Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia
Abstract
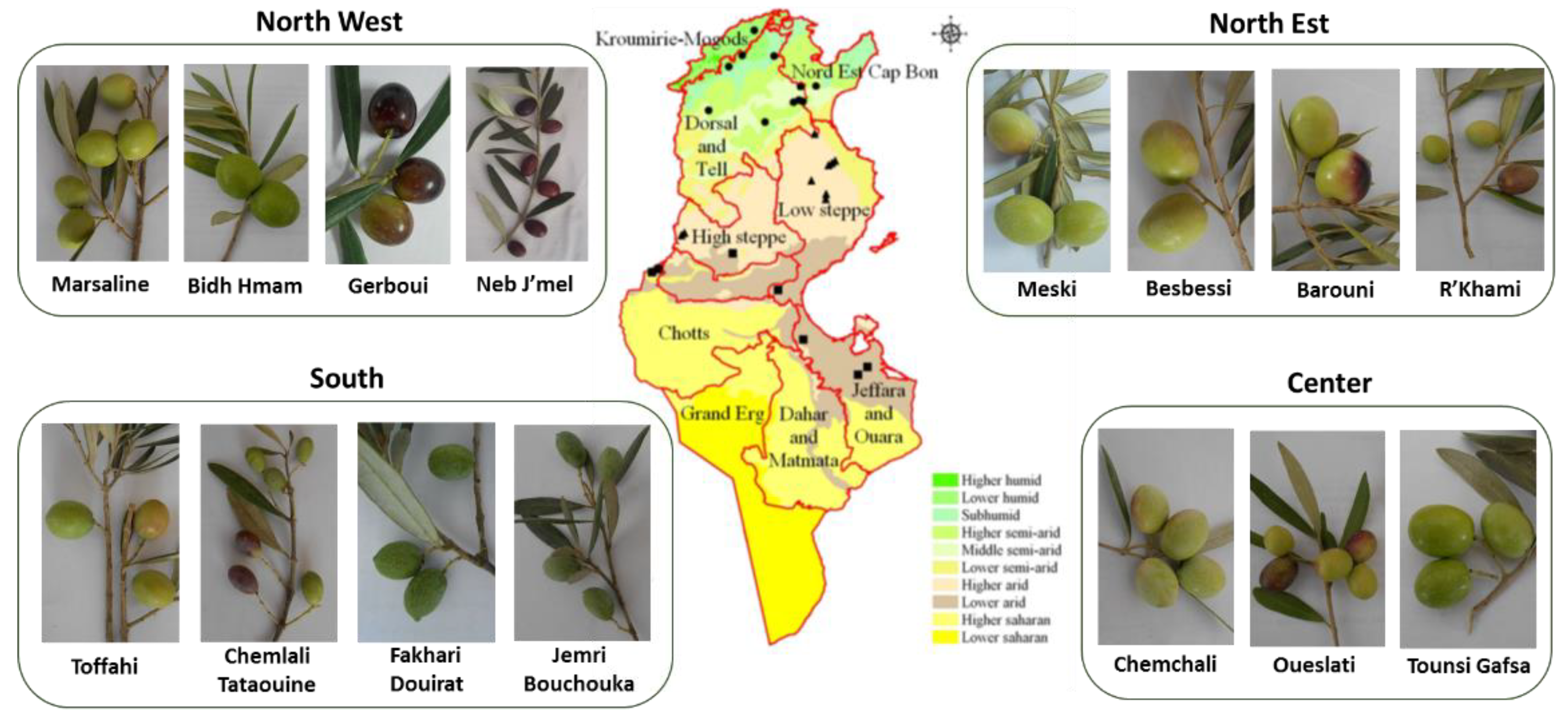
1. Introduction
2. Management of Ex Situ Collections Using SSR Markers
3. Olive Genetic Resources Characterization and Study of Genetic Diversity
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loumou, A.; Giourga, C. Olive groves: The life and identity of the Mediterranean. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeobotanical, historical and molecular evidence from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Besnard, G.; Terral, J.F.; Cornille, A. On the origins and domestication of the olive: A review and perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Diez, C.M.; Trujillo, I.; Barrio, E.; Belaj, A.; Barranco, D.; Rallo, L. Centennial olive trees as a reservoir of genetic diversity. Ann. Bot. 2011, 108, 797–807. [Google Scholar] [CrossRef]
- di Rienzo, V.; Sion, S.; Taranto, F.; Zammit-Mangion, M.; Miazzi, M. Genetic flow among olive populations within the Mediterranean basin. Peer J. 2018, 18, e5260. [Google Scholar] [CrossRef] [PubMed]
- Ross-Ibarra, J.; Morrell, P.L.; Gaut, B.S. Plant domestication, a unique opportunity to identify the genetic basis of adaptation Jeffrey Ross. Proc. Natl. Acad. Sci. USA 2007, 104, 8641–8648. [Google Scholar] [CrossRef]
- Bracci, T.; Busconi, M.; Fogher, C.; Sebastiani, L. Molecular studies in olive (Olea europaea L.): Overview on DNA markers applications and recent advances in genome analysis. Plant Cell Rep. 2011, 30, 449–462. [Google Scholar] [CrossRef] [PubMed]
- International Olive Oil Council (IOOC). 2018. Available online: http://www.internationaloliveoi.org/ (accessed on 15 October 2020).
