Nuclear Receptors and Development of Marine Invertebrates
Abstract
:1. Introduction
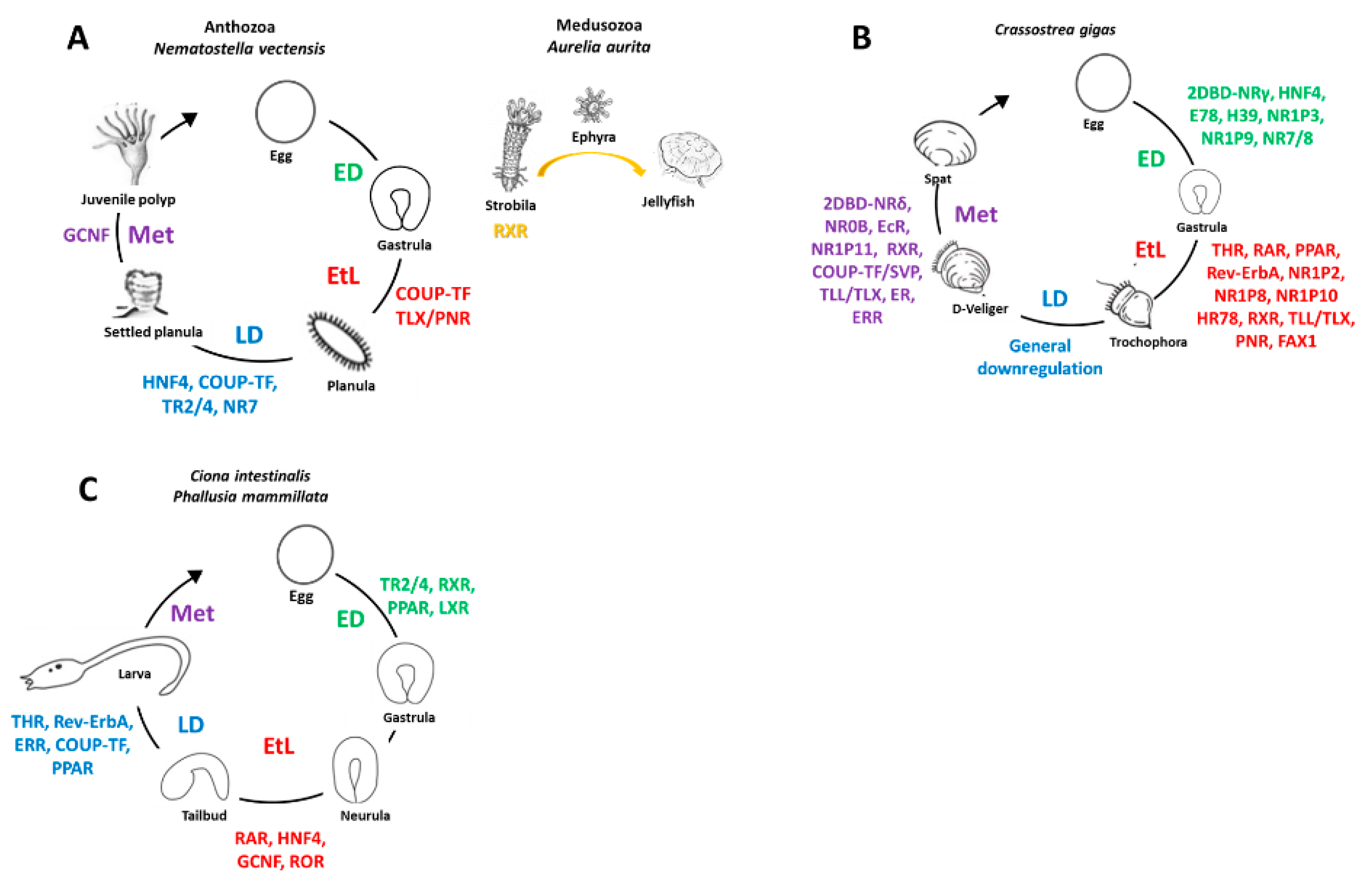
2. Development of Marine Invertebrates and Associated NR Cohorts
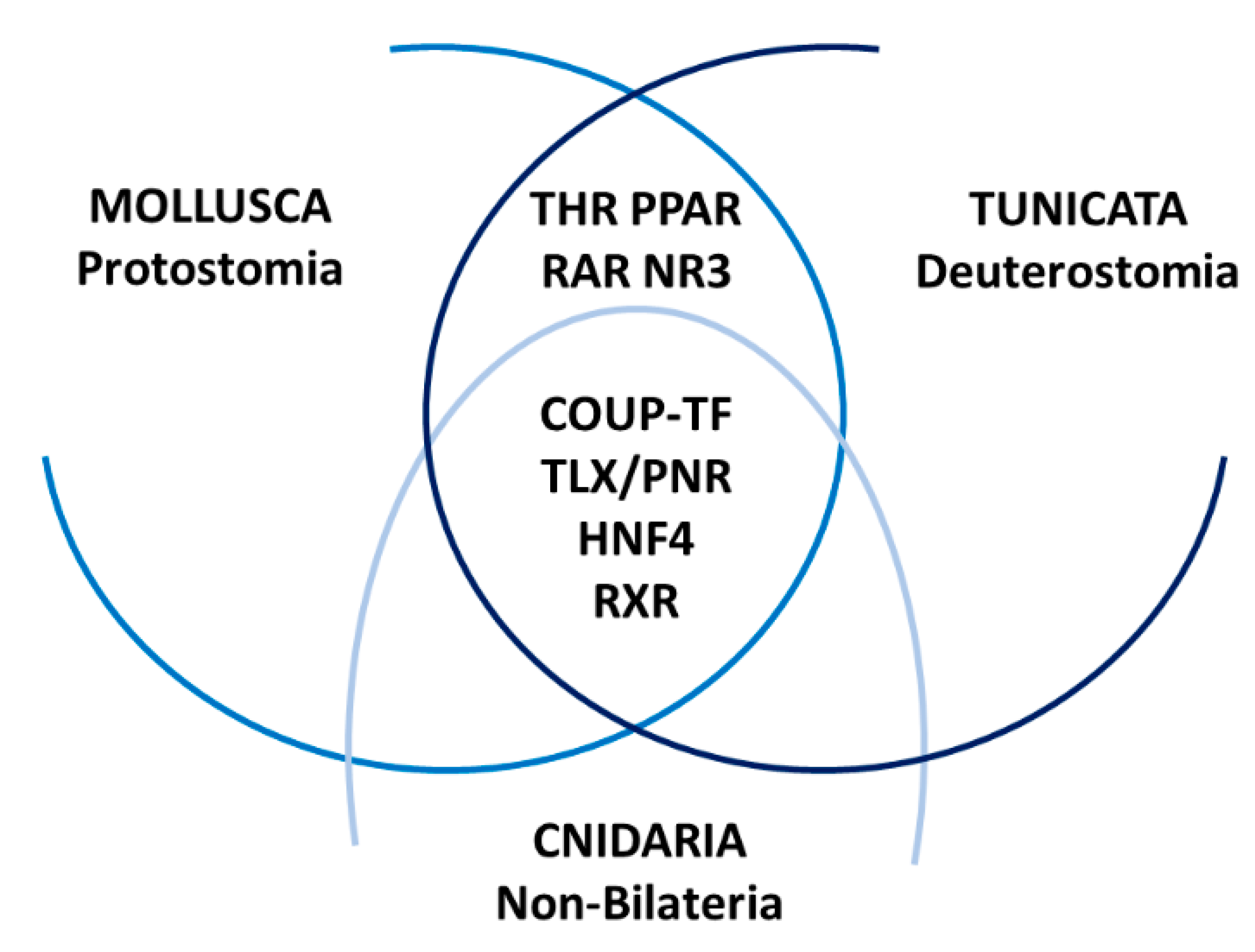
3. Development of Marine Invertebrates and NR Diversification
3.1. Chicken Ovalbumin Upstream Promoter Transcription Factor, COUP-TF
3.2. Tailless/Photoreceptor Cell-Specific Nuclear Receptor, TLX/PNR
3.3. Hepatocyte Nuclear Factor 4, HNF4
3.4. Retinoid X Receptor, RXR
4. Thyroid Hormone Receptor (THR) Signaling Regulates Developmental Transitions in Marine Invertebrates
5. Retinoic Acid Receptor (RAR)-Dependent Signaling Is Required for Neurogenesis in Marine Invertebrates
6. Retinoid X Receptor (RXR) Functions during Marine Invertebrate Development
7. Estrogen Receptor (ER), Estrogen-Related Receptor (ERR) and the Development of Marine Invertebrates
7.1. ERR Might Play a Role during Development of Marine Invertebrates
7.2. Developmental Functions of ER in Marine Invertebrates Remain Largely Elusive
8. Are NRs a Primary Target of EDCs in Marine Invertebrates?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Escriva, H.; Delaunay, F.; Laudet, V. Ligand binding and nuclear receptor evolution. BioEssays 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Escriva, H.; Bertrand, S.; Laudet, V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004, 40, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, D.L.; Heneghan, A.F.; Connaghan-Jones, K.D.; Miura, M.T. Nuclear receptor structure: Implications for function. Annu. Rev. Physiol. 2007, 69, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Bourguet, W.; Germain, P.; Gronemeyer, H. Nuclear receptor ligand-binding domains: Three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol. Sci. 2000, 21, 381–388. [Google Scholar] [CrossRef]
- Helsen, C.; Kerkhofs, S.; Clinckemalie, L.; Spans, L.; Laurent, M.; Boonen, S.; Vanderschueren, D.; Claessens, F. Structural basis for nuclear hormone receptor DNA binding. Mol. Cell. Endocrinol. 2012, 348, 411–417. [Google Scholar] [CrossRef]
- Claessens, F.; Gewirth, D.T. DNA recognition by nuclear receptors. Essays Biochem. 2004, 40, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Khorasanizadeh, S.; Rastinejad, F. Nuclear-receptor interactions on DNA-response elements. Trends Biochem. Sci. 2001, 26, 384–390. [Google Scholar] [CrossRef]
- Perlmann, T.; Umesono, K.; Rangarajan, P.N.; Forman, B.M.; Evans, R.M. Two distinct dimerization interfaces differentially modulate target gene specificity of nuclear hormone receptors. Mol. Endocrinol. 1996, 10, 958–966. [Google Scholar] [CrossRef]
- Germain, P.; Bourguet, W. Dimerization of nuclear receptors. In Methods in Cell Biology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 117, pp. 21–41. [Google Scholar] [CrossRef]
- Sainath, S.B.; André, A.; Castro, L.F.C.; Santos, M.M. The evolutionary road to invertebrate thyroid hormone signaling: Perspectives for endocrine disruption processes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 124–138. [Google Scholar] [CrossRef]
- Benoit, G.; Cooney, A.; Giguere, V.; Ingraham, H.; Lazar, M.; Muscat, G.; Perlmann, T.; Renaud, J.P.; Schwabe, J.; Sladek, F.; et al. International union of pharmacology. LXVI. Orphan nuclear receptors. Pharmacol. Rev. 2006, 58, 798–836. [Google Scholar] [CrossRef] [Green Version]
- Germain, P.; Staels, B.; Dacquet, C.; Spedding, M.; Laudet, V. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 2006, 58, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Laudet, V.; Hanni, C.; Coll, J.; Catzeflis, F.; Stehelin, D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992, 11, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Lecroisey, C.; Laudet, V.; Schubert, M. The cephalochordate amphioxus: A key to reveal the secrets of nuclear receptor evolution. Brief. Funct. Genom. 2012, 11, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridgham, J.T.; Eick, G.N.; Larroux, C.; Deshpande, K.; Harms, M.J.; Gauthier, M.E.A.; Ortlund, E.A.; Degnan, B.M.; Thornton, J.W. Protein evolution by molecular tinkering: Diversification of the nuclear receptor superfamily from a ligand-dependent ancestor. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Markov, G.V.; Laudet, V. Origin and evolution of the ligand-binding ability of nuclear receptors. Mol. Cell. Endocrinol. 2011, 334, 21–30. [Google Scholar] [CrossRef]
- Fonseca, E.S.S. Nuclear receptors in metazoan lineages: The cross-talk between evolution and endocrine disruption. Ph.D. Thesis, Faculdade de Ciências da Universidade do Porto, Porto, Portugal, 2020. [Google Scholar]
- Santos, M.M.; Ruivo, R.; Capitão, A.; Fonseca, E.; Castro, L.F.C. Identifying the gaps: Resources and perspectives on the use of nuclear receptor based-assays to improve hazard assessment of emerging contaminants. J. Hazard. Mater. 2018, 358, 508–511. [Google Scholar] [CrossRef]
