Did Amino Acid Side Chain Reactivity Dictate the Composition and Timing of Aminoacyl-tRNA Synthetase Evolution?
Abstract
:1. Introduction
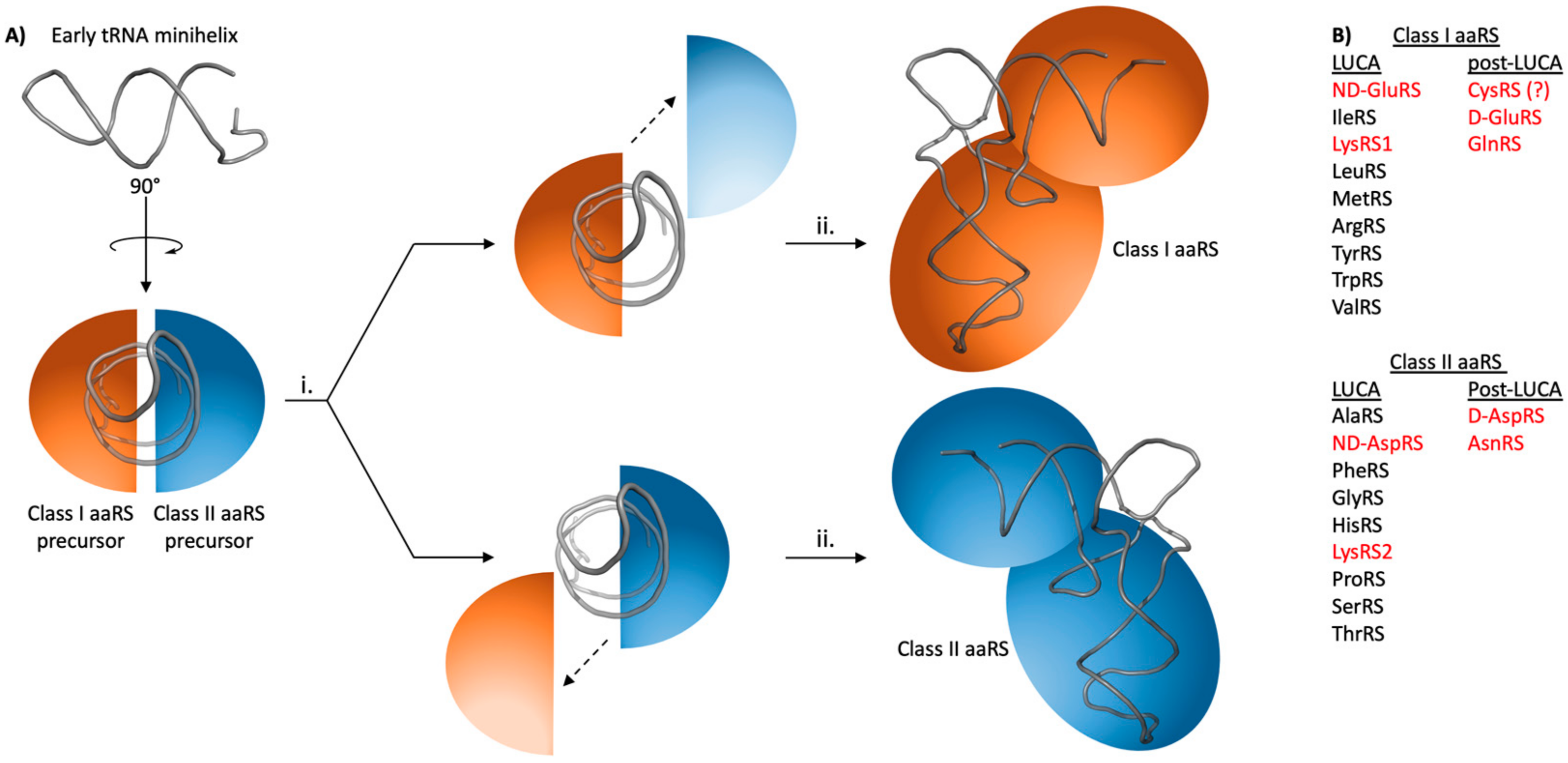
2. Early aaRS Evolution
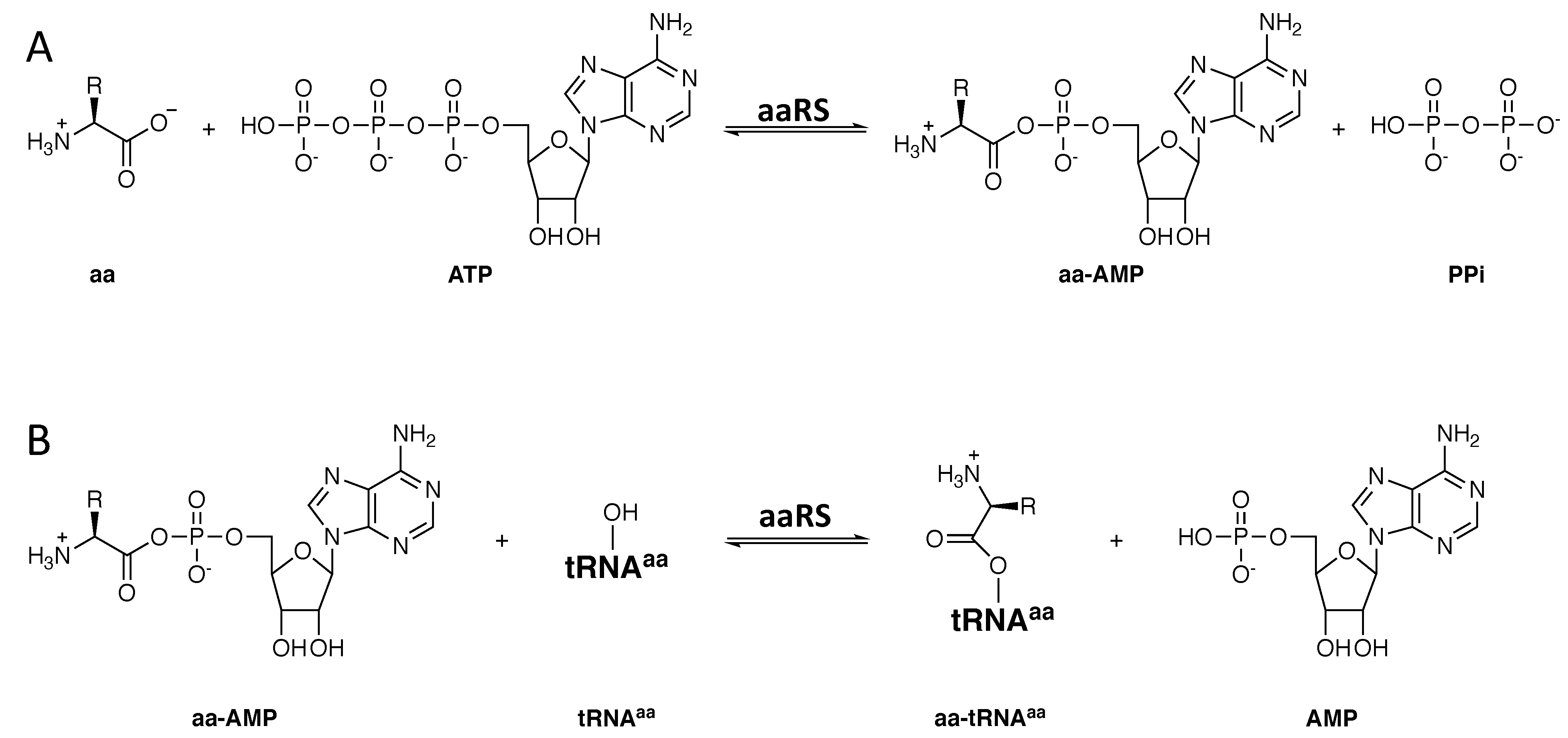
3. Generalities on How the aaRSs Recognize Cognate and Reject Non-Cognate Amino Acids
4. The Evolution of LysRS: Why Is Ornithine Not Part of the Genetic Code?
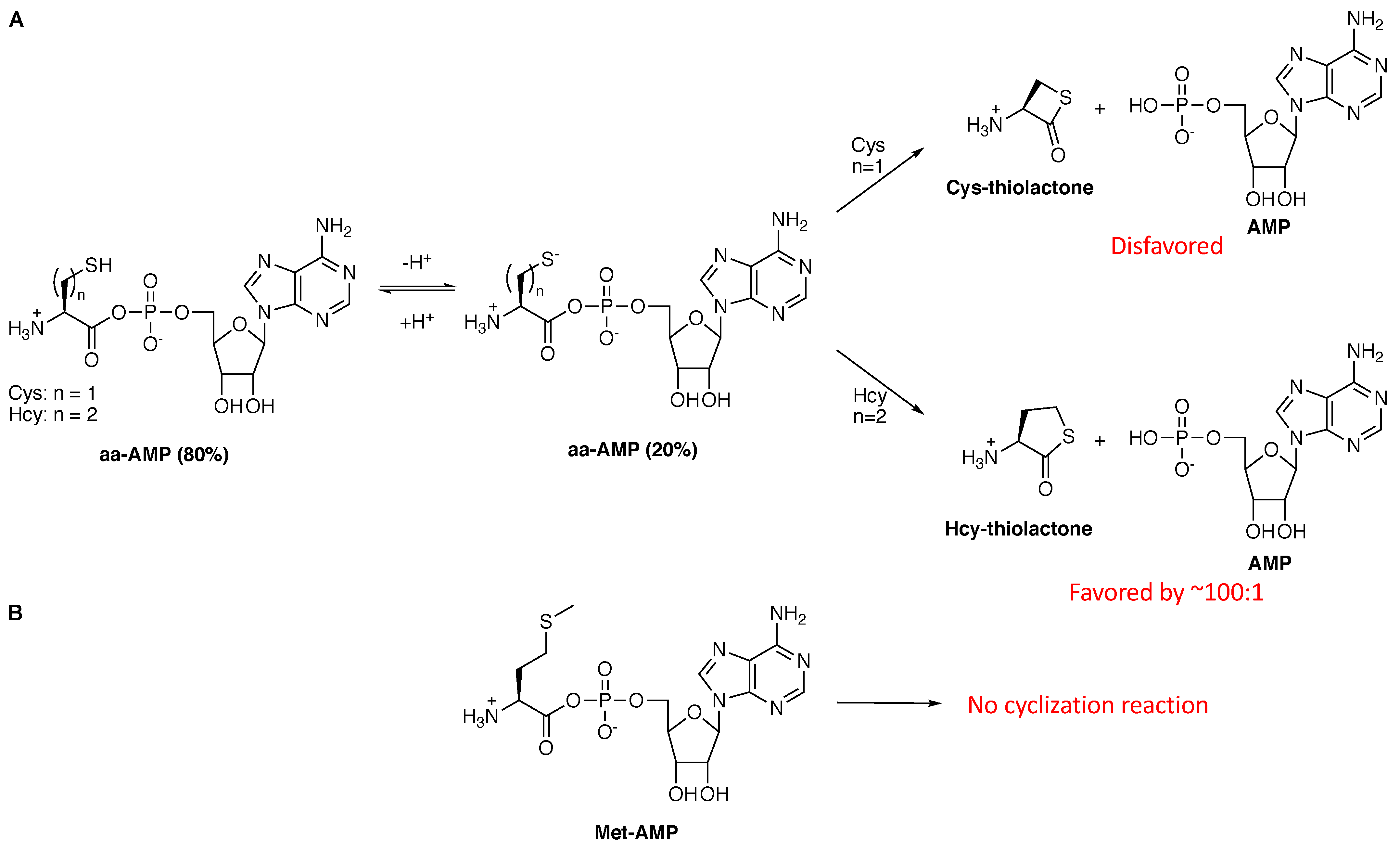
5. The Cases against Homocysteine, Homoserine, and Selenocysteine
6. The Post-LUCA Emergence of GlnRS and AsnRS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mushegian, A.R.; Koonin, E.V. A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc. Natl. Acad. Sci. USA 