- Food and Agriculture Organization of the United Nations (FAO). The Statistical Database (FAOSTAT); Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017; Available online: http://www.fao.org/faostat/en/#data (accessed on 10 October 2020).
- Tunisian Agriculture Ministry-General Direction of Agricultural Production. Available online: http://www.agriculture.tn (accessed on 20 September 2019).
- Hannachi, H.; Msallem, M.; Ben Elhadj, S.; El Gazzah, M. Influence du site géographique sur les potentialités agronomiques et technologiques de l’olivier (Olea europaea L.) en Tunisie. C. R. Biol. 2007, 330, 135–142. [Google Scholar] [CrossRef]
- Karray, B.; Msallam, M.; Ksantini, M.; Mahjoub Boujnah, D.; Grati Kamoun, N. L’Institut de l’Olivier—Tunisie: Programmes et Acquis de Recherches Pour la Rénovation de la Filière Huile D’olive et L’amélioration de ses Performances; Ministère de l’Agriculture; IRESA; Institut de l’Olivier: Tunis, Tunisia, 2009. [Google Scholar]
- Saddoud Debbabi, O.M.M.; Miazzi, O.; Elloumi, M.; Fendri, F.; Ben Amar, M.; Savoia, S.; Sion, H.; Souabni, S.; Rahmani Mnasri, S. Recovery, assessment, and molecular characterization of minor olive genotypes in Tunisia. Plants 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Trigui, A.; Msallem, M. Oliviers de Tunisie: Catalogue des variétés autochtones et types locaux. In Identification Variétale and Caractérisation Morpho-Pomologique des Ressources Génétiques Oléicoles de Tunisie; IRESA Press: Tunis, Tunisia, 2002; Volume I, p. 159. [Google Scholar]
- Dhillon, B.S.; Tyagi, R.K.; Lal, A.; Saxena, S. Plant Genetic Resource Management; Narosa Publishing House: New Delhi, India, 2004; p. 434. [Google Scholar]
- Borokini, T.I.; Okere, A.U.; Giwa, A.O.; Daramola, B.O.; Odofin, W.T. Biodiversity and conservation of plant genetic resources in Field Gene-bank of the National Centre for Genetic Resources and Biotechnology, Ibadan, Nigeria. Int. J. Biodivers. Conserv. 2010, 2, 37–50. [Google Scholar]
- Powell, W.; Machray, G.C.; Provan, J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996, 1, 215–222. [Google Scholar] [CrossRef]
- Schlötterer, C.; Tautz, D. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992, 20, 211–215. [Google Scholar] [CrossRef]
- Goldstein, D.B.; Schlötterer, C. Microsatellites: Evolution and applications. Q. Rev. Biol. 1999, 83, 633–634. [Google Scholar]
- Sefc, K.M.; Lopes, M.S.; Mendonça, D.; Santos, M.R.D.; Machado, M.L.D.C.; Machado, A.D.C. Identification of microsatellite loci in olive (Olea europaea L.) and their characterization in Italian and Iberian olive trees. Mol. Ecol. 2000, 9, 1171–1173. [Google Scholar] [CrossRef]
- Carriero, F.; Fontanazza, G.; Cellini, F.; Giorio, G. Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.). Theor. Appl. Genet. 2002, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; Baradat, P.; Bervillé, A. Genetic relationships in the olive (Olea europaea L.) reflect multilocal selection of cultivars. Theor. Appl. Genet. 2001, 102, 251–258. [Google Scholar] [CrossRef]
