Microsatellite DNA Analysis of Genetic Diversity and Parentage Testing in the Popular Dog Breeds in Poland
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
- Polymorphic information content—PIC [25],
- Power of discrimination—PD [26],where pjk is the allele frequency j,k for i-locus; CPD is the cumulative power of discrimination.
- Probability of identity—PID [27],where pi p,j is allele frequencies j,i; CPID is the cumulative probability of identity.
- Probability of parentage exclusion for each locus, when the genotypes of one and both parents are known (PE1 and PE2) and the cumulative probability of parentage exclusion (CPE) [28],where pjk is allele frequency j,k for i-locus; CPE1, CPE2 are cumulative probabilities of identity.
3. Results
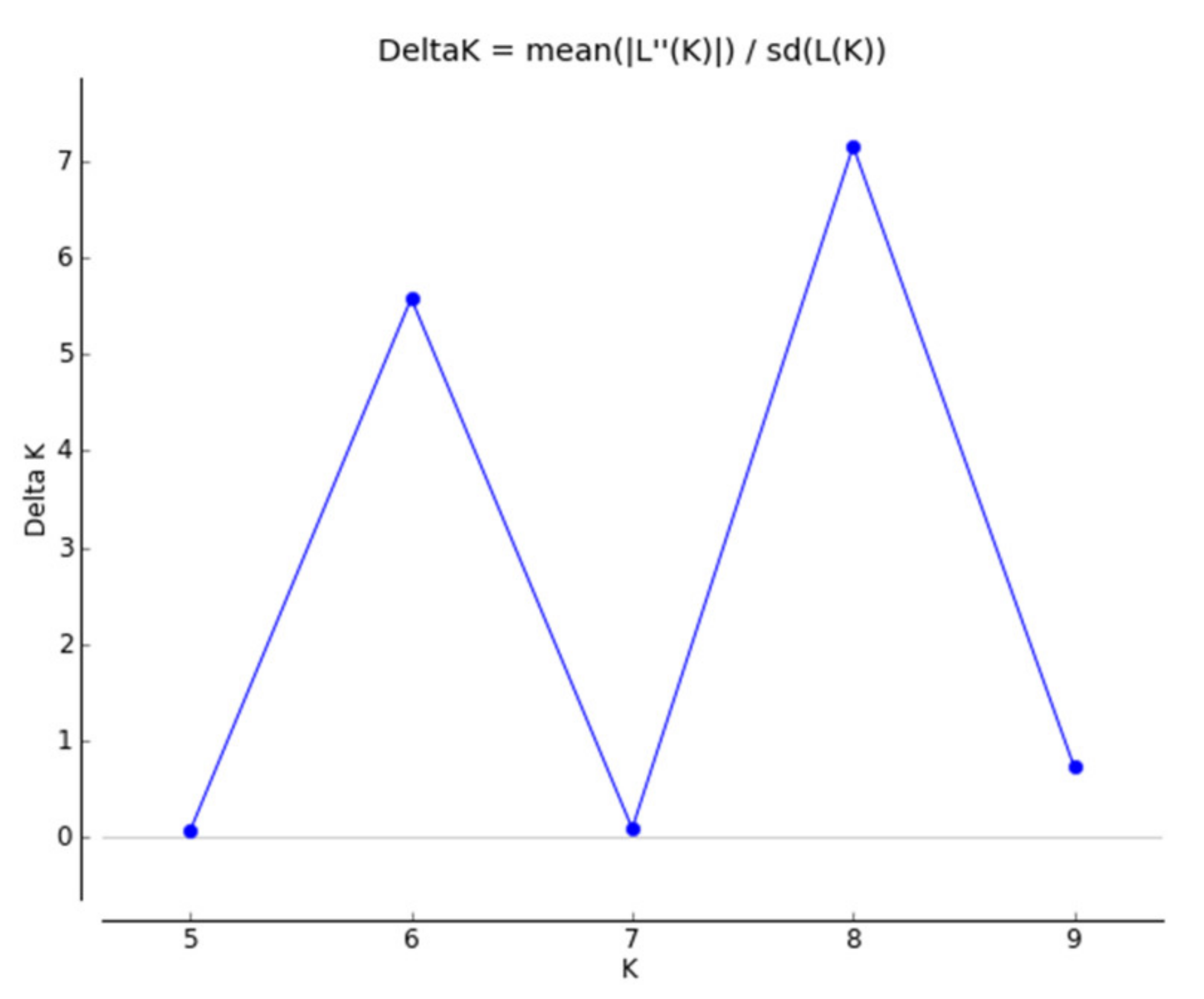
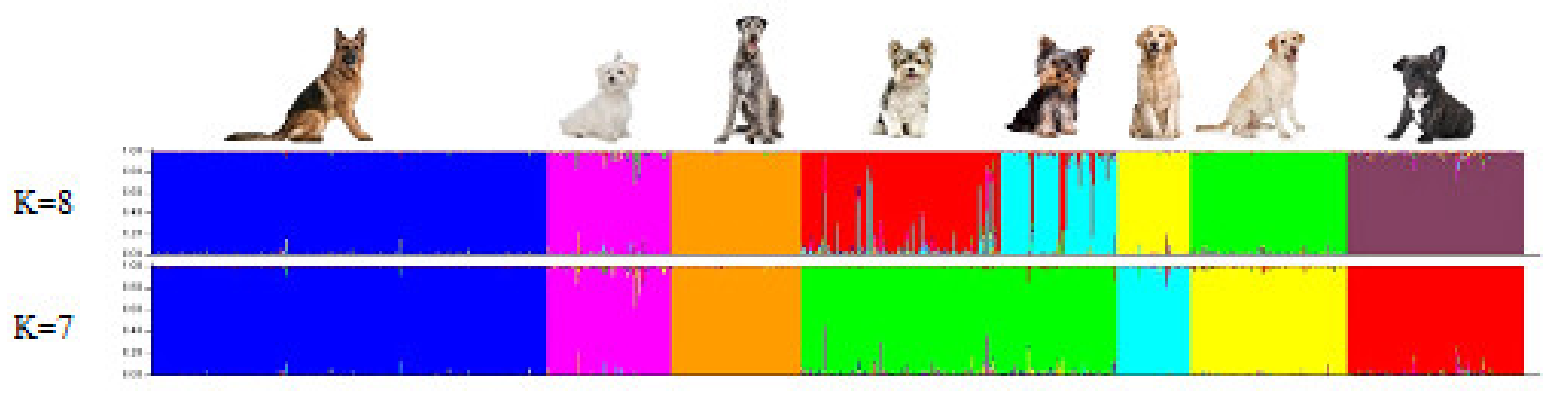