1996, 93, 10268–10273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, G.E. Origin and evolution of the ribosome. Cold Spring Harb. Perspect. Biol. 2010, 2, a003483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böck, A.; Forchhammer, K.; Heider, J.; Leinfelder, W.; Sawers, G.; Veprek, B.; Zinoni, F. Selenocysteine: The 21st amino acid. Mol. Microbiol. 1991, 5, 515–520. [Google Scholar] [CrossRef]
- Copeland, P.R. Making sense of nonsense: The evolution of selenocysteine usage in proteins. Genome Biol. 2005, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrel, G.; Gaci, N.; Peyret, P.; O’Toole, P.W.; Gribaldo, S.; Brugere, J.-F. Unique characteristics of the pyrrolysine system in the 7th order of methanogens: Implications for the evolution of a genetic code expansion cassette. Archaea 2014, 2014, 374146. [Google Scholar] [CrossRef]
- Heinemann, I.U.; O’Donoghue, P.; Madinger, C.; Benner, J.; Randau, L.; Noren, C.J.; Söll, D. The appearance of pyrrolysine in tRNAHis guanylyltransferase by neutral evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 21103–21108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazil, R. The Alphabet Soup of Life. Available online: https://www.chemistryworld.com/features/why-are-there-20-amino-acids/3009378.article#/ (accessed on 9 February 2021).