- Belaj, A.; Munoz-Dıez, C.; Baldoni, L.; Porceddu, A.; Barranco, D.; Satovic, Z. Genetic diversity and population structure of wild olives from the north-western Mediterranean assessed by SSR markers. Ann. Bot 2007, 100, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; El Bakkali, A.; Haouane, H.; Baali-Cherif, D.; Moukhli, A.; Khadari, B. Population genetics of Mediterranean and Saharan olives: Geographic patterns of differentiation and evidence for early generations of admixture. Ann. Bot. 2013, 112, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, I.; Vendramin, G.G.; Chiappetta, A. Genetic biodiversity of Italian olives (Olea europaea) germplasm analyzed by SSR markers. Sci. World J. 2014, 2014, 296590. [Google Scholar] [CrossRef]
- Boucheffa, S.; Tamendjari, A.; Sanchez-Gimeno, A.C.; Rovellini, O.; Venturini, S.; di Rienzo, V.; Miazzi, M.M.; Montemurro, C. Diversity Assessment of Algerian Wild and Cultivated Olives (Olea europaea L.) by Molecular, Morphological, and Chemical Traits. Eur. J. Lipid Sci. Tech. 2019, 121, 1800302. [Google Scholar]
- di Rienzo, V.; Miazzi, M.M.; Fanelli, V.; Sabetta, W.; Montemurro, C. The preservation and characterization of Apulian olive germplasm biodiversity. Acta Hortic. 2018, 1199, 1–6. [Google Scholar] [CrossRef]
- Erre, P.; Chessa, I.; Diez, C.M.; Belaj, A.; Rallo, L.; Trujillo, E. Genetic diversity and relationships between wild and cultivated olives (Olea europaea L.) in Sardinia assessed by SSR markers. Genet. Resour. Crop Evol. 2010, 57, 41–54. [Google Scholar] [CrossRef]
- Abdessemed, S.; Muzzalupo, I.; Benbouza, H. Assessment of genetic diversity among Algerian olive (Olea europaea L.) cultivars using SSR marker. Sci. Hortic. 2015, 192, 10–20. [Google Scholar] [CrossRef]
- De la Rosa, R.; James, C.M.; Tobutt, K.R. Using microsatellites for paternity testing in olive progenies. Hortic. Sci. 2004, 39, 351–354. [Google Scholar] [CrossRef]
- Besnard, G.; Cheptou, P.O.; Debbaoui, M.; Lafont, P.; Hugueny, B.; Dupin, J.; Baali-Cherif, D. Paternity tests support a diallelic self-incompatibility system in a wild olive (Olea europaea subsp. laperrinei, Oleaceae). Ecol. Evol. 2020, 10, 1876–1888. [Google Scholar] [CrossRef]
- Montemurro, C.; Dambruoso, G.; Bottalico, G.; Sabetta, W. Self-incompatibility assessment of some Italian olive genotypes (Olea europaea L.) and cross-derived seedling selection by SSR markers on seed endosperms. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- De la Rosa, R.; Angiolillo, A.; Guerrero, C.; Pellegrini, M.; Rallo, L.; Besnard, G.; Bervillé, A.; Martin, A.; Baldoni, L. A first genetic linkage map of olive (Olea europaea L.) cultivars using RAPD and AFLP markers. Theor. Appl. Genet. 2003, 106, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Collins, G.; Sedgley, M. A molecular linkage map of olive (Olea europaea L.) based on RAPD, microsatellite, and SCAR markers. Genome 2004, 47, 26–35. [Google Scholar] [CrossRef]
- Baldoni, L.; Cultrera, N.G.; Mariotti, R.; Ricciolini, C.; Arcioni, S.; Vendramin, G.G. A consensus list of microsatellite markers for olive genotyping. Mol. Breed. 2009, 24, 213–231. [Google Scholar] [CrossRef]
