A Novel Kelch-Like-1 Is Involved in Antioxidant Response by Regulating Antioxidant Enzyme System in Penaeus vannamei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. CDNA Synthesis and RT-qPCR
2.3. Identification PvKelch-Like-1
2.4. Polyclonal Antibody Preparation
2.5. Tissue-Specific Expression and Immunofluorescence
2.6. Cadmium Challenge
2.7. Overexpression of PvKelch-Like-1 in S2 Cell
2.8. Silenced PvKelch-Like-1 In Vivo
2.9. Comet Assays and Total Hemocyte Counts (THC)
2.10. ROS Accumulation
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. Characterization of PvKelch-Like-1
3.2. Preparation of Polyclonal Antibody
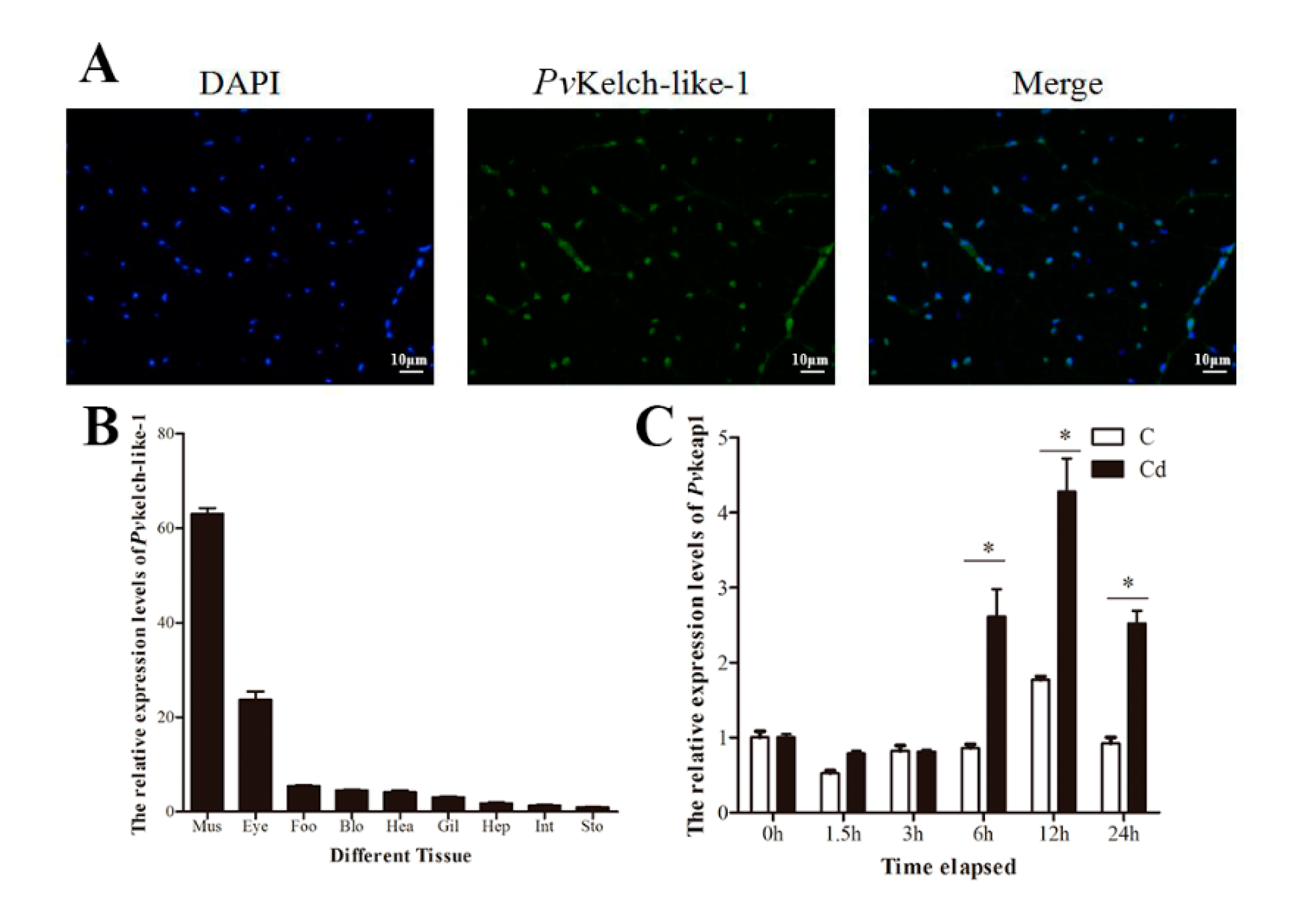
3.3. Subcellular Localization and Tissue-Specific Expression
3.4. Expression Pattern of PvKelch-Like-1 after Cadmium Challenge
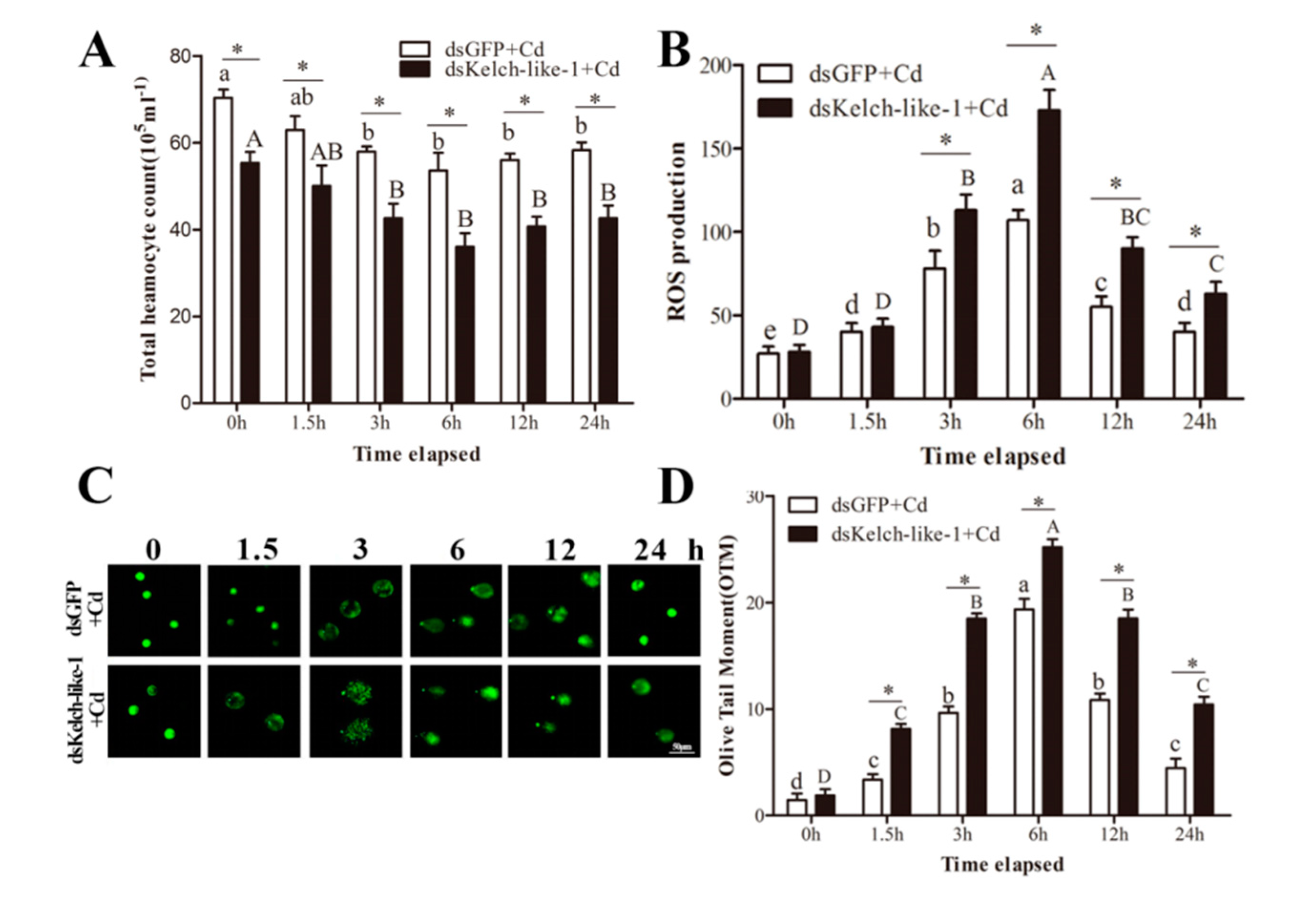
3.5. PvKelch-Like-1 Participates Injury Response under Cadmium Stress
3.6. PvKelch-Like-1 Responds to Cadmium Stress by Regulating Antioxidant Enzyme System
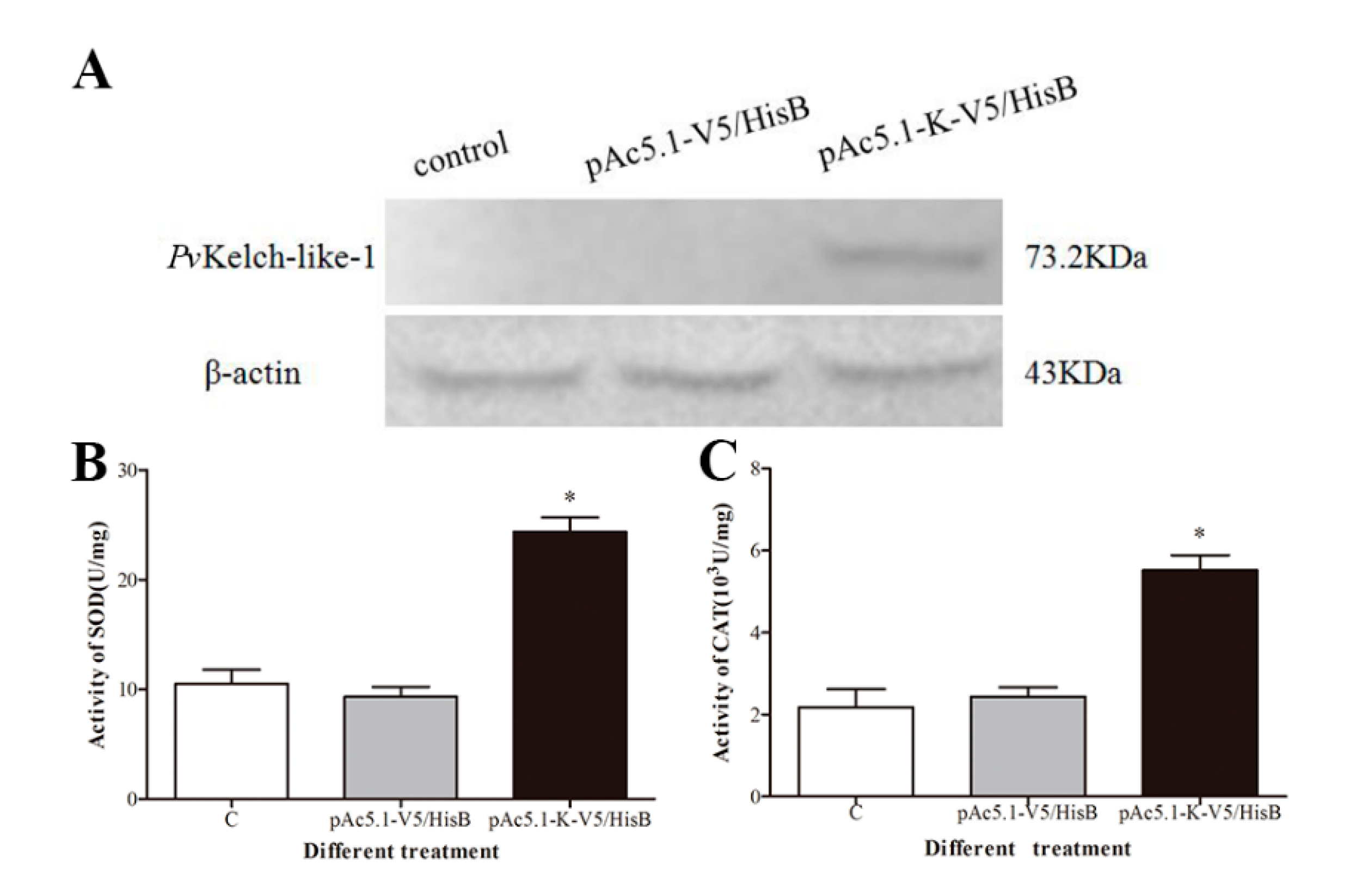
3.7. Effect of Enzyme Activity after Overexpression of PvKelch-Like-1 in S2 Cell
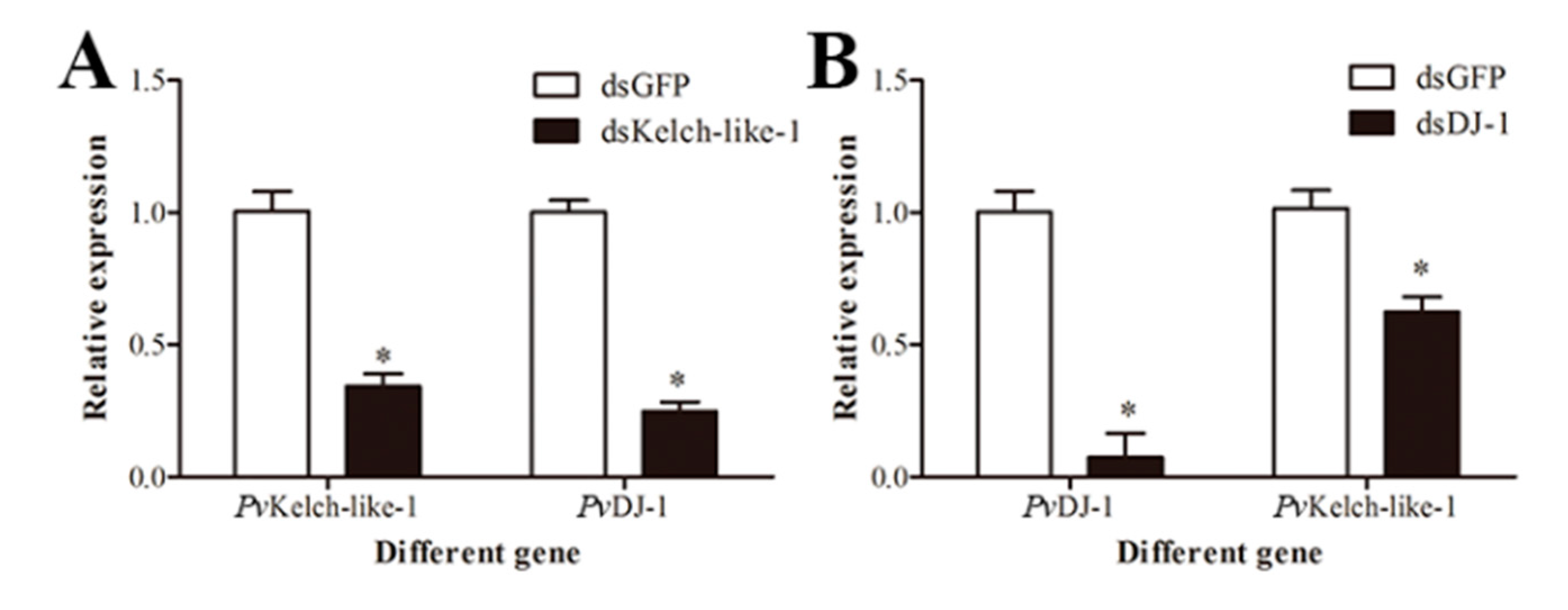
3.8. Relationship between PvKelch-Like-1 and PvDJ-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krumschnabel, G.; Ebner, H.L.; Hess, M.W.; Villunger, A. Apoptosis and necroptosis are induced in rainbow trout cell lines exposed to cadmium. Aquat. Toxicol. 2010, 99, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Zhi, L.; Zhang, G.Y.; Liu, X.J.; Min, J. Effect of dietary cadmium level on the growth, body composition and several hepatic enzymatic activities of juvenile yellow catfish, Pelteobagrus fulvidraco. Aquac. Res. 2010, 41, 1022–1029. [Google Scholar] [CrossRef]