3.1. Breed Relationships
3.2. Diversity Analysis
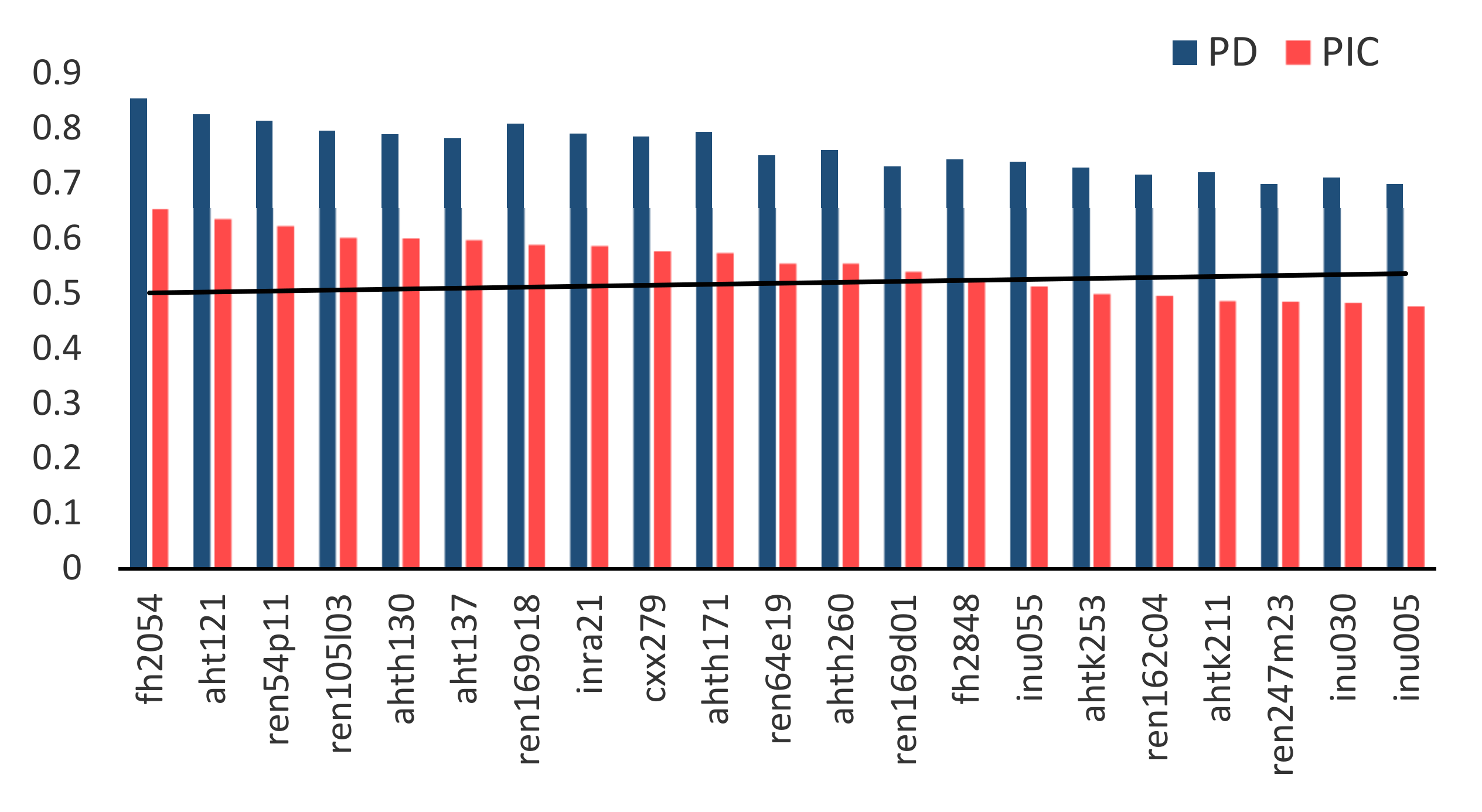
3.3. Parentage Testing and Individual Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wayne, R.K.; von Holdt, B.M. Evolutionary genomics of dog domestication. Mamm. Genome 2012, 23, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Karlsson, E.K.; Perri, A.; Webster, M.T.; Ho, S.Y.W.; Peters, J.; Stahl, P.W.; Piper, P.J.; Lingaas, F.; Fredholm, M.; et al. Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc. Natl. Acad. Sci. USA 2012, 109, 8878–8883. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Liu, H.; Leonard, A.; Griffioen, L. A search for genetic diversity among Italian Greyhounds from Continental Europe and the USA and the effect of inbreeding on susceptibility to autoimmune disease. Canine Genet. Epidemiol. 2015, 2, 17. [Google Scholar] [CrossRef]
- Keijser, S.F.A.; Fieten, H.; Vos-Loohuis, M.; Piek, C.J.; Anderson, H.; Donner, J.; Scholten, I.; Nielen, M.; Hesselink, J.W.; Van Steenbeek, F.G. Heterozygosity testing and multiplex DNA panel screening as a potential tool to monitor health and inbreeding in a small, closed dog population. Canine Genet. Epidemiol. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lampi, S.; Donner, J.; Anderson, H.; Pohjoismäki, J. Variation in breeding practices and geographic isolation drive subpopu-lation differentiation, contributing to the loss of genetic diversity within dog breed lineages. Canine Med. Genet. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- American Kennel Club. Available online: https://www.akc.org/press-center/articles-resources/facts-and-stats/breeds-year-recognized (accessed on 1 January 2021).