- Sarri, V.; Baldoni, L.; Porceddu, A.; Cultrera, N.G.M.; Contento, A.; Frediani, M.; Belaj, A.; Trujillo, I.; Cionini, P. Microsatellite markers are powerful tools for discriminating among olive cultivars and assigning them to geographically defined populations. Genome 2006, 49, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Claros, M.G.; Crespillo, R.; Aguilar, M.L.; Cánovas, F.M. DNA fingerprinting and classification of geographically related genotypes of olive-tree (Olea europaea L.). Euphytica 2000, 116, 131–142. [Google Scholar] [CrossRef]
- Mnasri, S.; Saddoud Debbabi, O.; M’barek, B.N. Molecular markers: An important tool to analyze the genetic diversity of local Tunisian olive varieties. Euro-Medit. J. Environ. Integr. 2019, 4. [Google Scholar] [CrossRef]
- Dervishi, A.; Jakše, J.; Ismaili, H.; Javornik, B.; Štajner, N. Comparative assessment of genetic diversity in Albanian olive (Olea europaea L.) using SSRs from anonymous and transcribed genomic regions. Tree Genet. Genomes 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Mariotti, R.; Cultrera, N.G.M.; Mousavi, S.; Baglivo, F.; Rossi, M.; Albertini, E.; Alagna, F.; Carbone, F.; Perrotta, G.; Baldoni, L. Development, evaluation, and validation of new EST-SSR markers in olive (Olea europaea L.). Tree Genet. Genomes 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Pasqualone, A.; di Rienzo, V.D.; Miazzi, M.M.; Fanelli, V.; Caponio, F.; Montemurro, C. High resolution melting analysis of DNA microsatellites in olive pastes and virgin olive oils obtained by talc addition. Eur. J. Lip. Sci. Tech. 2015, 117, 2044–2048. [Google Scholar] [CrossRef]
- Sabetta, W.; Miazzi, M.M.; Di Rienzo, V.; Pasqualone, A.; Montemurro, C. Development and application of protocols to certify the authenticity and traceability of Apulian typical products in olive sector. Riv. Ital. Delle Sostanze Grasse 2017, 94, 37–43. [Google Scholar]
- Trujillo, I.; Ojeda, M.A.; Urdiroz, N.M.; Potter, D.; Barranco, D.; Rallo, L.; Diez, C.M. Identification of the Worldwide Olive Germplasm Bank of Córdoba (Spain) using SSR and morphological markers. Tree Genet Gen. 2014, 10, 141–155. [Google Scholar] [CrossRef]
- Diez, C.M.; Moral, J.; Barranco, D.; Rallo, L. Genetic diversity and conservation of olive genetic resources. In Genetic Diversity and Erosion in Plants: Case Histories; Ahuja, M.R., Mohan Jain, S., Eds.; Sustainable Development and Biodiversity Series; Springer: Basel, Switzerland, 2016; Volume 8, pp. 337–356. [Google Scholar]
- Haouane, H.; El Bakkali, A.; Moukhli, A.; Tollon, C.; Santoni, S.; Oukabli, A.; El Modafar, C.; Khadari, B. Genetic structure and core collection of the World Olive Germplasm Bank of Marrakech: Towards the optimised management and use of Mediterranean olive genetic resources. Genetica 2011, 139, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- El Bakkali, A.; Haouane, H.; Moukhli, A.; Van Damme, P.; Costes, E.; Khadari, B. Construction of core collections suitable for association mapping to optimize use of Mediterranean olive (Olea europaea L.) genetic resources. PLoS ONE 2013, 8, e61263. [Google Scholar] [CrossRef][Green Version]