- Wu, J.P.; Chen, H.C.; Huang, D.J. Histopathological and biochemical evidence of hepatopancreatic toxicity caused by cadmium and zinc in the white shrimp, Litopenaeus vannamei. Chemosphere 2008, 73, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Chen, H.C.; Huang, D.J. Histopathological Alterations in Gills of White Shrimp, Litopenaeus vannamei (Boone) After Acute Exposure to Cadmium and Zinc. Bull. Environ. Contam. Toxicol. 2009, 82, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Boudet, L.C.; Polizzi, P.; Romero, M.B.; Robles, A.; Marcovecchio, J.E.; Gerpe, M.S. Histopathological and biochemical evidence of hepatopancreatic toxicity caused by cadmium in white shrimp, Palaemonetes argentinus. Ecotoxicol. Environ. Saf. 2015, 113, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Russell, E.G.; Cotter, T.G. New Insight into the Role of Reactive Oxygen Species (ROS) in Cellular Signal-Transduction Processes. Int. Rev. Cell Mol. Biol. 2015, 319, 221–254. [Google Scholar] [PubMed]
- Xu, Z.; Regenstein, J.M.; Xie, D.; Lu, W.; Ren, X.; Yuan, J.; Mao, L. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish. Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Huang, M.Z.; Liu, Y.; Xie, C.Y.; Wang, W.N. LvDJ-1 plays an important role in resistance against Vibrio alginolyticus in Litopenaeus vannamei. Fish Shellfish. Immunol. 2015, 44, 180–186. [Google Scholar] [CrossRef]
- Gan, L.; Johnson, D.A.; Johnson, J.A. Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur. J. Neurosci. 2010, 31, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kim, M.J.; Yoon, W.; Kim, E.Y.; Kim, H.; Lee, Y.; Min, B.; Kang, K.S.; Son, J.H.; Park, H.T.; et al. Isocitrate protects DJ-1 null dopaminergic cells from oxidative stress through NADP(+)—Dependent isocitrate dehydrogenase (IDH). PLoS Genet. 