Epidermal Growth Factor Receptor (EGFR) Gene Polymorphism May be a Modifier for Cadmium Kidney Toxicity
Abstract
:1. Introduction
2. Materials and Methods
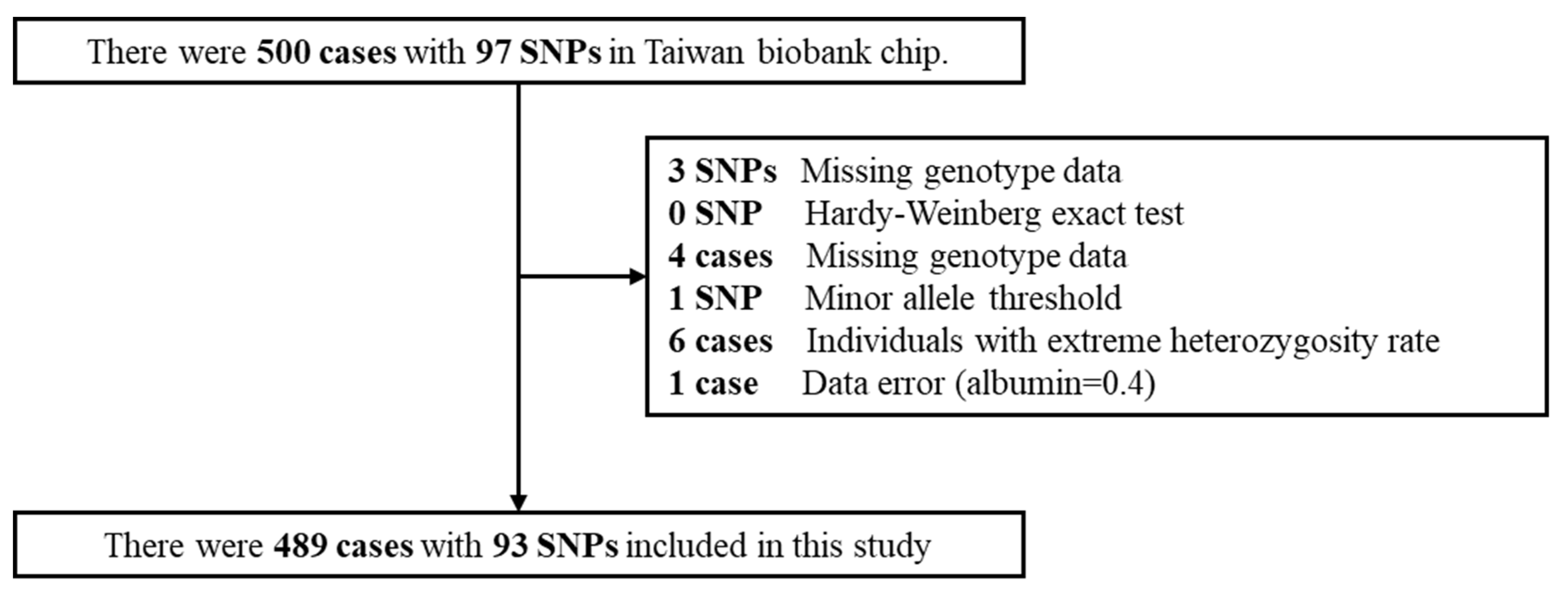
2.1. Taiwan Biobank
2.2. Plasma Cadmium
2.3. eGFR (Estimated Glomerular Filtration Rate) Equation
2.4. Statistical Analysis
3. Results
3.1. Characteristic and Laboratory Information
3.2. SNPs Information
3.3. Association between Plasma Cadmium, EGFR and eGFR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C.; Phelps, K.R. The Effect of Cadmium on GFR Is Clarified by Normalization of Excretion Rates to Creatinine Clearance. Int. J. Mol. Sci. 2021, 22, 1762. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Ujjin, P.; Vesey, D.A. A Comparison of the Nephrotoxicity of Low Doses of Cadmium and Lead. Toxics 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The Source and Pathophysiologic Significance of Excreted Cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Urinary Cadmium Threshold to Prevent Kidney Disease Development. Toxics 2018, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-L.; Kuo, C.-C.; Pan, W.-H.; Chung, Y.-T.; Chen, C.-Y.; Wu, T.-N.; Wang, S.-L. The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int. 2017, 92, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liao, Q.; Chillrud, S.N.; Yang, Q.; Huang, L.; Bi, J.; Yan, B. Environmental Exposure to Cadmium: Health Risk Assessment and its Associations with Hypertension and Impaired Kidney Function. Sci. Rep. 2016, 6, 29989. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, N.; Zhuang, S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013, 83, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Singh, A.B.; Harris, R.C. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp. Cell Res. 2009, 315, 602–610. [Google Scholar] [CrossRef] [Green Version]
- Melenhorst, W.B.; Mulder, G.M.; Xi, Q.; Hoenderop, J.G.; Kimura, K.; Eguchi, S.; Van Goor, H. Epidermal growth factor receptor signaling in the kidney: Key roles in physiology and disease. Hypertension 2008, 52, 987–993. [Google Scholar] [CrossRef]
- Bienaime, F.; Canaud, G.; El Karoui, K.; Gallazzini, M.; Terzi, F. Molecular pathways of chronic kidney disease progression. Nephrol. Ther. 2016, 12 (Suppl. 1), S35–S38. [Google Scholar] [CrossRef]
- Fisher, D.A.; Salido, E.C.; Barajas, L. Epidermal growth factor and the kidney. Annu. Rev. Physiol. 1989, 51, 67–80. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Wang, N.; Li, X. Association of genetic polymorphisms of EGFR with glioma in a Chinese population. Genet. Test. Mol. Biomark. 2015, 19, 59–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-B.; Peng, Y.-P.; Dou, C.-W.; Su, X.-L.; Gao, N.-K.; Tian, F.