- Diez, C.M.; Imperato, A.; Rallo, L.; Barranco, D.; Trujillo, I. Worldwide core collection of olive cultivars based on Simple Sequence Repeat and morphological markers. Crop Sci. 2012, 52, 211–221. [Google Scholar] [CrossRef]
- Brown, A.D.H. Core collections: A practical approach to genetic resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Belaj, A.; del Carmen Dominguez-García, M.; Atienza, S.G.; Urdíroz, N.M.; De la Rosa, R.; Satovic, Z.; Martín, A.; Kilian, A.; Trujillo, I.; Valpuesta, V.; et al. Developing a core collection of olive (Olea europaea L.) based on molecular markers (DArTs, SSRs, SNPs) and agronomic traits. Tree Genet. Genomes 2012, 8, 365–378. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, M.V.; Beuzon, C.; González-Plaza, J.J.; Fernández-Ocaña, A.M. Identification of an olive (Olea europaea L.) core collection with a new set of SSR markers. Genet. Resour. Crop Evol. 2020, 68, 1–17. [Google Scholar]
- Charafi, J.; El Meziane, A.; Moukhli, A.; Boulouha, B.; El Modafar, C.; Khadari, B. Menara gardens: A Moroccan olive germplasm collection identified by a SSR locus-based genetic study. Genet. Resour. Crop Evol. 2008, 55, 893–900. [Google Scholar] [CrossRef]
- Kaya, H.B.; Cetin, O.; Kaya, H.; Sahin, M.; Sefer, F.; Kahraman, A.; Tanyolac, B. SNP discovery by Illumina-based transcriptome sequencing of the olive and the genetic characterization of Turkish olive genotypes revealed by AFLP, SSR and SNP markers. PLoS ONE 2013, 8, e73674. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, A.; Ganopoulos, I.; Koubouris, G.; Tsaftaris, A.; Sergendani, C.; Kalivas, A.; Madesis, P. Microsatellite high-resolution melting (SSR-HRM) analysis for genotyping and molecular characterization of an Olea europaea germplasm collection. Plant Genet. Resour. 2014, 12, 273–277. [Google Scholar] [CrossRef]
- Khadari, B.; Breton, C.; Moutier, N.; Roger, J.; Besnard, G.; Bervillé, A.; Dosba, F. The use of molecular markers for germplasm management in French olive collection. Theor. Appl Genet 2003, 106, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Khadari, B.; Moutier, N.; Pinczon Du Sel, S.; Dosba, F. Molecular characterization of French olive cultivars using microsatellites: Towards the establishment of a reference genotype database. Oléag Corps Gras Lipides 2004, 11, 225–229. [Google Scholar] [CrossRef][Green Version]
- Khadari, B.; El Bakkali, A.; Essalouh, L.; Tollon, C.; Pinatel, C.; Besnard, G. Cultivated Olive Diversification at Local and Regional Scales: Evidence from the Genetic Characterization of French Genetic Resources. Front. Plant Sci. 2019, 10, 1593. [Google Scholar] [CrossRef]
- Haddad, B.; Gristina, A.S.; Mercati, F.; Saadi, A.E.; Aiter, N.; Martorana, A.; Sharaf, A.; Carimi, F. Molecular Analysis of the Official Algerian Olive Collection Highlighted a Hotspot of Biodiversity in the Central Mediterranean Basin. Genes 2020, 11, 303. [Google Scholar] [CrossRef]
- Zelasco, S.; Salimonti, A.; Baldoni, L.; Mariotti, R.; Preece, J.E.; Aradhya, M.; Koehmstedt, A.M. Efficiency of SSR markers for exploring olive germplasm diversity through a genetic comparison between the USDA-NCGR and the CRA-OLI olive collections. Acta Hortic. 2012, 1057, 585–592. [Google Scholar] [CrossRef]