2017, 13, e1006975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Yuan, Y.H.; Shao, Q.H.; Wang, Z.Z.; Zhu, C.G.; Shi, J.G.; Ma, K.L.; Yan, X.; Chen, N.H. DJ-1 regulating PI3K-Nrf2 signaling plays a significant role in bibenzyl compound 20C-mediated neuroprotection against rotenone-induced oxidative insult. Toxicol. Lett. 2017, 271, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Albagli, O.; Dhordain, P.; Deweindt, C.; Lecocq, G.; Leprince, D. The BTB/POZ domain: A new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1995, 6, 1193–1198. [Google Scholar]
- Chaharbakhshi, E.; Jemc, J.C. Broad-complex, tramtrack, and bric-a-brac (BTB) proteins: Critical regulators of development. Genesis 2016, 54, 505–518. [Google Scholar] [CrossRef]
- Xue, F.; Cooley, L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 1993, 72, 681–693. [Google Scholar] [CrossRef]
- Adams, J.; Kelso, R.; Cooley, L. The kelch repeat superfamily of proteins: Propellers of cell function. Trends Cell Biol. 2000, 10, 17–24. [Google Scholar] [CrossRef]
- Seng, S.; Avraham, H.K.; Jiang, S.; Venkatesh, S.; Avraham, S. KLHL1/MRP2 Mediates Neurite Outgrowth in a Glycogen Synthase Kinase 3β-Dependent Manner. Mol. Cell. Biol. 2006, 26, 8371–8384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumara, I.; Quadroni, M.; Frei, C.; Olma, M.H.; Sumara, G.; Ricci, R.; Peter, M. A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev. Cell 2007, 2, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Burles, K.; Couturier, B.; Randall, C.M.; Shisler, J.; Barry, M. Ectromelia virus encodes a BTB/kelch protein, EVM150, that inhibits NF-kappaB signaling. J. Virol. 2014, 88, 4853–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.D.; Lo, S.C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Kensler, T.W. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005, 18, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, M.; Hasson, T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Q.; Ou, M.; Li, Z.; Ren, Y.; Wei, W.; Qiao, X.; Hu, R.; Wu, X.; Liu, Y.; Wang, W. Functional analysis target of rapamycin (TOR) on the Penaeus vannamei in response to acute low temperature stress. Fish Shellfish. Immunol. 2019, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Camhi, S.L.; Lee, P.; Choi, A.M. The oxidative stress response. New Horiz. 1995, 3, 170–182. [Google Scholar] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Savín, T.; Saldierna, R.; Ahuejote-Sandoval, M. Superoxide radical production in response to environmental hypoxia in cultured shrimp. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 301–308. [Google Scholar] [CrossRef]
- Guo, H.; Li, K.; Wang, W.; Wang, C.; Shen, Y. Effects of copper on hemocyte apoptosis, ROS production, and gene expression in white shrimp Litopenaeus vannamei. Biol. Trace Elem. Res. 2017, 179, 318–326. [Google Scholar] [CrossRef]
- Mullett, S.J.; Hinkle, D.A. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009, 33, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, Y.Y.; Qiu, L.Y.; Yan, Y.F.; Liao, Z.P.; Chen, H.P. Involvement of DJ-1 in ischemic preconditioninginduced delayed cardioprotection in vivo. Mol. Med. Rep. 2017, 15, 995–1001. [Google Scholar] [CrossRef]
- Hudson, A.M.; Mannix, K.M.; Cooley, L. Actin Cytoskeletal Organization in Drosophila Germline Ring Canals Depends on Kelch Function in a Cullin-RING E3 Ligase. Genetics 2015, 201, 1117–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.N.; Cooley, L. Drosophila kelch is an