- Ribas de Pouplana, L. The evolution of aminoacyl-tRNA synthetases: From dawn to LUCA. In Biology of Aminoacyl-tRNA Synthetases; Ribas de Pouplana, L., Kaguni, L.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 48. [Google Scholar]
- Ibba, M.; Morgan, S.; Curnow, A.W.; Pridmore, D.R.; Vothknecht, U.C.; Gardner, W.; Lin, W.; Woese, C.R.; Soll, D. A euryarchaeal lysyl-tRNA synthetase: Resemblance to class I synthetases. Science 1997, 278, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Ambrogelly, A.; Korencic, D.; Ibba, M. Functional annotation of class I lysyl-tRNA synthetase phylogeny indicates a limited role for gene transfer. J. Bacteriol. 2002, 184, 4594–4600. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, K.; Söll, D. On the evolution of the tRNA-dependent amidotransferases, GatCAB and GatDE. J. Mol. Biol. 2008, 377, 831–844. [Google Scholar] [CrossRef] [Green Version]
- Curnow, A.W.; Hong, K.-W.; Yuan, R.; Kim, S.-I.; Martins, O.; Winkler, W.; Henkin, T.M.; Söll, D. Glu-tRNAGln amidotransferase: A novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. USA 1997, 94, 11819–11826. [Google Scholar] [CrossRef] [Green Version]
- Raczniak, G.; Becker, H.D.; Min, B.; Söll, D. A single amidotransferase forms asparaginyl-tRNA and glutaminyl-tRNA in Chlamydia trachomatis. J. Biol. Chem. 2001, 276, 45862–45867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriani, G.; Delarue, M.; Poch, O.; Gangloff, J.; Moras, D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 1990, 347, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.N.; Ohno, S. Two types of aminoacyl-tRNA synthetases could be originally encoded by complementary strands of the same nucleic acid. Orig. Life Evol. Biosph. 1995, 25, 565–589. [Google Scholar] [CrossRef]
- Ribas de Pouplana, L.; Schimmel, P. Two classes of tRNA synthetases suggested by sterically compatible dockings on tRNA acceptor stem. Cell 2001, 104, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Francklyn, C.; Schimmel, P. Enzymatic aminoacylation of an eight-base-pair microhelix with histidine. Proc. Natl. Acad. Sci. USA 1990, 87, 8655–8659. [Google Scholar] [CrossRef] [Green Version]
- Ribas de Pouplana, L.; Schimmel, P. A view into the origin of life: Aminoacyl-tRNA synthetases. Cell. Mol. Life Sci. 2000, 57, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.-F.; Ng, S.-K.; Mat, W.-K.; Hu, T.; Xue, H. Coevolution theory of the genetic code at age forty: Pathway to translation and synthetic life. Life 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgs, P.G.; Pudritz, R.E. A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code. Astrobiology 2009, 9, 483–490. [Google Scholar] [CrossRef]
- Fournier, G.P.; Andam, C.P.; Alm, E.J.; Gogarten, J.P. Molecular evolution of aminoacyl tRNA synthetase proteins in the early history of life. Orig. Life Evol. Biosph. 2011, 41, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Frenkel-Pinter, M.; Haynes, J.W.; Martin, C.; Petrov, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar] [CrossRef] [Green Version]
- Xavier, J.C.; Hordijk, W.; Kauffman, S.; Steel, M.; Martin, W.F. Autocatalytic chemical networks at the origin of metabolism. Proc. R. Soc. B 2020, 287, 20192377. [Google Scholar] [CrossRef]
- Takenaka, A.; Moras, D. Correlation between equi-partition of aminoacyl-tRNA synthetases and amino-acid biosynthesis pathways. Nucleic Acids Res. 2020, 48, 3277–3285. [Google Scholar] [CrossRef]