- Vogeler, S.; Galloway, T.S.; Lyons, B.P.; Bean, T.P. The nuclear receptor gene family in the Pacific oyster, Crassostrea gigas, contains a novel subfamily group. BMC Genom. 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Khalturin, K.; Billas, I.M.L.; Chebaro, Y.; Reitzel, A.M.; Tarrant, A.M.; Laudet, V.; Markov, G.V. NR3E receptors in cnidarians: A new family of steroid receptor relatives extends the possible mechanisms for ligand binding. J. Steroid Biochem. Mol. Biol. 2018, 184, 11–19. [Google Scholar] [CrossRef]
- Baker, M.E. Trichoplax, the simplest known animal, contains an estrogen-related receptor but no estrogen receptor: Implications for estrogen receptor evolution. Biochem. Biophys. Res. Commun. 2008, 375, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Niles, E.G.; Hirai, H.; LoVerde, P.T. Evolution of a novel subfamily of nuclear receptors with members that each contain two DNA binding domains. BMC Evol. Biol. 2007, 7. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Jobling, S.; Jones, C.S.; Noble, L.R.; Routledge, E.J.; Lockyer, A.E. The nuclear receptors of Biomphalaria glabrata and Lottia gigantea: Implications for developing new model organisms. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Xu, F.; Li, J.; Li, L.; Que, H.; Zhang, G. Evolution of a novel nuclear receptor subfamily with emphasis on the member from the Pacific oyster Crassostrea gigas. Gene 2015, 567, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Markov, G.V.; Laudet, V. Evolution of nuclear receptors and ligand signaling: Toward a soft key–lock model? In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 125, pp. 1–38. [Google Scholar] [CrossRef]
- Robinson-Rechavi, M.; Garcia, H.E.; Laudet, V. The nuclear receptor superfamily. J. Cell Sci. 2003, 116, 585–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, S.; Brunet, F.G.; Escriva, H.; Parmentier, G.; Laudet, V.; Robinson-Rechavi, M. Evolutionary genomics of nuclear receptors: From twenty-five ancestral genes to derived endocrine systems. Mol. Biol. Evol. 2004, 21, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Laumer, C.E.; Fernández, R.; Lemer, S.; Combosch, D.; Kocot, K.M.; Riesgo, A.; Andrade, S.C.S.; Sterrer, W.; Sørensen, M.V.; Giribet, G. Revisiting metazoan phylogeny with genomic sampling of all phyla. Proc. R. Soc. B 2019, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotnỳ, J.P.; Chughtai, A.A.; Kostrouchová, M.; Kostrouchová, V.; Kostrouch, D.; Kaššák, F.; Kaňa, R.; Schierwater, B.; Kostrouchová, M.; Kostrouch, Z. Trichoplax adhaerens reveals a network of nuclear receptors sensitive to 9-cis-retinoic acid at the base of metazoan evolution. PeerJ 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Reitzel, A.M.; Tarrant, A.M. Nuclear receptor complement of the cnidarian Nematostella vectensis: Phylogenetic relationships and developmental expression patterns. BMC Evol. Biol. 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Brunet, F.; Paris, M.; Bertrand, S.; Benoit, G.; Laudet, V. Nuclear hormone receptor signaling in amphioxus. Dev. Genes Evol. 2008, 218, 651–665. [Google Scholar] [CrossRef]
- Dehal, P.; Boore, J.L. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005, 3. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.E. Steroid receptors and vertebrate evolution. Mol. Cell. Endocrinol. 2019, 496, 110526. [Google Scholar] [CrossRef] [Green Version]
- Robinson-Rechavi, M.; Maina, C.V.; Gissendanner, C.R.; Laudet, V.; Sluder, A. Explosive lineage-specific expansion of the orphan nuclear receptor HNF4 in nematodes. J. Mol. Evol. 2005, 60, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Kostrouchova, M.; Kostrouch, Z. Nuclear receptors in nematode development: Natural experiments made by a phylum. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; De Tomaso, A.; Davidson, B.; Di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science 2002, 298, 2157–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, A.C.; Cooney, A.J. The varied roles of nuclear receptors during vertebrate embryonic development. Nucl. Recept. Signal. 2003, 1, nrs.01007. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Evans, R.M. The RXR heterodimers and orphan receptors. Cell 1995, 83, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors, RXR, and the big bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Gronemeyer, H.; Gustafsson, J.Å.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Toporova, L.; Balaguer, P. Nuclear receptors are the major targets of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2020, 502, 110665. [Google Scholar] [CrossRef]
- Balaguer, P.; Delfosse, V.; Bourguet, W. Mechanisms of endocrine disruption through nuclear receptors and related pathways. Curr. Opin. Endocr. Metab. Res. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Katsiadaki, I. Are marine invertebrates really at risk from endocrine-disrupting chemicals? Curr. Opin. Environ. Sci. Health 2019, 11, 37–42. [Google Scholar] [CrossRef]
