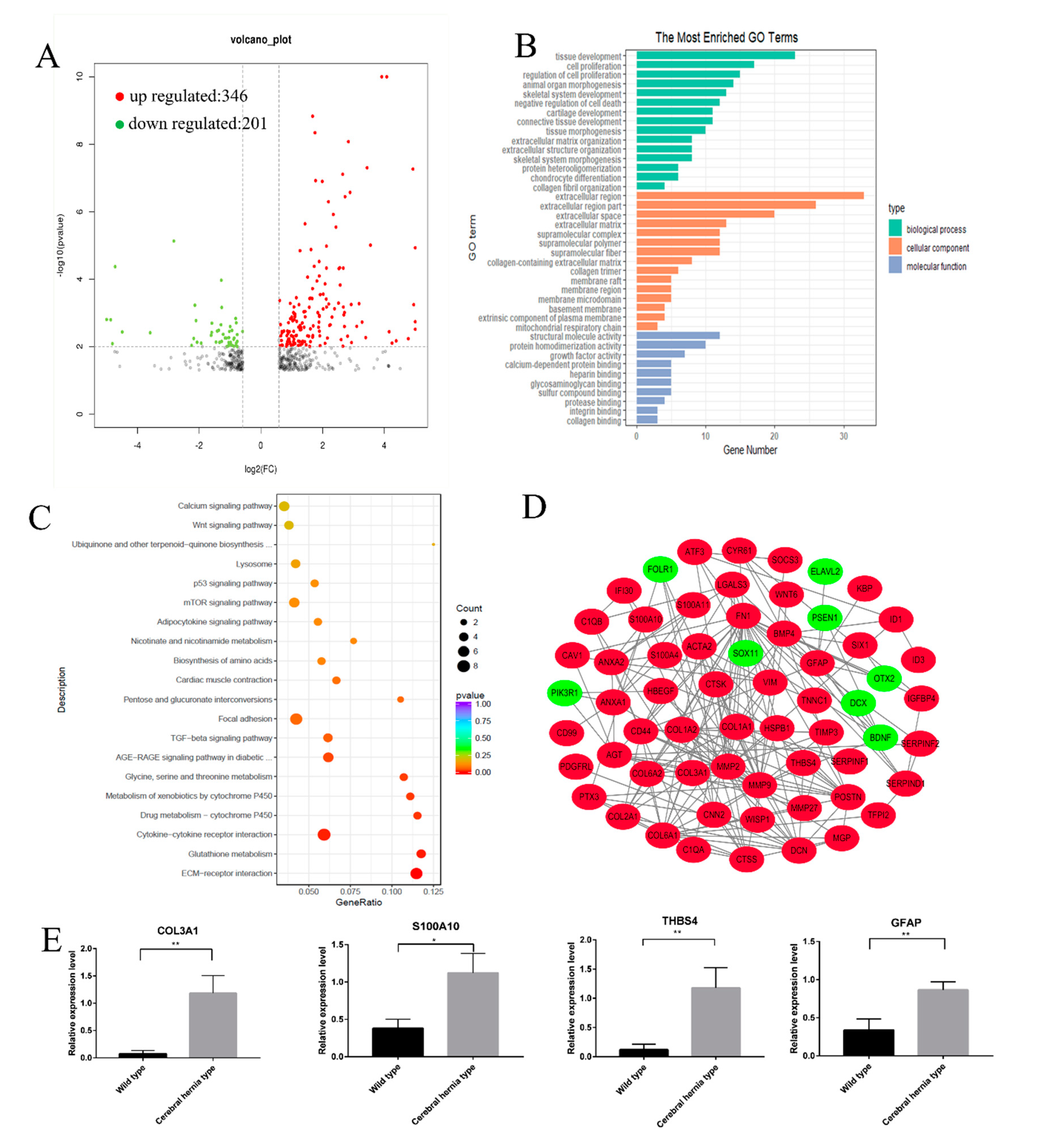
3.3. Functional Annotation of DEGs in Deformed Telencephalon in Chickens with Cerebral Hernia
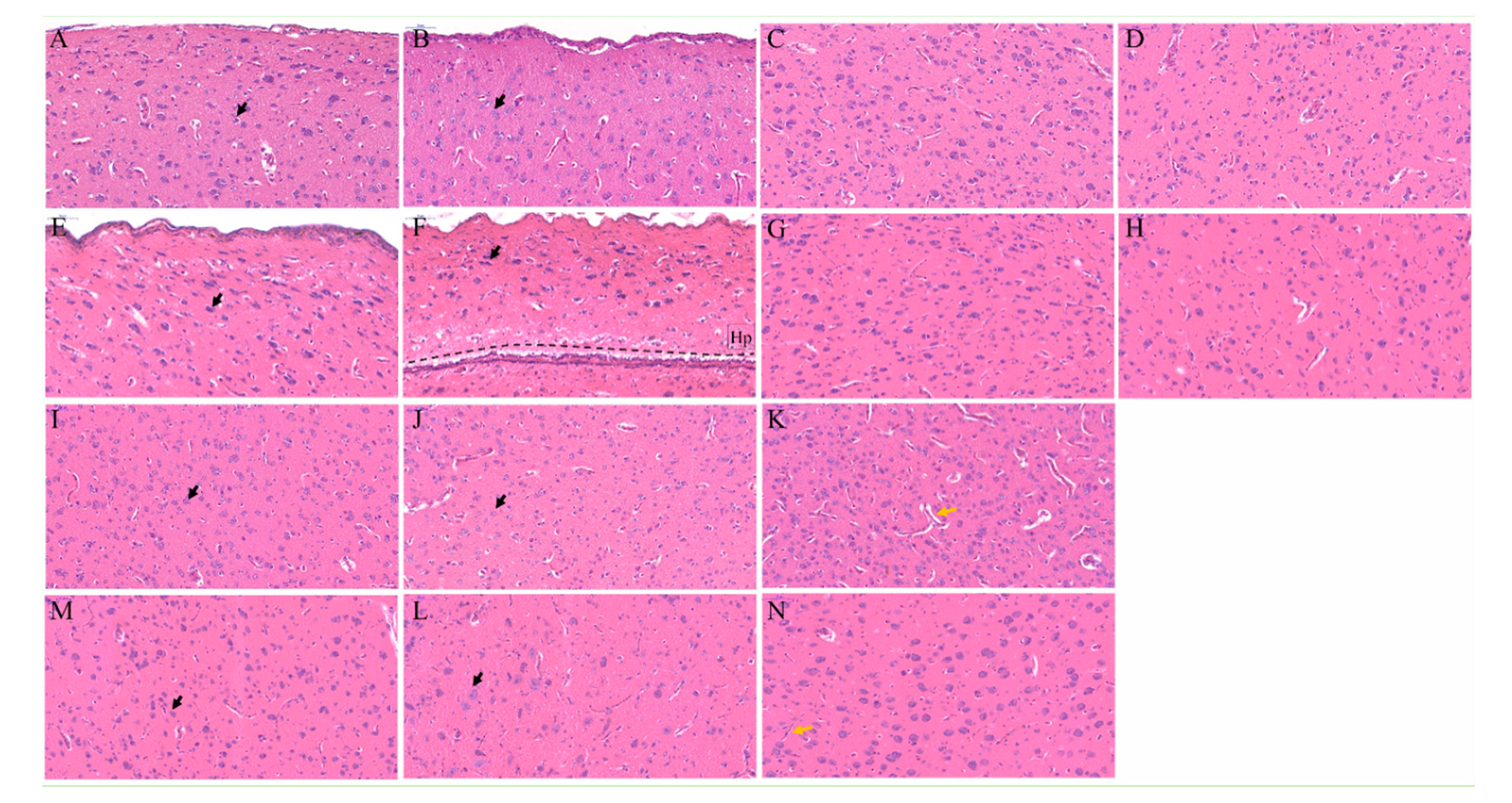
3.4. Inspection of Neurons in Chicken Telencephalon with or without Cerebral Hernia by Immunohistochemistry
3.5. Detection of Astrocytes in Chicken Telencephalon with or without Cerebral Hernia by Immunohistochemistry
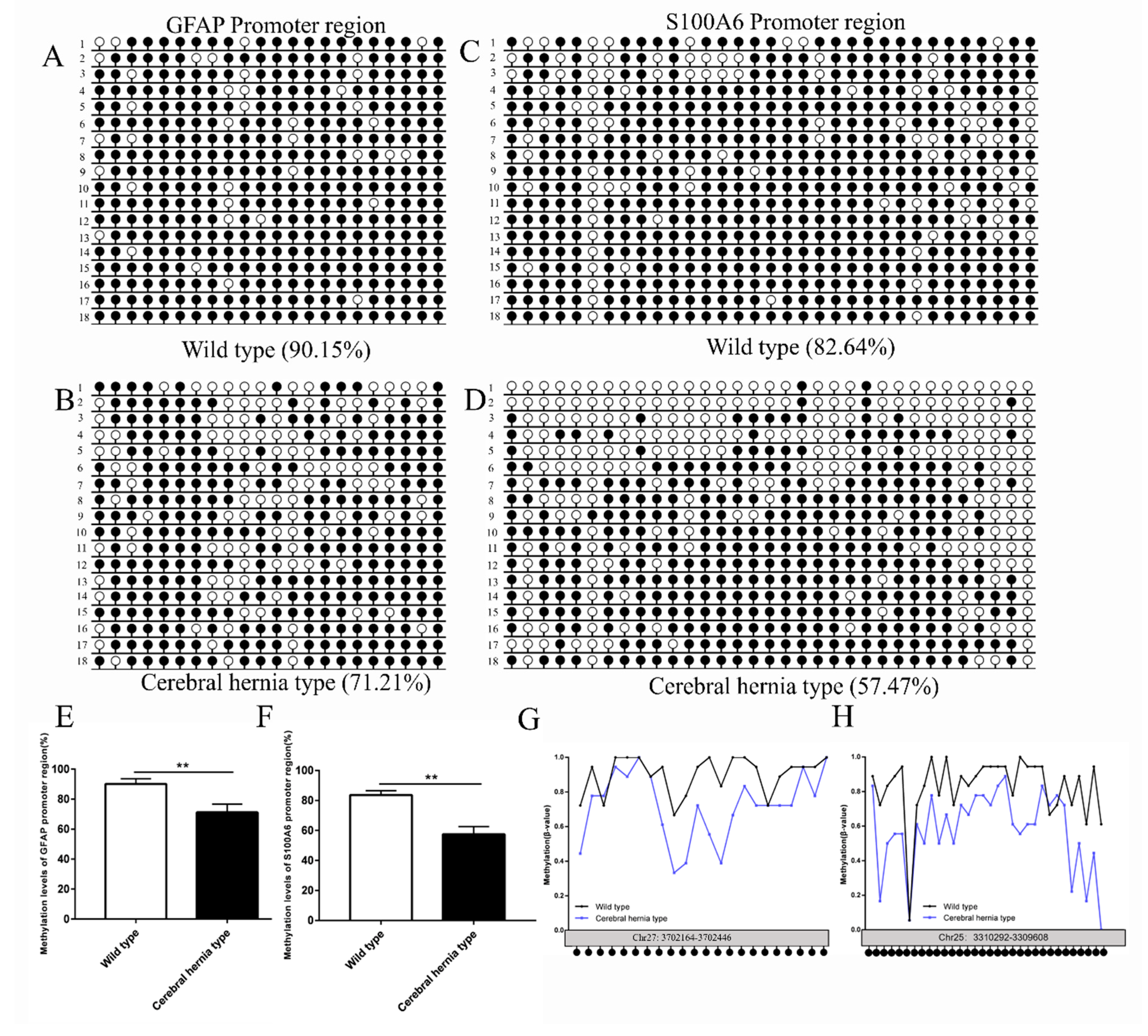
3.6. Evaluation of DNA Methylation of GFAP and S100A6 Genes in the Telencephalon of Chickens with or without Cerebral Hernia
4. Discussion
4.1. Chickens with Cerebral Hernia Showed Behavior Abnormalities
4.2. Cerebral Hernia Phenotype in Chickens Is Partially Caused by the Misplaced Sphenoid Bone
4.3. Functional Impact of Abnormalities in Structure and Cell State of Telencephalon
4.4. Functional Analysis of DEGs in the Telencephalon of Chickens with or without Cerebral Hernia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshimura, K.; Kinoshita, K.; Mizutani, M.; Matsuda, Y.; Saito, N. Inheritance and developmental pattern of cerebral hernia in the crested polish chicken. J. Exp. Zool. Part B Mol. Dev. Evol. 2012, 318, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Frahm, H.D.; Rehkamper, G. Allometric comparison of the brain and brain structures in the white crested polish chicken with uncrested domestic chicken breeds. Brain Behav. Evol. 1998, 52, 292–307. [Google Scholar] [CrossRef]
- Fisher, R.A. Crest and hernia in fowls due to a single gene without dominance. Science 1934, 80, 288–289. [Google Scholar] [CrossRef] [Green Version]
- The variation of animals and plants under domestication. Br. Foreign Med. Chir. Rev. 1868, 42, 143–166.
- Wang, Y.; Gao, Y.; Imsland, F.; Gu, X.; Feng, C.; Liu, R.; Song, C.; Tixier-Boichard, M.; Gourichon, D.; Li, Q.; et al. The crest phenotype in chicken is associated with ectopic expression of hoxc8 in cranial skin. PLoS ONE 2012, 7, e34012. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.L.; Eckard, D.A.; Chason, D.P.; Brecheisen, M.A.; Batnitzky, S. Imaging of acquired cerebral herniations. Neuroimaging Clin. N. Am. 2002, 12, 217–228. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, S.; Zhang, Y.; Guo, Q.; Wang, S.; Bi, Y.; Dai, W.; Shen, X.; Gu, T.; Pan, R.; et al. Embryonic morphology observation and hoxc8 gene expression in crest cushions of chinese crested duck. Gene 2019, 688, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Krautwald-Junghanns, M.E.; Portmann, S.; Brinkmeier, J.; Kummerfeld, N.; Sohn, H.G.; Dorsch, B. The use of conventional radiography and computer-assisted tomography as instruments for demonstration of gross pathological lesions in the cranium and cerebrum in the crested breed of the domestic duck (anas platyrhynchos f.Dom.). Avian Pathol. 2000, 29, 101–108. [Google Scholar] [CrossRef]
- Kahle, K.T.; Kulkarni, A.V.; Limbrick, D.D., Jr.; Warf, B.C. Hydrocephalus in children. Lancet 2016, 387, 788–799. [Google Scholar] [CrossRef]
- Jarvis, E.D.; Yu, J.; Rivas, M.V.; Horita, H.; Feenders, G.; Whitney, O.; Jarvis, S.C.; Jarvis, E.R.; Kubikova, L.; Puck, A.E.; et al. Global view of the functional molecular organization of the avian cerebrum: Mirror images and functional columns. J. Comp. Neurol. 2013, 521, 3614–3665. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Winkler, C.M.; Pfenning, A.R.; Jarvis, E.D. Molecular profiling of the developing avian telencephalon: Regional timing and brain subdivision continuities. J. Comp. Neurol. 2013, 521, 3666–3701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dicke, U.; Roth, G. Neuronal factors determining high intelligence. Philos. Trans. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150180. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.; Dicke, U. Evolution of the brain and intelligence. Science 1975, 9, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, P. The Long Evolution of Brains and Minds; Springer: New York, NY, USA, 2013. [Google Scholar]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 2018, 174, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Messing, A.; Su, M.; Brenner, M. Gfap promoter elements required for region-specific and astrocyte-specific expression. Glia 2008, 56, 481–493. [Google Scholar] [CrossRef]