- Gospodinov, A.; Kunnev, D. Universal codons with enrichment from GC to AU nucleotide composition reveal a chronological assignment from early to late along with LUCA formation. Life 2020, 10, 81. [Google Scholar] [CrossRef]
- Tawfik, D.S.; Gruic-Sovulj, I. How evolution shapes enzyme selectivity—lessons from aminoacyl-tRNA synthetases and other amino acid utilizing enzymes. FEBS J. 2020, 287, 1284–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Musier-Forsyth, K. Trans-editing of Cys-tRNAPro by Haemophilus influenzae YbaK protein. J. Biol. Chem. 2004, 279, 42359–42362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donoghue, P.; Luthey-Schulten, Z. On the evolution of structure in aminoacyl-tRNA synthetases. Microbiol. Mol. Biol. Rev. 2003, 67, 550–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibba, M.; Söll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef] [PubMed]
- Weinger, J.S.; Strobel, S.A. Participation of the tRNA A76 hydroxyl groups throughout translation. Biochemistry 2006, 45, 5939–5948. [Google Scholar] [CrossRef] [Green Version]
- Wolfson, A.D.; Uhlenbeck, O.C. Modulation of tRNAAla identity by inorganic pyrophosphatase. Proc. Natl. Acad. Sci. USA 2002, 99, 5965–5970. [Google Scholar] [CrossRef] [Green Version]
- Peacock, J.R.; Walvoord, R.R.; Chang, A.Y.; Kozlowski, M.C.; Gamper, H.; Hou, Y.M. Amino acid-dependent stability of the acyl linkage in aminoacyl-tRNA. RNA 2014, 20, 758–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loftfield, R.B.; Vanderjagt, D. The frequency of errors in protein biosynthesis. Biochem. J. 1972, 128, 1353–1356. [Google Scholar] [CrossRef]
- Freist, W.; Sternbach, H.; Cramer, F. Isoleucyl-tRNA synthetase from baker’s yeast and from Escherichia coli MRE 600. Discrimination of 20 amino acids in aminoacylation of tRNA(Ile)-C-C-A. Eur. J. Biochem. 1988, 173, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Festschrift fuer Prof. Dr. Arthur Stoll Siebzigsten; Stoll, A., Ed.; Birkhayser: Basel, Switzerland, 1958; pp. 597–602. [Google Scholar]
- Schmidt, E.; Schimmel, P. Mutational isolation of a sieve for editing in a transfer RNA synthetase. Science 1994, 264, 265–267. [Google Scholar] [CrossRef]
- Nureki, O.; Vassylyev, D.G.; Tateno, M.; Shimada, A.; Nakama, T.; Fukai, S.; Konno, M.; Hendrickson, T.L.; Schimmel, P.; Yokoyama, S. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science 1998, 280, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A.R.; Dingwall, C. Evidence for the double-sieve editing mechanism in protein synthesis. Steric exclusion of isoleucine by valyl-tRNA synthetases. Biochemistry 1979, 18, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, T.L.; Schimmel, P. Transfer RNA-dependent amino acid discrimination by aminoacyl-tRNA synthetases. In Translational Mechanisms; Lapointe, J., Brakier-Gringas, L., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 34–64. [Google Scholar]
- Wong, F.C.; Beuning, P.J.; Silvers, C.; Musier-Forsyth, K. An isolated class II aminoacyl-tRNA synthetase insertion domain is functional in amino acid editing. J. Biol. Chem. 2003, 278, 52857–52864. [Google Scholar] [CrossRef] [Green Version]
- Hati, S.; Ziervogel, B.; Sternjohn, J.; Wong, F.C.; Nagan, M.C.; Rosen, A.E.; Siliciano, P.G.; Chihade, J.W.; Musier-Forsyth, K. Pre-transfer editing by class II prolyl-tRNA synthetase: Role of aminoacylation active site in "selective release" of noncognate amino acids. J. Biol. Chem. 2006, 281, 27862–27872. [Google Scholar] [CrossRef] [Green Version]