- Fernandez, M.A. Populations collapses in marine invertebrates due to endocrine disruption: A cause for concern? Front. Endocrinol. Lausanne 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Ford, A.T.; Leblanc, G.A. Endocrine disruption in invertebrates: A survey of research progress. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vogeler, S.; Bean, T.P.; Lyons, B.P.; Galloway, T.S. Dynamics of nuclear receptor gene expression during Pacific oyster development. BMC Dev. Biol. 2016, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bodofsky, S.; Koitz, F.; Wightman, B. Conserved and exapted functions of nuclear receptors in animal development. Nucl. Recept. Res. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handberg-Thorsager, M.; Gutierrez-Mazariegos, J.; Arold, S.T.; Nadendla, E.K.; Bertucci, P.Y.; Germain, P.; Tomançak, P.; Pierzchalski, K.; Jones, J.W.; Albalat, R.; et al. The ancestral retinoic acid receptor was a low-affinity sensor triggering neuronal differentiation. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- André, A.; Ruivo, R.; Fonseca, E.; Froufe, E.; Castro, L.F.C.; Santos, M.M. The retinoic acid receptor (RAR) in molluscs: Function, evolution and endocrine disruption insights. Aquat. Toxicol. 2019, 208, 80–89. [Google Scholar] [CrossRef]
- Gutierrez-Mazariegos, J.; Nadendla, E.K.; Lima, D.; Pierzchalski, K.; Jones, J.W.; Kane, M.; Nishikawa, J.I.; Hiromori, Y.; Nakanishi, T.; Santos, M.M.; et al. A mollusk retinoic acid receptor (RAR) ortholog sheds light on the evolution of ligand binding. Endocrinology 2014, 155, 4275–4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, G.; Roux, N.; Laudet, V. Evolution of ligands, receptors and metabolizing enzymes of thyroid signaling. Mol. Cell. Endocrinol. 2017, 459, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.D.L.; Gazo, I.; Besnardeau, L.; Hebras, C.; McDougall, A.; Dumollard, R. Potential roles of nuclear receptors in mediating neurodevelopmental toxicity of known endocrine-disrupting chemicals in ascidian embryos. Mol. Reprod. Dev. 2019, 86, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G. Origin of thyroid hormone signalling in metazoans and implications in their metamorphosis. Ph.D. Thesis, Ecole Normale Supérieure de Lyon—ENS Lyon, Lyon, France, 2015. [Google Scholar]
- André, A.; Ruivo, R.; Gesto, M.; Castro, L.F.C.; Santos, M.M. Retinoid metabolism in invertebrates: When evolution meets endocrine disruption. Gen. Comp. Endocrinol. 2014, 208, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Vogeler, S.; Galloway, T.S.; Isupov, M.; Bean, T.P. Cloning retinoid and peroxisome proliferatoractivated nuclear receptors of the Pacific oyster and in silico binding to environmental chemicals. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Fonseca, E.; Ruivo, R.; Borges, D.; Franco, J.N.; Santos, M.M.; Castro, L.F.C. Of retinoids and organotins: The evolution of the retinoid x receptor in metazoa. Biomolecules 2020, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Pechenik, J.A. On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar. Ecol. Prog. Ser. 1999, 177, 269–297. [Google Scholar] [CrossRef] [Green Version]
- Hickman, C.S. Larvae in invertebrate development and evolution. In The Origin and Evolution of Larval Forms; Elsevier: Amsterdam, The Netherlands, 1999; pp. 21–59. [Google Scholar] [CrossRef]
- Hadfield, M.G. Why and how marine-invertebrate larvae metamorphose so fast. Semin. Cell Dev. Biol. 2000, 11, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Leclère, L.; Horin, C.; Chevalier, S.; Lapébie, P.; Dru, P.; Peron, S.; Jager, M.; Condamine, T.; Pottin, K.; Romano, S.; et al. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat. Ecol. Evol. 2019, 3, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Alié, A.; Hiebert, L.S.; Scelzo, M.; Tiozzo, S. The eventful history of nonembryonic development in tunicates. J. Exp. Zool. Part B Mol. Dev. Evol. 2020. [Google Scholar] [CrossRef]
- Scelzo, M.; Alié, A.; Pagnotta, S.; Lejeune, C.; Henry, P.; Gilletta, L.; Hiebert, L.S.; Mastrototaro, F.; Tiozzo, S. Novel budding mode in Polyandrocarpa zorritensis: A model for comparative studies on asexual development and whole body regeneration. Evodevo 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.; Wang, W.; Graspeuntner, S.; Li, Y.; Insua, S.; Herbst, E.M.; Dirksen, P.; Böhm, A.M.; Hemmrich, G.; Sommer, F.; et al. Regulation of polyp-to-jellyfish transition in Aurelia aurita. Curr. Biol. 2014, 24, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Brekhman, V.; Malik, A.; Haas, B.; Sher, N.; Lotan, T. Transcriptome profiling of the dynamic life cycle of the scypohozoan jellyfish Aurelia aurita. BMC Genom. 2015, 16. [Google Scholar] [CrossRef] [Green Version]
- Wray, G.A.; Raff, R.A. The evolution of developmental strategy in marine invertebrates. Trends Ecol. Evol. 1991, 6, 45–50. [Google Scholar] [CrossRef]
- Taneja, R. Nuclear Receptors in Development; Elsevier: Amsterdam, The Netherlands, 2006; Volume 16, ISBN 9780444528735. [Google Scholar]
- Bunce, C.M.; Campbell, M.J. Nuclear receptors an introductory overview. In Nuclear Receptors; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–13. [Google Scholar] [CrossRef]