oligomeric ring canal actin organizer. J. Cell Biol. 1997, 138, 799–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sihvola, V.; Levonen, A.L. Keap1 as the redox sensor of the antioxidant response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm. Res. 2010, 27, 999–1013. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, W.; Tang, M.; Wang, Y.; Fan, X.; Mo, X.; Li, Y.; Ying, Z.; Wan, Y.; Ocorr, K.; et al. KBTBD7, a novel human BTB-kelch protein, activates transcriptional activities of SRE and AP-1. BMB Rep. 2010, 43, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Luhrig, S.; Kolb, S.; Mellies, N.; Nolte, J. The novel BTB-kelch protein, KBTBD8, is located in the Golgi apparatus and translocates to the spindle apparatus during mitosis. Cell Div. 2013, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Li, Y.; Zhou, X.; Deng, Y.; Wang, Z.; Yuan, W.; Li, D.; Zhu, C.; Zhao, X.; Mo, X.; et al. A novel human BTB-kelch protein KLHL31, strongly expressed in muscle and heart, inhibits transcriptional activities of TRE and SRE. Mol. Cells Springer Sci. Bus. Media BV 2008, 26, 443–453. [Google Scholar]
- Bredholt, G.; Storstein, A.; Haugen, M.; Krossnes, B.K.; Husebye, E.; Knappskog, P.; Vedeler, C.A. Detection of Autoantibodies to the BTB-kelch Protein KLHL7 in Cancer Sera. Scand. J. Immunol. 2006, 64, 325–335. [Google Scholar] [CrossRef]
- Bautista-Covarrubias, J.C.; Velarde-Montes, G.J.; Voltolina, D.; García-de la Parra, L.M.; Soto-Jiménez, M.F.; Frías-Espericueta, M.G. Humoral and haemocytic responses of Litopenaeus vannamei to Cd exposure. Sci. World J. 2014, 2014, 903452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.; Wang, W.N.; Wang, A.L.; Tian, T.T.; Wang, P.; Zheng, Y.; Liu, Y. Effects of cadmium on respiratory