- Sivashanmugam, M.; Jaidev, J.; Umashankar, V.; Sulochana, K.N. Ornithine and its role in metabolic diseases: An appraisal. Biomed. Pharmacother. 2017, 86, 185–194. [Google Scholar] [CrossRef]
- Wang, S.; Liang, H.; Liu, L.; Jiang, X.; Wu, S.; Gao, H. Promiscuous enzymes cause biosynthesis of diverse siderophores in Shewanella oneidensis. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Levengood, J.; Ataide, S.F.; Roy, H.; Ibba, M. Divergence in noncognate amino acid recognition between class I and class II lysyl-tRNA synthetases. J. Biol. Chem. 2004, 279, 17707–17714. [Google Scholar] [CrossRef] [Green Version]
- Perona, J.J.; Gruic-Sovulj, I. Synthetic and editing mechanisms of aminoacyl-tRNA synthetases. Top. Curr. Chem. 2013, 344, 1–41. [Google Scholar] [CrossRef]
- DeTar, D.F.; Brooks, W. Cyclization and polymerization of ω-(Bromoalkyl)dimethylamines. J. Org. Chem. 1978, 43, 2245–2248. [Google Scholar] [CrossRef]
- Galli, C.; Illuminati, G.; Mandolini, L.; Tamborra, P. Ring-closure reactions. 7. Kinetics and activation parameters of lactone formation in the range of 3- to 23-membered rings. J. Am. Chem. Soc. 1977, 99, 2591–2597. [Google Scholar] [CrossRef]
- Jakubowski, H. Misacylation of tRNALys with noncognate amino acids by lysyl-tRNA synthetase. Biochemistry 1999, 38, 8088–8093. [Google Scholar] [CrossRef]
- Jakubowski, H. Aminoacyl thioester chemistry of class II aminoacyl-tRNA synthetases. Biochemistry 1997, 36, 11077–11085. [Google Scholar] [CrossRef]
- Jakubowski, H.; Goldman, E. Editing of errors in selection of amino acids for protein synthesis. Microbiol. Rev. 1992, 56, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Quality control in tRNA charging—editing of homocysteine. Acta Biochim. Pol. 2011, 58, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, H. Homocysteine editing, thioester chemistry, coenzyme A, and the origin of coded peptide synthesis. Life 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Gillet, S.; Hountondji, C.; Schmitter, J.-M.; Blanquet, S. Covalent methionylation of Escherichia coli methionyl-tRNA synthethase: Identification of the labeled amino acid residues by matrix-assisted laser desorption-ionization mass spectrometry. Protein Sci. 1997, 6, 2426–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [Green Version]
- Maroney, M.J.; Hondal, R.J. Selenium versus sulfur: Reversibility of chemical reactions and resistance to permanent oxidation in proteins and nucleic acids. Free Radic. Biol. Med. 2018, 127, 228–237. [Google Scholar] [CrossRef]
- Palioura, S.; Sherrer, R.L.; Steitz, T.A.; Söll, D.; Simonovic, M. The human SepSecS-tRNASec complex reveals the mechanism of selenocysteine formation. Science 2009, 325, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Lamour, V.; Quevillon, S.; Diriong, S.; N’Guyen, V.C.; Lipinski, M.; Mirande, M. Evolution of the Glx-tRNA synthetase family: The glutaminyl enzyme as a case of horizontal gene transfer. Proc. Natl. Acad. Sci. USA 1994, 91, 8670–8674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiba, K.; Motegi, H.; Yoshida, M.; Noda, T. Human asparaginyl-tRNA synthetase: Molecular cloning and the inference of the evolutionary history of Asx-tRNA synthetase family. Nucleic Acids Res. 1998, 26, 5045–5051. [Google Scholar] [CrossRef] [Green Version]