- Martin, A.; Hofmann, H.D.; Kirsch, M. Glial reactivity in ciliary neurotrophic factor-deficient mice after optic nerve lesion. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 5416–5424. [Google Scholar] [CrossRef]
- Yamada, J.; Jinno, S. S100a6 (calcyclin) is a novel marker of neural stem cells and astrocyte precursors in the subgranular zone of the adult mouse hippocampus. Hippocampus 2014, 24, 89–101. [Google Scholar] [CrossRef]
- Yamashita, N.; Ilg, E.C.; Schäfer, B.W.; Heizmann, C.W.; Kosaka, T. Distribution of a specific calcium-binding protein of the s100 protein family, s100a6 (calcyclin), in subpopulations of neurons and glial cells of the adult rat nervous system. J. Comp. Neurol. 1999, 404, 235–257. [Google Scholar] [CrossRef]
- Yamada, J.; Jinno, S. Upregulation of calcium binding protein, s100a6, in activated astrocytes is linked to glutamate toxicity. Neuroscience 2012, 226, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Liang, M.; Yang, G.; Ye, Y.; Xu, H.; He, X.; Huang, J.H. Expression of s100a6 in rat hippocampus after traumatic brain injury due to lateral head acceleration. Int. J. Mol. Sci. 2014, 15, 6378–6390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.J.; Jin, Y.; Allen, E.G.; Jin, P. Diverse and dynamic DNA modifications in brain and diseases. Hum. Mol. Genet. 2019, 28, R241–R253. [Google Scholar]
- Setoguchi, H.; Namihira, M.; Kohyama, J.; Asano, H.; Sanosaka, T.; Nakashima, K. Methyl-cpg binding proteins are involved in restricting differentiation plasticity in neurons. J. Neurosci. Res. 2006, 84, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.I.; Torrey, S.; Widowski, T.M.; Turner, P.V.; Knezacek, T.D.; Nicholds, J.; Crowe, T.G.; Schwean-Lardner, K. Evaluation of carbon dioxide induction methods for the euthanasia of day-old cull broiler chicks. Poult. Sci. 2019, 98, 2043–2053. [Google Scholar] [CrossRef]
- Shojadoost, B.; Vince, A.R.; Prescott, J.F. The successful experimental induction of necrotic enteritis in chickens by clostridium perfringens: A critical review. Vet. Res. 2012, 43, 74. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Kloeze, H.; Dam, A.; Ward, D.; Leung, N.; Brown, E.E.; Whiteman, A.; Chiappetta, M.E.; Hunter, D.B. Mass depopulation of laying hens in whole barns with liquid carbon dioxide: Evaluation of welfare impact. Poult. Sci. 2012, 91, 1558–1568. [Google Scholar] [CrossRef]