- Lombardo, L.; Fila, G.; Lombardo, N.; Epifani, C.; Duffy, D.H.; Godino, G.; Salimonti, A.; Zelasco, S. Uncovering Olive Biodiversity through Analysis of Floral and Fruiting Biology and Assessment of Genetic Diversity of 120 Italian Cultivars with Minor or Marginal Diffusion. Biology 2019, 8, 62. [Google Scholar] [CrossRef]
- Sion, S.; Taranto, F.; Montemurro, C.; Mangini, G.; Camposeo, S.; Falco, V.; Gallo, A.; Mita, G.; Saddoud Debbabi, O.; Ben Amar, F.; et al. Genetic Characterization of Apulian Olive Germplasm as Potential Source in New Breeding Programs. Plants 2019, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Miazzi, M.M.; di Rienzo, V.; Mascio, I.; Montemurro, C.; Sion, S.; Sabetta, W.; Vivaldi, G.A.; Camposeo, S.; Caponio, F.; Squeo, G.; et al. Re.Ger.O.P.: An Integrated Project for the Recovery of Ancient and Rare Olive Germplasm. Front. Plant Sci. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Fendri, M.; Ferreira, E.; Calado, L.; Abreu, I.; Rodríguez-García, M.; Amorim, M.I.; Alché, J.D. Discrimination of Portuguese and Spanish olive cultivars using microsatellite markers. Acad. J. Agric. Res. 2014, 2, 58–61. [Google Scholar]
- Veloso, M.M.; Simões-Costa, M.C.; Carneiro, L.C.; Guimarães, J.B.; Mateus, C.; Fevereiro, P.; Candido, P.R. Olive Tree (Olea europaea L.) diversity in traditional small farms of Ficalho, Portugal. Diversity 2018, 10, 5. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Muto, A.; Badolati, G.; Veizi, A.; Chiappetta, A. Genotyping of Albania olive (Olea europaea) germplasm by SSR molecular markers. Emir. J. Food Agric. 2018, 30, 573–580. [Google Scholar] [CrossRef]
- Hannachi, H.; Breton, C.; Msallem, M.; El Hadj, B.S.; El Gazzah, M.; Berville, A. Differences between native and introduced olive cultivars as revealed by morphology of drupes, oil composition and SSR polymorphisms: A case study in Tunisia. Sci Hortic. 2008, 116, 280–290. [Google Scholar] [CrossRef]
- Rekik, I.; Salimonti, A.; Grati Kamoun, N.; Muzzalupo, I.; Lepais, O.; Gerber, S.; Perri, E.; Rebai, A. Characterization and Identification of Tunisian Olive Tree Varieties by Microsatellite Markers. Hortic. Sci. 2008, 43, 1371–1376. [Google Scholar] [CrossRef]
- Abdelhamid, S.; Grati-Kamoun, N.; Marra, F.; Caruso, T. Genetic similarity among Tunisian cultivated olive estimated through SSR markers. Sci. Agric. 2013, 70, 33–38. [Google Scholar] [CrossRef][Green Version]
- Taranto, F.; D’Agostino, N.; Pavan, S.; Perri, E.; Fanelli, V.; Di Rienzo, V.; Sabetta, W.; Miazzi, M.M.; Zelasco, S.; Montemurro, C. Single nucleotide polymorphism (SNP) diversity in an olive germplasm collection. Acta Hortic. 2018, 1199, 27–31. [Google Scholar] [CrossRef]
- D’Agostino, N.; Taranto, F.; Camposeo, S.; Mangini, G.; Fanelli, V.; Gadaleta, S.; Miazzi, M.M.; Ciani, E.; Montemurro, C. GBS-derived SNP catalogue unveiled wide genetic variability and geographical relationships of Italian olive cultivars. Sci. Rep. 2018, 8, 15877. [Google Scholar] [CrossRef]
- Ben Amar, F.; Ayachi Mezghani, M.; Yengui Majeed, A.M.; Benbelkacem, N. Diversité génétique des variétés d’ olivier originaires de pays arabes présentes dans la collection nationale de l’ olivier de Boughrara (Sfax, Tunisie). Olivae 2014, 120, 17–21. [Google Scholar]