- Yaguchi, S.; Morino, Y.; Sasakura, Y. Development of marine invertebrates. In Japanese Marine Life; Springer: Singapore, 2020; pp. 109–124. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Q.; Xu, F.; Li, L.; Li, J.; Que, H.; Zhang, G. Functional characterization of retinoid X receptor with an emphasis on the mediation of organotin poisoning in the Pacific oyster (Crassostrea gigas). Gene 2020, 753. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A.; Tsai, M.J.; Tsai, S.Y. COUP-TF orphan nuclear receptors in development and differentiation. Cell. Mol. Life Sci. 2000, 57, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Tsai, S.Y.; Tsai, M.J. COUP-TFs and eye development. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauchat, D.; Escriva, H.; Miljkovic-Licina, M.; Chera, S.; Langlois, M.C.; Begue, A.; Laudet, V.; Galliot, B. The orphan COUP-TF nuclear receptors are markers for neurogenesis from cnidarians to vertebrates. Dev. Biol. 2004, 275, 104–123. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Zhang, C.L. TLX: A master regulator for neural stem cell maintenance and neurogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Kaltenbach, S.L.; Yu, J.K.; Holland, N.D. The origin and migration of the earliest-developing sensory neurons in the peripheral nervous system of amphioxus. Evol. Dev. 2009, 11, 142–151. [Google Scholar] [CrossRef]
- Watt, A.J.; Garrison, W.D.; Duncan, S.A. HNF4: A central regulator of hepatocyte differentiation and function. Hepatology 2003, 37, 1249–1253. [Google Scholar] [CrossRef]
- Laws, K.M.; Drummond-Barbosa, D. Control of germline stem cell lineages by diet and physiology. In Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2017; Volume 59, pp. 67–99. [Google Scholar] [CrossRef] [Green Version]
- Weber, H.; Holewa, B.; Jones, E.A.; Ryffel, G.U. Mesoderm and endoderm differentiation in animal cap explants: Identification of the HNF4-binding site as an activin A responsive element in the Xenopus HNFIα promoter. Development 1996, 122, 1975–1984. [Google Scholar]
- Chen, W.S.; Manova, K.; Weinstein, D.C.; Duncan, S.A.; Plump, A.S.; Prezioso, V.R.; Bachvarova, R.F.; Darnell, J.E. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994, 8, 2466–2477. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Sladek, F.M.; Darnell, J.E. The expression pattern of a Drosophila homolog to the mouse transcription factor HNF-4 suggests a determinative role in gut formation. EMBO J. 1993, 12, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell 2008, 134, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, K.R.; Hollenberg, A.N. The actions of thyroid hormone signaling in the nucleus. Mol. Cell. Endocrinol. 2017, 458, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Heyland, A. Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Mol. Cell. Endocrinol. 2017, 459, 14–20. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Milanesi, A.; Brent, G.A. Thyroid hormones. In Hormonal Signaling in Biology and Medicine: Comprehensive Modern Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 487–506. ISBN 9780128138151. [Google Scholar]
- Wu, W.; Niles, E.G.; LoVerde, P.T. Thyroid hormone receptor orthologues from invertebrate species with emphasis on Schistosoma mansoni. BMC Evol. Biol. 2007, 7. [Google Scholar] [CrossRef] [Green Version]
- Carosa, E.; Fanelli, A.; Ulisse, S.; Di Lauro, R.; Rall, J.E.; Jannini, E.A. Ciona intestinalis nuclear receptor 1: A member of steroid/thyroid hormone receptor family. Proc. Natl. Acad. Sci. USA 1998, 95, 11152–11157. [Google Scholar] [CrossRef] [Green Version]
- Patricolo, E.; Cammarata, M.; Dagati, P. Presence of thyroid hormones in ascidian larvae and their involvement in metamorphosis. J. Exp. Zool. 2001, 290, 426–430. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, P.; Cammarata, M. Comparative analysis of thyroxine distribution in ascidian larvae. Cell Tissue Res. 2006, 323, 529–535. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Zhao, B.; Lun, L. Up-regulation of C/EBP by thyroid hormones: A case demonstrating the vertebrate-like thyroid hormone signaling pathway in amphioxus. Mol. Cell. Endocrinol. 2009, 313, 57–63. [Google Scholar] [CrossRef]
- Paris, M.; Hillenweck, A.; Bertrand, S.; Delous, G.; Escriva, H.; Zalko, D.; Cravedi, J.P.; Laudet, V. Active metabolism of thyroid hormone during metamorphosis of amphioxus. Integr. Comp. Biol. 2010, 50, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.; Heyland, A. Thyroid hormones accelerate initiation of skeletogenesis via MAPK (ERK1/2) in larval sea urchins (Strongylocentrotus purpuratus). Front. Endocrinol. Lausanne 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, G.; Morishita, Y.; Fini, J.B.; Lorin, T.; Gillet, B.; Hughes, S.; Tohmé, M.; Deléage, G.; Demeneix, B.; Arvan, P.; et al. Thyroglobulin represents a novel molecular architecture of vertebrates. J. Biol. Chem. 2016, 291, 16553–16566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klootwijk, W.; Friesema, E.C.H.; Visser, T.J. A nonselenoprotein from amphioxus deiodinates TRIAC but not T3: Is TRIAC the primordial bioactive thyroid hormone? Endocrinology 2011, 152, 3259–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Xu, F.; Qu, T.; Zhang, R.; Li, L.; Que, H.; Zhang, G. Identification of thyroid hormones and functional characterization of thyroid hormone receptor in the pacific oyster Crassostrea gigas provide insight into evolution of the thyroid hormone system. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Ershov, N.I.; Vorontsova, E.V.; Shilov, A.G.; Merkulova, T.I.; Mordvinov, V.A. Identification of thyroid hormone receptor homologs in the fluke Opisthorchis felineus (Platyhelminthes). Mol. Biochem. Parasitol. 2014, 194, 64–68. [Google Scholar] [CrossRef]