- She, Y.; Shao, L.; Zhang, Y.; Hao, Y.; Cai, Y.; Cheng, Z.; Deng, C.; Liu, X. Neuroprotective effect of glycosides in buyang huanwu decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 2019, 242, 112051. [Google Scholar] [CrossRef]
- Carr, I.M.; Valleley, E.M.; Cordery, S.F.; Markham, A.F.; Bonthron, D.T. Sequence analysis and editing for bisulphite genomic sequencing projects. Nucleic Acids Res. 2007, 35, e79. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with hisat2 and hisat-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Brazda, V.; Coufal, J. Recognition of local DNA structures by p53 protein. Int. J. Mol. Sci. 2017, 18, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartkowska, K.; Swiatek, I.; Aniszewska, A.; Jurewicz, E.; Turlejski, K.; Filipek, A.; Djavadian, R.L. Stress-dependent changes in the cacybp/sip interacting protein s100a6 in the mouse brain. PLoS ONE 2017, 12, e0169760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, T.; Brinkmeier, J.; Portmann, S.; Krautwald-Junghanns, M.E.; Kummerfeld, N.; Boos, A. Osteological investigations of the incidence of cranial alterations in domestic ducks (anas platyrhynchos f. Dom.) with feather crests. Ann. Anat. Anat. Anz. 2001, 183, 73–80. [Google Scholar] [CrossRef]
- Frahm, H.D.; Rehkamper, G.; Werner, C.W. Brain alterations in crested versus non-crested breeds of domestic ducks (anas platyrhynchos f.D.). Poult. Sci. 2001, 80, 1249–1257. [Google Scholar] [CrossRef]
- Davenport, C.B. Inheritance in Poultry; Carnegie Institution of Washington: Washington, DC, USA, 1906; Volume 74, p. 583. [Google Scholar]
- Jamil, R.T.; Waheed, A.; Callahan, A.L. Anatomy, sphenoid bone. In Statpearls; Statpearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Costea, C.; Turliuc, S.; Cucu, A.; Dumitrescu, G.; Carauleanu, A.; Buzduga, C.; Sava, A.; Costache, I.; Turliuc, D. The “polymorphous” history of a polymorphous skull bone: The sphenoid. Anat. Sci. Int. 2018, 93, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Aksoy, F.; Yenigun, A.; Goktas, S.S.; Ozturan, O. Association of accessory sphenoid septa with variations in neighbouring structures. J. Laryngol. Otol. 2017, 131, 51–55. [Google Scholar] [CrossRef] [Green Version]