- Roy, H.; Becker, H.D.; Reinbolt, J.; Kern, D. When contemporary aminoacyl-tRNA synthetases invent their cognate amino acid metabolism. Proc. Natl. Acad. Sci. USA 2003, 100, 9837–9842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnayake, U.M.; Wood, W.N.; Hendrickson, T.L. Indirect tRNA aminoacylation during accurate translation and phenotypic mistranslation. Curr. Opin. Chem. Biol. 2017, 41, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Ajami, A.M. Glutamine: The emperor or his clothes? J. Nutr. 2001, 131, 2449S–2459S. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bachhawat, A.K. Pyroglutamic acid: Throwing light on a lightly studied metabolite. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- Fischer, W.H.; Spiess, J. Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. Proc. Natl. Acad. Sci. USA 1987, 84, 3628–3632. [Google Scholar] [CrossRef] [Green Version]
- Abraham, G.N.; Podell, D.N. Pyroglutamic acid. Non-metabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol. Cell Biochem. 1981, 38, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Haenni, A.-L.; Chapeville, F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim. Biophys. Acta BBA Nucleic Acids Protein Synth. 1966, 114, 135–148. [Google Scholar] [CrossRef]
- Singleton, M.; Isupov, M.; Littlechild, J. X-ray structure of pyrrolidone carboxyl peptidase from the hyperthermophilic archaeon Thermococcus litoralis. Structure 1999, 7, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Sheppard, K.; Tumbula-Hansen, D.; Söll, D. Gln-tRNAGln formation from Glu-tRNAGln requires cooperation of an asparaginase and a Glu-tRNAGln kinase. J. Biol. Chem. 2005, 280, 8150–8155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampias, T.; Sheppard, K.; Söll, D. The archaeal transamidosome for RNA-dependent glutamine biosynthesis. Nucleic Acids Res. 2010, 38, 5774–5783. [Google Scholar] [CrossRef]
- Wilcox, M. Gamma-glutamyl phosphate attached to glutamine-specific tRNA. A precursor of glutaminyl-tRNA in Bacillus subtilis. Eur. J. Biochem. 1969, 11, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M. Gamma-phosphoryl ester of glu-tRNA-GLN as an intermediate in Bacillus subtilis glutaminyl-tRNA synthesis. Cold Spring Harb. Symp. Quant. Biol. 1969, 34, 521–528. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Zhong, H.; Yu, H.-T. Theoretical study of fragmentation pathways and product distribution of deprotonated aspartic acid. Comput. Theor. Chem. 2017, 1099, 1–7. [Google Scholar] [CrossRef]
- Su, H.-W.; Zhu, J.-H.; Li, H.; Cai, R.-J.; Ealand, C.; Wang, X.; Chen, Y.-X.; Kayani, M.U.R.; Zhu, T.F.; Moradigaravand, D.; et al. The essential mycobacterial amidotransferase GatCAB is a modulator of specific translational fidelity. Nat. Microbiol. 2016, 1, 16147. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickson, T.L.; Wood, W.N.; Rathnayake, U.M. Did Amino Acid Side Chain Reactivity Dictate the Composition and Timing of Aminoacyl-tRNA Synthetase Evolution? Genes 2021, 12, 409. https://doi.org/10.3390/genes12030409
Hendrickson TL, Wood WN, Rathnayake UM. Did Amino Acid Side Chain Reactivity Dictate the Composition and Timing of Aminoacyl-tRNA Synthetase Evolution? Genes. 2021; 12(3):409. https://doi.org/10.3390/genes12030409
Chicago/Turabian StyleHendrickson, Tamara L., Whitney N. Wood, and Udumbara M. Rathnayake. 2021. "Did Amino Acid Side Chain Reactivity Dictate the Composition and Timing of Aminoacyl-tRNA Synthetase Evolution?" Genes 12, no. 3: 409. https://doi.org/10.3390/genes12030409
APA StyleHendrickson, T. L., Wood, W. N., & Rathnayake, U. M. (2021). Did Amino Acid Side Chain Reactivity Dictate the Composition and Timing of Aminoacyl-tRNA Synthetase Evolution? Genes, 12(3), 409. https://doi.org/10.3390/genes12030409