- Cornford, E.M. Schistosomatium douthitti: Effects of thyroxine. Exp. Parasitol. 1974, 36, 210–221. [Google Scholar] [CrossRef]
- Holzer, G.; Laudet, V. Thyroid hormones: A triple-edged sword for life history transitions. Curr. Biol. 2015, 25, R344–R347. [Google Scholar] [CrossRef] [Green Version]
- Osz, J.; McEwen, A.G.; Bourguet, M.; Przybilla, F.; Peluso-Iltis, C.; Poussin-Courmontagne, P.; Mély, Y.; Cianférani, S.; Jeffries, C.M.; Svergun, D.I.; et al. Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR–RXR. Nucleic Acids Res. 2020, 48, 9969–9985. [Google Scholar] [CrossRef]
- Zieger, E.; Schubert, M. New insights into the roles of retinoic acid signaling in nervous system development and the establishment of neurotransmitter systems. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2017; Volume 330, pp. 1–84. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Albalat, R.; Cañestro, C. Identification of Aldh1a, Cyp26 and RAR orthologs in protostomes pushes back the retinoic acid genetic machinery in evolutionary time to the bilaterian ancestor. Chem. Biol. Interact. 2009, 178, 188–196. [Google Scholar] [CrossRef]
- Urushitani, H.; Katsu, Y.; Ohta, Y.; Shiraishi, H.; Iguchi, T.; Horiguchi, T. Cloning and characterization of the retinoic acid receptor-like protein in the rock shell, Thais clavigera. Aquat. Toxicol. 2013, 142–143, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.S.S.; Hiromori, Y.; Kaite, Y.; Ruivo, R.; Franco, J.N.; Nakanishi, T.; Santos, M.M.; Castro, L.F.C. An orthologue of the retinoic acid receptor (RAR) is present in the ecdysozoa phylum Priapulida. Genes 2019, 10, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagatomo, K.I.; Ishibashi, T.; Satou, Y.; Satoh, N.; Fujiwara, S. Retinoic acid affects gene expression and morphogenesis without upregulating the retinoic acid receptor in the ascidian Ciona intestinalis. Mech. Dev. 2003, 120, 363–372. [Google Scholar] [CrossRef]
- Kaneko, N.; Katsuyama, Y.; Kawamura, K.; Fujiwara, S. Regeneration of the gut requires retinoic acid in the budding ascidian Polyandrocarpa misakiensis. Dev. Growth Differ. 2010, 52, 457–468. [Google Scholar] [CrossRef]
- Schubert, M.; Gibert, Y. Retinoids in embryonic development. Biomolecules 2020, 10, 1278. [Google Scholar] [CrossRef]
- Schubert, M.; Holland, N.D.; Laudet, V.; Holland, L.Z. A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev. Biol. 2006, 296, 190–202. [Google Scholar] [CrossRef]
- Halme, A.; Cheng, M.; Hariharan, I.K. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr. Biol. 2010, 20, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Bui-Göbbels, K.; Quintela, R.M.; Bräunig, P.; Mey, J. Is retinoic acid a signal for nerve regeneration in insects? Neural Regen. Res. 2015, 10, 901–903. [Google Scholar] [CrossRef]
- Estephane, D.; Anctil, M. Retinoic acid and nitric oxide promote cell proliferation and differentially induce neuronal differentiation in vitro in the cnidarian Renilla koellikeri. Dev. Neurobiol. 2010, 70, 842–852. [Google Scholar] [CrossRef]
- Lefebvre, P.; Benomar, Y.; Staels, B. Retinoid X receptors: Common heterodimerization partners with distinct functions. Trends Endocrinol. Metab. 2010, 21, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Krężel, W.; Rühl, R.; de Lera, A.R. Alternative retinoid X receptor (RXR) ligands. Mol. Cell. Endocrinol. 2019, 491, 110436. [Google Scholar] [CrossRef] [PubMed]
- Heyman, R.A.; Mangelsdorf, D.J.; Dyck, J.A.; Stein, R.B.; Eichele, G.; Evans, R.M.; Thaller, C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 1992, 68, 397–406. [Google Scholar] [CrossRef]
- Reitzel, A.M.; Macrander, J.; Mane-Padros, D.; Fang, B.; Sladek, F.M.; Tarrant, A.M. Conservation of DNA and ligand binding properties of retinoid X receptor from the placozoan Trichoplax adhaerens to human. J. Steroid Biochem. Mol. Biol. 2018, 184, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Kim, G.J.; Choi, E.J.; Yang, H.O.; Lee, D.S.; Sohn, Y.C. 9-cis-retinoic acid induces growth inhibition in retinoid-sensitive breast cancer and sea urchin embryonic cells via retinoid X receptor α and replication factor C3. Mol. Endocrinol. 2012, 26, 1821–1835. [Google Scholar] [CrossRef] [Green Version]
- Capitão, A.; Lopes-Marques, M.; Páscoa, I.; Ruivo, R.; Mendiratta, N.; Fonseca, E.; Castro, L.F.C.; Santos, M.M. The Echinodermata PPAR: Functional characterization and exploitation by the model lipid homeostasis regulator tributyltin. Environ. Pollut. 2020, 263. [Google Scholar] [CrossRef]