- di Rocc, C.; Samii, A.; Tamburrini, G.; Massimi, L.; Giordano, M. Sphenoid dysplasia in neurofibromatosis type 1: A new technique for repair. Child’s Nerv. Syst. ChNS Off. J. Soc. Pediatric Neurosurg. 2017, 33, 983–986. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, M.L.; MacKenzie, E.J.; Durbin, D.R.; Aitken, M.E.; Jaffe, K.M.; Paidas, C.N.; Slomine, B.S.; Dorsch, A.M.; Christensen, J.R.; Ding, R.; et al. Health-related quality of life during the first year after traumatic brain injury. Arch. Pediatrics Adolesc. Med. 2006, 160, 252–260. [Google Scholar] [CrossRef]
- Lighthall, J.W. Controlled cortical impact: A new experimental brain injury model. J. Neurotrauma 1988, 5, 1–15. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar]
- Pfenning, A.R.; Hara, E.; Whitney, O.; Rivas, M.V.; Wang, R.; Roulhac, P.L.; Howard, J.T.; Wirthlin, M.; Lovell, P.V.; Ganapathy, G.; et al. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science 2014, 346, 1256846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel, J.F.; Molnar, Z. The impact of gene expression analysis on evolving views of avian brain organization. J. Comp. Neurol. 2013, 521, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Azim, S.; Banday, A.R.; Tabish, M. Alternatively spliced three novel transcripts of gria1 in the cerebellum and cortex of mouse brain. Neurochem. Res. 2012, 37, 193–201. [Google Scholar] [CrossRef]
- O’Connor, J.A.; Hemby, S.E. Elevated gria1 mrna expression in layer ii/iii and v pyramidal cells of the dlpfc in schizophrenia. Schizophr. Res. 2007, 97, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delabio, R.; Rasmussen, L.; Mizumoto, I.; Viani, G.A.; Chen, E.; Villares, J.; Costa, I.B.; Turecki, G.; Linde, S.A.; Smith, M.C.; et al. Psen1 and psen2 gene expression in alzheimer’s disease brain: A new approach. J. Alzheimer’s Dis. JAD 