burst, intracellular Ca2+ and DNA damage in the white shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2009, 149, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.A.; Wang, A.L.; Miao, Y.T.; Li, B. Flow Cytometric Analysis of In Vitro Cytotoxicity of Cadmium in Haemocytes from the Tiger Shrimp, Penaeus monodon. Bull. Environ. Contam. Toxicol. 2013, 90, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Guzman, G.; Sanchez-Martinez, J.G.; Campa-Cordova, A.I.; Luna-Gonzalez, A.; Ascencio, F. Penaeid shrimp immune system. Thai J. Vet. Med. 2009, 39, 205–215. [Google Scholar]
- Alday-Sanz, V.; Roque, A.; Turnbull, J. Clearing mechanisms of Vibrio vulnificus biotype I in the black tiger shrimp Penaeus monodon. Dis. Aquat. Org. 2002, 48, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keating, J.; Delaney, M.; Meehan-Meola, D.; Warren, W.; Alcivar, A.; Alcivar-Warren, A. Histological findings, cadmium bioaccumulation, and isolation of expressed sequence tags (ESTs) in cadmium-exposed, specific pathogen-free shrimp, Litopenaeus vannamei postlarvae. J. Shellfish. Res. 2007, 26, 1225–1237. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chen, S.J.; Chen, M.; Tian, L.X.; Niu, J.; Liu, Y.J.; Xu, D.H. Effect of cadmium-polluted diet on growth, salinity stress, hepatotoxicity of juvenile Pacific white shrimp (Litopenaeus vannamei): Protective effect of Zn(II)-curcumin. Ecotoxicol. Environ. Saf. 2016, 125, 176–183. [Google Scholar] [CrossRef]
- Qian, Z.; Liu, T.; Liu, Q.; He, S.; Liu, Y.; Hou, F.; Wang, X.; Mi, X.; Cai, C.; Liu, X. p53 is involved in shrimp survival via its regulation roles on MnSOD and GPx in response to acute environmental stresses. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 159, 38–51. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.; Reibert, B.; Amann, T.; Constien, R.; Rondinone, C.M.; Valverde, A.M. In vivo siRNA delivery of Keap1 modulates death and survival signaling pathways and attenuates concanavalin-A-induced acute liver injury in mice. Dis. Models Mech. 2014, 7, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Lee, H.; Lee, E.J.; Kim, M.; Kim, T.G.; Kim, H.P.; Oh, S.H. Keap1 knockdown in melanocytes induces cell proliferation and survival via HO-1-associated beta-catenin signaling. J. Dermatol. Sci. 2017, 88, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, H.; Shinohara, F.; Kajiya, M.; Kodama, T. The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J. Biol. Chem. 2013, 288, 23009–23020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, X.; Yan, Y.Y.; Tong, Y.H.; Fan, Y.; Zeng, J.M.; Wang, L.L.; Lin, N.M. Overexpression of Keap1 inhibits the cell proliferation and metastasis and overcomes the drug resistance in human lung cancer A549 cells. Chin. J. Oncol. 2016, 38, 404–410. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.-L.; Liang, Q.-J.; Liu, Y.; Wang, W.-N. A Novel Kelch-Like-1 Is Involved in Antioxidant Response by Regulating Antioxidant Enzyme System in Penaeus vannamei. Genes 2020, 11, 1077. https://doi.org/10.3390/genes11091077
Qiao X-L, Liang Q-J, Liu Y, Wang W-N. A Novel Kelch-Like-1 Is Involved in Antioxidant Response by Regulating Antioxidant Enzyme System in Penaeus vannamei. Genes. 2020; 11(9):1077. https://doi.org/10.3390/genes11091077
Chicago/Turabian StyleQiao, Xue-Li, Qing-Jian Liang, Yuan Liu, and Wei-Na Wang. 2020. "A Novel Kelch-Like-1 Is Involved in Antioxidant Response by Regulating Antioxidant Enzyme System in Penaeus vannamei" Genes 11, no. 9: 1077. https://doi.org/10.3390/genes11091077