- Iwema, T.; Billas, I.M.L.; Beck, Y.; Bonneton, F.; Nierengarten, H.; Chaumot, A.; Richards, G.; Laudet, V.; Moras, D. Structural and functional characterization of a novel type of ligand-independent RXR-USP receptor. EMBO J. 2007, 26, 3770–3782. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, G.; LeBlanc, G.A. Cloning and characterization of the retinoid X receptor from a primitive crustacean Daphnia magna. Gen. Comp. Endocrinol. 2007, 150, 309–318. [Google Scholar] [CrossRef]
- Germain, P.; Chambon, P.; Eichele, G.; Evans, R.M.; Lazar, M.A.; Leid, M.; De Lera, A.R.; Lotan, R.; Mangelsdorf, D.J.; Gronemeyer, H. International union of pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006, 58, 760–772. [Google Scholar] [CrossRef] [Green Version]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2019; Volume 116, pp. 135–170. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, M.; Lin, A.Y. A review on sex steroid hormone estrogen receptors in mammals and fish. Int. J. Endocrinol. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Bardet, P.L.; Laudet, V.; Vanacker, J.M. Studying non-mammalian models? Not a fool’s ERRand! Trends Endocrinol. Metab. 2006, 17, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crevet, L.; Vanacker, J.M. Regulation of the expression of the estrogen related receptors (ERRs). Cell. Mol. Life Sci. 2020, 77, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Huss, J.M.; Garbacz, W.G.; Xie, W. Constitutive activities of estrogen-related receptors: Transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1912–1927. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, M.; Leary, C.M.; Furlow, J.D.; Gorski, J.; Ahrens, H.; Mueller, G.C.; Schuh, T.J. A ligand-induced conformational change in the estrogen receptor is localized in the steroid binding domain. Biochemistry 1992, 31, 5303–5311. [Google Scholar] [CrossRef]
- Katsu, Y.; Cziko, P.A.; Chandsawangbhuwana, C.; Thornton, J.W.; Sato, R.; Oka, K.; Takei, Y.; Baker, M.E.; Iguchi, T. A second estrogen receptor from Japanese lamprey (Lethenteron japonicum) does not have activities for estrogen binding and transcription. Gen. Comp. Endocrinol. 2016, 236, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Bridgham, J.T.; Keay, J.; Ortlund, E.A.; Thornton, J.W. Vestigialization of an allosteric switch: Genetic and structural mechanisms for the evolution of constitutive activity in a steroid hormone receptor. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.D.L.; Gazo, I.; Nabi, D.; Besnardeau, L.; Hebras, C.; McDougall, A.; Dumollard, R. Bisphenols disrupt differentiation of the pigmented cells during larval brain formation in the ascidian Phallusia mammillata. Aquat. Toxicol. 2019, 216, 105314. [Google Scholar] [CrossRef] [PubMed]
- Bardet, P.L.; Schubert, M.; Horard, B.; Holland, L.Z.; Laudet, V.; Holland, N.D.; Vanacker, J.M. Expression of estrogen-receptor related receptors in amphioxus and zebrafish: Implications for the evolution of posterior brain segmentation at the invertebrate-to-vertebrate transition. Evol. Dev. 2005, 7, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Palanker, L.; Necakov, A.S.; Sampson, H.M.; Ni, R.; Hu, C.; Thummel, C.S.; Krause, H.M. Dynamic regulation of Drosophila nuclear receptor activity in vivo. Development 2006, 133, 3549–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tennessen, J.M.; Baker, K.D.; Lam, G.; Evans, J.; Thummel, C.S. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011, 13, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Paris, M.; Pettersson, K.; Schubert, M.; Bertrand, S.; Pongratz, I.; Escriva, H.; Laudet, V. An amphioxus orthologue of the estrogen receptor that does not bind estradiol: Insights into estrogen receptor evolution. BMC Evol. Biol. 2008, 8, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markov, G.V.; Gutierrez-Mazariegos, J.; Pitrat, D.; Billas, I.M.L.; Bonneton, F.; Moras, D.; Hasserodt, J.; Lecointre, G.; Laudet, V. Origin of an ancient hormone/receptor couple revealed by resurrection of an ancestral estrogen. Sci. Adv. 2017, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.L.; Walker, C.; Azizi, B.; Tolbert, L.; Williams, L.D.; Snell, T.W. Conservation of estrogen receptor function in invertebrate reproduction. BMC Evol. Biol. 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Keay, J.; Thornton, J.W. Hormone-activated estrogen receptors in annelid invertebrates: Implications for evolution and endocrine disruption. Endocrinology 2009, 150, 1731–1738. [Google Scholar] [CrossRef] [Green Version]
- García-Alonso, J.; Hoeger, U.; Rebscher, N. Regulation of vitellogenesis in Nereis virens (Annelida: Polychaeta): Effect of estradiol-17β on eleocytes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 143, 55–61. [Google Scholar] [CrossRef]