- Fendri, M.; Trujillo, I.; Trigui, A.; Rodriguez-Garcia, I.M.; Ramirez, J.D.A. Simple Sequence Repeat identification and endocarp characterization of olive accessions in a Tunisian germplasm collection. Hortic. Sci. 2010, 45, 1429–1436. [Google Scholar] [CrossRef]
- De Ollas, C.; Morillón, R.; Fotopoulos, V.; Puértolas, J.; Ollitrault, P.; Gómez-Cadenas, A.; Arbona, V. Facing Climate Change: Biotechnology of Iconic Mediterranean Woody Crops. Front. Plant Sci. 2019, 10, 427. [Google Scholar] [CrossRef]
- Taamalli, W.; Geuna, F.; Banfi, R.; Bassi, D.; Daoud, D.; Zarrouk, M. Using microsatellite markers to characterize the main Tunisian olive cultivars Chemlali and Chetoui. J. Hortic. Sci. Biotech. 2007, 82, 25–28. [Google Scholar] [CrossRef]
- Taamalli, W.; Geuna, F.; Bassi, D.; Daoud, D. SSR Marker Based DNA Fingerprinting of Tunisian Olive (Olea europaea L.) Varieties. J. Agric. 2008, 7, 176–181. [Google Scholar]
- Mohamed, M.B.; Zelasco, S.; Guasmi, S.B.A.F.; Triki, T.; Conforti, F.L.; Naziha, G.K. Exploring olive tree genetic variability in the south east of Tunisia. GMR 2017, 16, gmr16039850. [Google Scholar] [CrossRef]
- Ben Ayed, R.; Sans-Grout, C.; Moreau, F.; Grati-Kamoun, N.; Rebai, A. Genetic Similarity Among Tunisian Olive Cultivars and Two Unknown Feral Olive Trees Estimated Through SSR Markers. Biochem. Genet 2014, 52, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Saddoud Debbabi, O.; Montemurro, C.; Ben Maachia, S.; Ben Amar, F.; Fanelli, F.; Gadaleta, S.; El Riachy, M.; Chehade, A.; Siblini, M.; Boucheffa, S.; et al. A Hot Spot of Olive Biodiversity in the Tunisian Oasis of Degache. Diversity 2020, 12, 358. [Google Scholar] [CrossRef]
- Barranco, D.; Rallo, L. Olive cultivars in Spain. Hortic. Tech. 2000, 10, 107–110. [Google Scholar] [CrossRef]
- de Castro, A.I.; Rallo, P.; Suárez, M.P.; Torres-Sánchez, J.; Casanova, L.; Jiménez-Brenes, F.M.; Morales-Sillero, A.; Jiménez, M.R.; López-Granados, F. High-Throughput System for the Early Quantification of Major Architectural Traits in Olive Breeding Trials Using UAV Images and OBIA Techniques. Front. Plant Sci. 2019, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Dridi, J.; Fendri, M.; Breton, C.M.; Msallem, M. Characterization of olive progenies derived from a Tunisian breeding program by morphological traits and SSR markers. Sci. Hortic. 2018, 236, 12–136. [Google Scholar] [CrossRef]

| SSR ID | Bibliographic Reference | Repeat Motif | Primer Sequence (5′-3′) | Ta |
|---|---|---|---|---|
| DCA03 | Sefc et al. (2000) [21] | (GA)19 | cccaagcggaggtgtatattgttac | 50 °C |
| tgcttttgtgtttgagatgttg | ||||
| DCA05 | Sefc et al. (2000) [21] | (GA)15 | aacaaatcccatacgaactgcc | 50 °C |
| cgtgttgctgtgaagaaaatcg | ||||
| DCA09 | Sefc et al. (2000) [21] | (GA)23 | aatcaaagtcttccttctcatttcg | 55 °C |
| gatccttccaaaagtataacctctc | ||||
| DCA15 | Sefc et al. (2000) [21] | (CA)3G(AC)14 | gatcttgtctgtatatccacac | 50 °C |
| tataccttttccatcttgacgc | ||||
| DCA16 | Sefc et al. (2000) [21] | (GT)13(GA)29 | ttaggtgggattctgtagatggttg | 50 °C |