-M.; Bai, J. Comprehensive study on associations between nine SNPs and glioma risk. Asian Pac. J. Cancer Prev. 2012, 13, 4905–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, U.; Schwartzbaum, J.; Wiklund, F.; Sjöström, S.; Liu, Y.; Tsavachidis, S.; Ahlbom, A.; Auvinen, A.; Collatz-Laier, H.; Feychting, M.; et al. A comprehensive study of the association between the EGFR and ERBB2 genes and glioma risk. Acta Oncol. 2010, 49, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Peng, Y.; Zhao, X. An Updated and Comprehensive Meta-Analysis of Association Between Seven Hot Loci Polymorphisms from Eight GWAS and Glioma Risk. Mol. Neurobiol. 2016, 53, 4397–4405. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.; Li, J.; Yan, M.; Xie, Z.; Zhu, Y.; Chen, C.; Jin, T. Effect of epidermal growth factor receptor gene polymorphisms on prognosis in glioma patients. Oncotarget 2016, 7, 63054–63064. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Y.; Jiang, J.; Guo, L.; Li, Y.; Huang, H.; Shen, L.; Luan, M.; Li, M.; Du, H.; Ma, C.; et al. Genetic Association of Curative and Adverse Reactions to Tyrosine Kinase Inhibitors in Chinese advanced Non-Small Cell Lung Cancer patients. Sci. Rep. 2016, 6, 23368. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xu, X.; Zhou, Y. Association between EGFR polymorphisms and the risk of lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 15245–15249. [Google Scholar]
- Zhang, X.; Fan, J.; Li, Y.; Lin, S.; Shu, P.; Ni, J.; Qin, S.; Zhang, Z. Polymorphisms in epidermal growth factor receptor (EGFR) and AKT1 as possible predictors of clinical outcome in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Tumor Biol. 2016, 37, 1061–1069. [Google Scholar] [CrossRef]
- Jou, Y.-S.; Lo, Y.-L.; Hsiao, C.-F.; Chang, G.-C.; Tsai, Y.-H.; Su, W.-C.; Chen, Y.-M.; Huang, M.-S.; Chen, H.-L.; Chen, C.-J.; et al. Association of an EGFR intron 1 SNP with never-smoking female lung adenocarcinoma patients. Lung Cancer 2009, 64, 251–256. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Zhou, S.; Li, X.; Wu, L. The Impact of EGFR Gene Polymorphisms on the Risk of Alzheimer’s Disease in a Chinese Han Population: A Case-Controlled Study. Med. Sci. Monit. 2018, 24, 5035–5040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Freedman, J.A.; Liu, H.; Moorman, P.G.; Hyslop, T.; George, D.J.; Lee, N.H.; Patierno, S.R.; Wei, Q. Associations between RNA splicing regulatory variants of stemness-related genes and racial disparities in susceptibility to prostate cancer. Int. J. Cancer 2017, 141, 731–743. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, X.; Li, J.; Xu, J.; Yu, X.; Li, T.; Mo, Z.; Hu, Y. RTK/ERK pathway under natural selection associated with prostate cancer. PLoS ONE 2013, 8, e78254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhan, Z.; Wu, J.; Zhang, C.; Yang, Y.; Tong, S.; Sun, Z.; Qin, L.; Yang, X.; Dong, W. Association among polymorphisms in EGFR gene exons, lifestyle and risk of gastric cancer with gender differences in Chinese Han subjects. PLoS ONE 2013, 8, e59254. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, R.L.; Jiang, B.H. Roles of EGFR, PI3K, AKT, and mTOR in heavy metal-induced cancer. Curr. Cancer Drug Targets 2013, 13, 252–266. [Google Scholar] [CrossRef]
- Misra, U.K.; Gawdi, G.; Pizzo, S.V. Induction of mitogenic signalling in the 1LN prostate cell line on exposure to submicromolar concentrations of cadmium+. Cell. Signal. 2003, 15, 1059–1070. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, L.-Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.-H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Taal, M.W. Chronic kidney disease: Towards a risk-based approach. Clin. Med. 2016, 16 (Suppl. 6), s117–s120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shull, G.H. The Composition of a Field of Maize. J. Hered. 1908, 4, 296–301. [Google Scholar] [CrossRef]
- National Health Research Institutes. Taiwan Chronic Kidney Disease Clinical Guidelines; National Health Research Institutes, Ed.; Hsing-Jien Kung: Miaoli County, Taiwan, 2015. [Google Scholar]

| Variables | n or Mean * | |
|---|---|---|
| Participants | 489 | |
| male | 273 (55.8%) | |
| female | 216 (44.2%) | |
| Age | 48.3 | |
| male | 47.5 ± 11.0 | |
| female | 49.3 ± 10.7 | |
| Weight | 66.9 ± 12.2 | |
| male | 73.2 ± 10.5 | |
| female | 58.9 ± 9.1 | |
| Height | 165.1 ± 8.6 | |
| male | 170.8 ± 5.9 | |
| female | 157.9 ± 5.4 | |
| Body-mass index, BMI | 24.4 ± 3.4 | |
| male | 25.1 ± 3.2 | |
| female | 23.6 ± 3.6 | |
| Alcohol | ||
| never or quit | 437 (89.4%) | |
| current | 52 (10.6%) | |
| Smoking status | ||
| never or quit | 435 (88.9%) | |
| current | 54 (11.0%) | |
| Hypertension | 55 (11.2%) | |
| male | 41 (8.4%) | |
| female | 14 (2.9%) | |
| Diabetes | 27 (5.5%) | |
| male | 17 (3.5%) | |
| female | 10 (2.0%) | |
| Kidney stone | 36 (7.4%) | |
| male | 25 (5.1%) | |
| female | 11 (2.2%) | |
| Variables | Mean | Standard Deviation | Median | IQR * | |
|---|---|---|---|---|---|
| Cadmium(ug/L) | 0.022 | 0.008 | 0.022 | 0.011 | |
| male | 0.021 | 0.007 | 0.022 | 0.012 | |
| female | 0.023 | 0.009 | 0.022 | 0.010 | |
| BUN(mg/dL) | 13.05 | 3.49 | 12.60 | 4.70 | |
| male | 13.59 | 3.40 | 13.00 | 4.80 | |
| female | 12.36 | 3.48 | 12.00 | 4.65 | |
| Creatinine(mg/dL) | 0.78 | 0.19 | 0.62 | 0.79 | |
| male | 0.90 | 0.15 | 0.89 | 0.15 | |
| female | 0.62 | 0.11 | 0.61 | 0.13 | |
| CKD-EPI a | 101.37 | 14.28 | 102.74 | 18.06 | |
| male | 98.21 | 14.40 | 99.50 | 18.12 | |
| female | 105.37 | 13.11 | 106.12 | 18.39 | |