- Lidke, A.K.; Bannister, S.; Löwer, A.M.; Apel, D.M.; Podleschny, M.; Kollmann, M.; Ackermann, C.F.; García-Alonso, J.; Raible, F.; Rebscher, N. 17β-Estradiol induces supernumerary primordial germ cells in embryos of the polychaete Platynereis dumerilii. Gen. Comp. Endocrinol. 2014, 196, 52–61. [Google Scholar] [CrossRef]
- Balbi, T.; Ciacci, C.; Canesi, L. Estrogenic compounds as exogenous modulators of physiological functions in molluscs: Signaling pathways and biological responses. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 222, 135–144. [Google Scholar] [CrossRef]
- Tran, T.K.A.; MacFarlane, G.R.; Kong, R.Y.C.; O’Connor, W.A.; Yu, R.M.K. Potential mechanisms underlying estrogen-induced expression of the molluscan estrogen receptor (ER) gene. Aquat. Toxicol. 2016, 179, 82–94. [Google Scholar] [CrossRef]
- Balbi, T.; Franzellitti, S.; Fabbri, R.; Montagna, M.; Fabbri, E.; Canesi, L. Impact of bisphenol A (BPA) on early embryo development in the marine mussel Mytilus galloprovincialis: Effects on gene transcription. Environ. Pollut. 2016, 218, 996–1004. [Google Scholar] [CrossRef]
- Yan, X.H.; Liu, H.L.; Huang, H.; Li, X.B.; Guo, Y.W. Steroids with aromatic A-rings from the Hainan soft coral Dendronephthya studeri Ridley. J. Nat. Prod. 2011, 74, 175–180. [Google Scholar] [CrossRef]
- Satya, S.; Wade, M.; Hass, U.; Holbech, H.; Løfstedt, M.; Vinggaard, A.M.; Tyle, K.H.; Nielsen, P.J.; Holmer, M.L.; Christiansen, S. Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; Organisation for Economic Cooporation and Development: Paris, France, 2014. [Google Scholar] [CrossRef]
- Horiguchi, T. Contamination by organotins and its population-level effects involved by imposex in prosobranch gastropods. In Biological Effects by Organotins; Springer: Tokyo, Japan, 2017; pp. 73–99. ISBN 978-4-431-56451-5. [Google Scholar] [CrossRef]
- Cuvillier-Hot, V.; Lenoir, A. Invertebrates facing environmental contamination by endocrine disruptors: Novel evidences and recent insights. Mol. Cell. Endocrinol. 2020, 504, 110712. [Google Scholar] [CrossRef] [PubMed]
- Zou, E. Invisible endocrine disruption and its mechanisms: A current review. Gen. Comp. Endocrinol. 2020, 293, 113470. [Google Scholar] [CrossRef] [PubMed]
- Hartenstein, V. The neuroendocrine system of invertebrates: A developmental and evolutionary perspective. J. Endocrinol. 2006, 190, 555–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingfield, J.C. Environmental endocrinology: Insights into the diversity of regulatory mechanisms in life cycles. Integr. Comp. Biol. 2018, 58, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.O. Comparative endocrinology: Past, present, and future. Integr. Comp. Biol. 2018, 58, 1033–1042. [Google Scholar] [CrossRef] [PubMed]



| Subfamily | Group | Name | NRNC Symbol | Abbreviation | Physiological Ligand |
|---|---|---|---|---|---|
| # * | A | NRs with two DBDs | NR#A1 | 2DBD-NRα | |
| B | NR#B1 | 2DBD-NRβ | |||
| C | NR#C1 | 2DBD-NRγ | |||
| D | NR#D1 | 2DBD-NRδ | |||
| 0 | A * | Zygotic gap protein | NR0A1 | KNI | x |
| Zygotic gap protein- related | NR0A2 | KNRL | x | ||
| Egon | NR0A3 | EG | x | ||
| ODR-7 | NR0A4 | ODR-7 | x | ||
| Trithorax | NR0A5 | TRX | x | ||
| B | Dosage-sensitive sex reversal-adrenal hypoplasia congenital critical region on the X chromosome, gene 1 | NR0B1 | DAX1 | x | |
| Small heterodimer partner | NR0B2 | SHP | x | ||
| 1 | A | Thyroid hormone receptor | NR1A1,2 | THRα,β | T3 |
| B | Retinoic acid receptor | NR1B1-3 | RARα-γ | All-trans-RA | |
| C | Peroxisome proliferator-activated receptor | NR1C1-3 | PPARα-γ | Fatty acids, Prostaglandins | |
| D | Rev-ErbA | NR1D1,2 | Rev-ErbAα,β | x | |
| E * | Ecdysone-regulated E78 gene | NR1E | E78 | ||
| F | RAR-related orphan receptor | NR1F1-3 | RORα-γ | x | |
| HR3 * | NR1F4 | HR3 | |||
| G * | CNR14-like | NR1G1 | Sex-1 | x | |
| H | Liver X receptor-like | NR1H1 * | EcR | Ecdysteroids | |
| NR1H2,3 | LXRα,β | Oxysterols | |||
| NR1H4,5 | FXRα,β | x | |||
| I | Vitamin D receptor-like | NR1I1 | VDR | Vitamin D | |
| NR1I2 | PXR | Xenobiotics | |||
| NR1I3 | CAR | Androstane | |||
| J * | NHR96 | NR1J1 | DHR96 | ||
| K * | VDR/PXRα,β | NR1K1,2 | VDRα,β-like | ||
| L * | HNR-like 97 | NR1L | HR97 | ||
| M * | HNR-like 19 | NR1M1 | HR10 | ||
| N * | HNR-like 11 | NR1N1 | HR11 | ||
| O * | NR1O | ||||
| P * | NR1P1-11 | ||||
| 2 | A | Hepatocyte nuclear factor 4 | NR2A1-3 | HNF4α,γ | Fatty acids |
| NR2A4 * | HNF4 | ||||
| B | Retinoid X receptor | NR2B1-3 | RXRα-γ | x | |
| NR2B4 | USP | x | |||
| C | Testicular receptor | NR2C1 | TR2 | x | |
| NR2C2 | TR4 | x | |||
| D * | DHR78 | NR2D1 | HR78 | ||
| E | Tailless / Photoreceptor cell-specific nuclear receptor | NR2E1 | TLX | x | |
| NR2E2 * | TLL | x | |||
| NR2E3 | PNR/HR51 * | x | |||
| Dissatisfaction nuclear receptor * | NR2E4 | DSF | |||
| Nuclear hormone receptor FAX-1 * | NR2E5 | FAX1 | |||
| F | Chicken ovalbumin upstream promoter transcription factor | NR2F1,2 | COUP-TFI,II | x | |
| Seven-up * | NR2F3 | SVP | |||
| Chicken ovalbumin upstream promoter transcription factor III * | NR2F4 | COUP-TFIII | |||
| Seven-up related protein 46 * | NR2F5 | SVP-46 | |||
| V-erbA-related protein 2 | NR2F6 | EAR-2 | x | ||