2014, 42, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, M.; Petersen, A.J.; Naumenko, N.; Puttonen, K.; Lehtonen, S.; Gubert Olive, M.; Shakirzyanova, A.; Leskela, S.; Sarajarvi, T.; Viitanen, M.; et al. Psen1 mutant ipsc-derived model reveals severe astrocyte pathology in alzheimer’s disease. Stem Cell Rep. 2017, 9, 1885–1897. [Google Scholar] [CrossRef] [Green Version]
- Bezprozvanny, I.; Mattson, M.P. Neuronal calcium mishandling and the pathogenesis of alzheimer’s disease. Trends Neurosci. 2008, 31, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Spampinato, S.F.; Merlo, S.; Sano, Y.; Kanda, T.; Sortino, M.A. Astrocytes contribute to abeta-induced blood-brain barrier damage through activation of endothelial mmp9. J. Neurochem. 2017, 142, 464–477. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Su, P.; Meng, S.; Aschner, M.; Cao, Y.; Luo, W.; Zheng, G.; Liu, M. Role of matrix metalloproteinase-2/9 (mmp2/9) in lead-induced changes in an in vitro blood-brain barrier model. Int. J. Biol. Sci. 2017, 13, 1351–1360. [Google Scholar] [CrossRef] [Green Version]
- Winger, R.C.; Koblinski, J.E.; Kanda, T.; Ransohoff, R.M.; Muller, W.A. Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood-brain barrier. J. Immunol. 2014, 193, 2427–2437. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.J. Star power: Astrocytes regulate behavior. Cell 2019, 177, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, L.H.; Zhong, X.Y.; Xing, X.F.; Liu, Y.Q.; Niu, Z.J.; Peng, Y.; Du, H.; Zhang, G.G.; Hu, Y.; et al. S100a6 overexpression is associated with poor prognosis and is epigenetically up-regulated in gastric cancer. Am. J. Pathol. 2010, 177, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Melville, Z.; Aligholizadeh, E.; McKnight, L.E.; Weber, D.J.; Pozharski, E.; Weber, D.J. X-ray crystal structure of human calcium-bound s100a1. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankratova, S.; Klingelhofer, J.; Dmytriyeva, O.; Owczarek, S.; Renziehausen, A.; Syed, N.; Porter, A.E.; Dexter, D.T.; Kiryushko, D. The s100a4 protein signals through the erbb4 receptor to promote neuronal survival. Theranostics 2018, 8, 3977–3990. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, A.; Liebmann, T.; Knudsen, M.; Schintu, N.; Svenningsson, P.; Greengard, P. Cell- and region-specific expression of depression-related protein p11 (s100a10) in the brain. J. Comp. Neurol. 2017, 525, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, X.; Zhou, H.; Zheng, L.; Shi, J. S100a11 protects against neuronal cell apoptosis induced by cerebral ischemia via inhibiting the nuclear translocation of annexin a1. Cell Death Dis. 2018, 9, 657. [Google Scholar] [CrossRef]
- Lindsey, J.C.; Lusher, M.E.; Anderton, J.A.; Gilbertson, R.J.; Ellison, D.W.; Clifford, S.C. Epigenetic deregulation of multiple s100 gene family members by differential hypomethylation and hypermethylation events in medulloblastoma. Br. J. Cancer 2007, 97, 267–274. [Google Scholar] [CrossRef] [Green Version]


| Description | Genes |
|---|---|
| Neuron apoptotic process | PSEN1 CEBPB BDNF |
| Neuron death | PSEN1 CEBPB BDNF |
| Positive regulation of neuron differentiation | PSEN1 BDNF |
| Neuron projection | PSEN1 BDNF NFIB RHOC GABRQ PTGS2 |
| Dendritic tree | PSEN1 BDNF |
| Regulation of immune system process | CEBPB CD28 PSEN1 ANXA1 CAV1 |
| Leukocyte mediated immunity | CD28 |
| Immune response-regulating signaling pathway | CD28 PSEN1 |
| Activation of immune response | CD28 PSEN1 |
| T cell proliferation | CD28 CEBPB ANXA1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Zhou, X.; Zheng, X.; Li, S.; Mou, C. Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia. Genes 2020, 11, 1008. https://doi.org/10.3390/genes11091008
Tao Y, Zhou X, Zheng X, Li S, Mou C. Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia. Genes. 2020; 11(9):1008. https://doi.org/10.3390/genes11091008
Chicago/Turabian StyleTao, Yingfeng, Xiaoliu Zhou, Xinting Zheng, Shijun Li, and Chunyan Mou. 2020. "Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia" Genes 11, no. 9: 1008. https://doi.org/10.3390/genes11091008
APA StyleTao, Y., Zhou, X., Zheng, X., Li, S., & Mou, C. (2020). Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia. Genes, 11(9), 1008. https://doi.org/10.3390/genes11091008