| ttttaggtgagttcatagaattagc | ||||
| DCA17 | Sefc et al. (2000) [21] | (GT)9(AT)7AGATA(GA)38 | gatcaaattctaccaaaaatata | 50 °C |
| taatttttggcacgtagtattgg | ||||
| DCA18 | Sefc et al. (2000) [21] | (CA)4CT(CA)3(GA)19 | aagaaagaaaaaggcagaattaagc | 50 °C |
| gttttcgtctctctacataagtgac | ||||
| EMOL | De la Rosa et al. (2002) [34] | (GA)12 | ctttccaatatgggctctcg | 55 °C |
| atggcactttacgggaaaaa | ||||
| tgccaattatggggctaact | ||||
| GAPU101 | Carriero et al. (2002) [22] | (GA)8(G)3(AG)3 | catgaaaggagggggacata | 57–60 °C |
| ggcacttgttgtgcagattg | ||||
| GAPU71b | Carriero et al. (2002) [22] | GA(AG)6(AAG)8 | gatcaaaggaagaaggggataaa | 57–60 °C |
| acaacaaatccgtacgcttg | ||||
| UDO28 | Cipriani et al. (2002) [22] | (CA)23(TA)3 | ctgcagcttctgcccatac | 57 °C |
| gcagatcatcatttggcact | ||||
| UDO43 | Cipriani et al. (2002) [22] | (GT)12 | tcggctttacaacccatttc | 57 °C |
| tgccaattatggggctaact |
| N° | Accession Name | N° | Accession Name |
|---|---|---|---|
| 1 | BAROUNI | 28 | JEDDARIA CHAAL |
| 2 | BELDI | 29 | JEMRI_BOUCHOUKA |
| 3 | BESBESSI | 30 | LATTOUT SNED |
| 4 | BIDH_HMAM | 31 | LQAM EL KOTTI |
| 5 | CHAHLEYA | 32 | MALLAHI EL MOUAMMAR |
| 6 | CHEMCHALI_GAFSA | 33 | MARSALINE |
| 7 | CHEMLALI BALHI | 34 | MARSALINE |
| 8 | CHEMLALI BOUCHOUKA | 35 | MBAZZEL KBIR |
| 9 | CHEMLALI CHOUAMEKH | 36 | MESKI |
| 10 | CHEMLALI LACH4HAB | 37 | NEB TATAOUINE |
| 11 | CHEMLALI ONTHA TATAOUINE | 38 | NEB_JEMAL_TATAOUINE |
| 12 | CHEMLALI SIG | 39 | OUESLATI2 |
| 13 | CHEMLALI_JERBA | 40 | RKHAMI3 |
| 14 | CHEMLALI_ONTHA | 41 | SAYALI |
| 15 | CHEMLALI_SFAX | 42 | SEMNI JBENIANA |
| 16 | CHEMLALI_TATAOUINE | 43 | TOFFAHI |
| 17 | CHEMLALI_TATAOUINE | 44 | TOUNSI |
| 18 | CHEMLALI_ZARZIS | 45 | TOUNSI GAFSA |
| 19 | CHETOUI2 | 46 | ZALMATI |
| 20 | DHOKKAR NAFTI | 47 | ZARBOUT LOUZIR |
| 21 | ECH CHAHLA | 48 | ZARRAZI EJJBAL |
| 22 | FAKHARI | 49 | ZARRAZI KGH |
| 23 | FAKHARI TATAOUINE | 50 | ZARRAZI_ZARZIS |
| 24 | GERBOUI2 | 51 | ZEITOUN BOUBAZZOULA |
| 25 | HORR CHARQIA | 52 | ZEITOUN EL MANACHER |
| 26 | HORR EL KOTTI | 53 | ZEITOUN KHDIM EL BEY |
| 27 | INJASSI HCHICHINA | 54 | ZEYETI EL KOTTI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saddoud Debbabi, O.; Rahmani Mnasri, S.; Ben Amar, F.; Ben Naceur, M.; Montemurro, C.; Miazzi, M.M. Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes 2021, 12, 286. https://doi.org/10.3390/genes12020286
Saddoud Debbabi O, Rahmani Mnasri S, Ben Amar F, Ben Naceur M, Montemurro C, Miazzi MM. Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes. 2021; 12(2):286. https://doi.org/10.3390/genes12020286
Chicago/Turabian StyleSaddoud Debbabi, Olfa, Sameh Rahmani Mnasri, Fathi Ben Amar, M’barek Ben Naceur, Cinzia Montemurro, and Monica Marilena Miazzi. 2021. "Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia" Genes 12, no. 2: 286. https://doi.org/10.3390/genes12020286
APA StyleSaddoud Debbabi, O., Rahmani Mnasri, S., Ben Amar, F., Ben Naceur, M., Montemurro, C., & Miazzi, M. M. (2021). Applications of Microsatellite Markers for the Characterization of Olive Genetic Resources of Tunisia. Genes, 12(2), 286. https://doi.org/10.3390/genes12020286