| 3 | A | Estrogen receptor | NR3A1,2 | ERα,β | Estradiol |
| B | Estrogen-related receptor | NR3B1-3 | ERRα-γ | x | |
| NR3B4 * | ERR | x | |||
| C | Steroid receptor / Ketosteroid receptors | NR3C1 | Glucocorticoid receptor, GR | Cortisol | |
| NR3C2 | Mineralocorticoid receptor, MR | Aldosterone | |||
| NR3C3 | Progesterone receptor, PR | Progesterone | |||
| NR3C4 | Androgen receptor, AR | Testosterone | |||
| D * | Estrogen receptor-like in Protostomia | NR3D | ER-like | ||
| E * | Estrogen receptor-like in Cnidaria | NR3E | ER-like | ||
| F * | Estrogen receptor-like in Placozoa | NR3F | ER-like | ||
| 4 | A | Nerve growth factor IB | NR4A1 | NGFIB | x |
| Nuclear receptor related 1 | NR4A2 | NURR1 | x | ||
| Neuron-derived orphan receptor 1 | NR4A3 | NOR1 | x | ||
| DHR38 * | NR4A4 | HR38 | |||
| 5 | A | Steroidogenic factor 1 | NR5A1 | SF1 | Phosphatidylinositols |
| Liver receptor homolog-1 | NR5A2 | LRH1 | Phosphatidylinositols | ||
| NHR FTZ1-α * | NR5A3 | FTZ1-α | |||
| B * | NHR39/FTZ1-β | NR5B1 | HR39 | ||
| 6 | A | Germ cell nuclear factor | NR6A1 | GCNF | x |
| HR4 * | NR6A2 | HR4 | |||
| 7/8 * | A | NR7/8A1 |
| Taxon | Clade | Phylum | Receptor Activity | Developmental Function |
|---|---|---|---|---|
| Deuterostomia | Chordata | Tunicata | Unknown | Suspected role in metamorphosis |
| Cephalochordata | Activated by TRIAC | Pivotal regulator of metamorphosis | ||
| Ambulacraria | Echinodermata | Presumably ligand-activated and/or controlled by alternative signaling pathways | Suspected role in growth, metamorphosis, skeletogenesis | |
| Protostomia | Lophotrochozoa | Annelida | Ligand-activated by T3 or TRIAC | Regulator of developmental transition from trochophore to crawling larva |
| Mollusca | Presumably ligand-activated and/or controlled by alternative signaling pathways | Suspected role in growth and developmental transitions | ||
| Platyhelminthes | Presumably ligand-activated and/or controlled by alternative signaling pathways | Suspected role in growth | ||
| Non-Bilateria | Radiata | Cnidaria | Absent from the genome | THs with a role in metamorphosis, strobilation, skeletogenesis |
| Taxon | Clade | Phylum | Receptor Activity | Developmental Function |
|---|---|---|---|---|
| Deuterostomia | Chordata | Tunicata | Ligand-activated by retinoic acid | Neurogenesis, budding |
| Cephalochordata | Ligand-activated by retinoic acid | Neurogenesis, axial patterning | ||
| Ambulacraria | Echinodermata | Ligand-activated by high concentrations of retinoic acid | Presumably involved in developmental growth | |
| Protostomia | Ecdysozoa | Priapulida | Ligand-activated by high concentrations of retinoic acid | Unknown |
| Hexapoda | Absent from the genome | RA with role in nervous system regeneration, tissue repair | ||
| Lophotrochozoa | Annelida | Ligand-activated by high concentrations of retinoic acid | Neurogenesis | |
| Mollusca | Ligand-binding pocket occluded and potential activation by liganded RXR | Neurogenesis | ||
| Non-Bilateria | Radiata | Cnidaria | Absent from the genome | RA with role in neurogenesis, metamorphosis, strobilation |
| Taxon | Clade | Phylum | Receptor Activity | Developmental Function |
|---|---|---|---|---|
| Deuterostomia | Chordata | Tunicata | ERR: Orphan receptor ER: Lost | ERR: Suggested role in sensory cell differentiation in the larval brain |
| Cephalochordata | ERR: Orphan receptor ER: Unknown | ERR: Suspected role in establishment of neuromuscular contacts ER: Unknown | ||
| Protostomia | Ecdysozoa | Arthropoda | ERR: Orphan receptor ER: Lost | ERR: Control of metabolism underlying larval growth and cell proliferation |
| Lophotrochozoa | Annelida | ERR: Unknown ER: Ligand-activated receptor binding estrogens | ER: Regulation of formation and proliferation of primordial germ cells | |
| Mollusca | ERR: Orphan receptor ER: Occluded ligand binding pocket, but constitutive transcriptional activity | ERR: Unknown ER: Unknown | ||
| Rotifera | ERR: Unknown ER: Ligand-activated receptor binding estrogens | ERR: Unknown ER: Unknown | ||
| Non-Bilateria | Radiata | Cnidaria | ER-like: Ligand-activated receptor binding paraestrol A, an ancestral estrogen | ER-like: Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglioli, A.; Canesi, L.; Gomes, I.D.L.; Schubert, M.; Dumollard, R. Nuclear Receptors and Development of Marine Invertebrates. Genes 2021, 12, 83. https://doi.org/10.3390/genes12010083
Miglioli A, Canesi L, Gomes IDL, Schubert M, Dumollard R. Nuclear Receptors and Development of Marine Invertebrates. Genes. 2021; 12(1):83. https://doi.org/10.3390/genes12010083
Chicago/Turabian StyleMiglioli, Angelica, Laura Canesi, Isa D. L. Gomes, Michael Schubert, and Rémi Dumollard. 2021. "Nuclear Receptors and Development of Marine Invertebrates" Genes 12, no. 1: 83. https://doi.org/10.3390/genes12010083
APA StyleMiglioli, A., Canesi, L., Gomes, I. D. L., Schubert, M., & Dumollard, R. (2021). Nuclear Receptors and Development of Marine Invertebrates. Genes, 12(1), 83. https://doi.org/10.3390/genes12010083