| Variables/Model | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E. | β | S.E. | β | S.E. | β | S.E. | β | S.E. | β | S.E. | |
| Sex (Male/Female) | −6.08 | 1.34 * | −6.21 | 1.33 * | −5.83 | 1.34 * | −5.96 | 1.34 * | −5.95 | 1.34 * | −6.08 | 1.33 * |
| Hypertension (Yes/No) | −5.89 | 2.00 * | −6.18 | 2.01 * | −6.24 | 2.02 * | −6.19 | 2.02 * | −6.68 | 2.04 * | −5.89 | 2.00 * |
| Diabetes (Yes/No) | −12.11 | 4.44 * | −12.54 | 4.43 * | −11.83 | 4.44 * | −11.82 | 4.44 * | −9.58 | 4.67 * | −12.43 | 4.45 * |
| Kidney stone (Yes/No) | −1.41 | 2.35 | −1.63 | 2.35 | −1.64 | 2.35 | −1.53 | 2.35 | −1.54 | 2.35 | −1.72 | 2.37 |
| Smoking | 2.24 | 2.05 | 2.32 | 2.05 | 2.01 | 2.07 | 2.15 | 2.05 | 1.81 | 2.06 | 2.16 | 2.05 |
| drinking | −0.89 | 2.09 | −1 | 2.09 | −1.02 | 2.09 | −0.97 | 2.09 | −0.84 | 2.09 | −0.91 | 2.09 |
| BMI | −0.48 | 0.18 * | −0.44 | 0.18 * | −0.46 | 0.18 * | −0.45 | 0.18 * | −0.47 | 0.18 * | −0.45 | 0.18 * |
| Cadmium | −134.93 | 76.3 a | −128.32 | 76.18 | −149.75 | 77.03 b | −154.92 | 76.77 * | −155.78 | 76.62 * | −440.02 | 167.44 * |
| rs13244925 (AA/CC) | - | - | 4.89 | 2.31 * | - | - | - | - | - | - | ||
| rs13244925 (AC/CC) | - | - | −0.65 | 1.29 | - | - | - | - | - | - | ||
| rs6948867 (AA/GG) | - | - | - | - | 5.54 | 2.54 * | - | - | - | - | ||
| rs6948867 (AG/GG) | - | - | - | - | 1.46 | 1.32 | - | - | - | - | ||
| rs35891645 (TC/CC) | - | - | - | - | - | - | 1.34 | 1.32 | - | - | ||
| rs35891645 (TT/CC) | - | - | - | - | - | - | 4.96 | 2.5 * | - | - | ||
| rs6593214 (AA/TT) | - | - | - | - | - | - | - | - | 5.16 | 2.61 * | ||
| rs6593214 (AT/TT) | - | - | - | - | - | - | - | - | 1.53 | 1.32 | ||
| rs845555 (CT/TT) | −9.14 | 4.33 * | ||||||||||
| rs845555 (CC/TT) | 1.17 | 6.34 | ||||||||||
| Cd*rs845555-CT | 448.94 | 190.89 * | ||||||||||
| Cd*rs845555-CC | 3.42 | 274.06 | ||||||||||
| intercept | 120.21 | 4.82 | 119.27 | 4.89 | 119.21 | 4.88 | 119.22 | 4.89 | 119.63 | 4.89 | 125.57 | 5.82 |
| R-square | 0.120 | 0.132 | 0.13 | 0.129 | 0.128 | 0.135 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-T.; Chen, T.-H.; Yang, C.-C.; Luo, K.-H.; Chen, T.-H.; Chuang, H.-Y. Epidermal Growth Factor Receptor (EGFR) Gene Polymorphism May be a Modifier for Cadmium Kidney Toxicity. Genes 2021, 12, 1573. https://doi.org/10.3390/genes12101573
Lin C-T, Chen T-H, Yang C-C, Luo K-H, Chen T-H, Chuang H-Y. Epidermal Growth Factor Receptor (EGFR) Gene Polymorphism May be a Modifier for Cadmium Kidney Toxicity. Genes. 2021; 12(10):1573. https://doi.org/10.3390/genes12101573
Chicago/Turabian StyleLin, Chun-Ting, Ting-Hao Chen, Chen-Cheng Yang, Kuei-Hau Luo, Tzu-Hua Chen, and Hung-Yi Chuang. 2021. "Epidermal Growth Factor Receptor (EGFR) Gene Polymorphism May be a Modifier for Cadmium Kidney Toxicity" Genes 12, no. 10: 1573. https://doi.org/10.3390/genes12101573