- The Fédération Cynologique Internationale. Available online: http://www.fci.be/en/Presentation-of-our-organi-sation-4.html (accessed on 27 April 2020).
- Mellanby, R.J.; Ogden, R.; Clements, D.N.; French, A.T.; Gow, A.G.; Powell, R.; Corcoran, B.; Schoeman, J.P.; Summers, K.M. Population structure and genetic heterogeneity in popular dog breeds in the UK. Vet. J. 2013, 196, 92–97. [Google Scholar] [CrossRef]
- Bigi, D.; Marelli, S.P.; Randi, E.; Polli, M. Genetic characterization of four native Italian shepherd dog breeds and analysis of their relationship to cosmopolitan dog breeds using microsatellite markers. Animal 2015, 9, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Arata, S.; Asahi, A.; Takeuchi, Y.; Mori, Y. Microsatellite loci analysis for individual identification in Shiba Inu. J. Vet. Med. Sci. 2016, 78, 439–441. [Google Scholar] [CrossRef][Green Version]
- Radko, A.; Rubiś, D.; Szumiec, A. Analysis of microsatellite DNA polymorphism in the Tatra Shepherd Dog. J. Appl. Anim. Res. 2017, 46, 254–256. [Google Scholar] [CrossRef]
- Turcsán, B.; Tátrai, K.; Petró, E.; Topál, J.; Balogh, L.; Egyed, B.; Kubinyi, E. Comparison of Behavior and Genetic Structure in Populations of Family and Kenneled Beagles. Front. Vet. Sci. 2020, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Goleman, M.; Balicki, I.; Radko, A.; Jakubczak, A.; Fornal, A. Genetic diversity of the Polish Hunting Dog population based on pedigree analyses and molecular studies. Livest. Sci. 2019, 229, 114–117. [Google Scholar] [CrossRef]
- Van Asch, B.; Alves, C.; Gusmão, L.; Pereira, V.; Pereira, F.; Amorim, A. A new autosomal STR nineplex for canine iden-tification and parentage testing. Electrophoresis 2009, 30, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, V.; Stevanovic, J.; Savic, M.; Petrujkic, B.; Simeunovic, P.; Milosevic, I.; Stanimirovic, Z. Validation of 10 mi-crosatellite loci for their use in parentage verification and individual identification in the Yugoslavian Shepherd Dog Sharplanina. Ann. Anim. Sci. 2013, 13, 715–722. [Google Scholar] [CrossRef]
- ISAG Panel DOG. 2005. Available online: www.isag.us/Docs/2005ISAGPanelDOG.pdf (accessed on 1 July 2005).
- Goleman, M.; Balicki, I.; Radko, A.; Rozempolska-Rucińska, I.; Zięba, G. Pedigree and Molecular Analyses in the Assessment of Genetic Variability of the Polish Greyhound. Animals 2021, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Dog Way. Available online: https://dogway.pl/blog/p/najpopularniejsze-rasy-psow-w-polsce/ (accessed on 7 May 2019).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Res. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Reynolds, J.; Weir, B.S.; Cockerham, C.C. Eestimation of the coancestry coefficient: Basis for a short-term genetic distance. Genetics 1983, 105, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and genetic distance. Genetics 1974, 76, 379–390. [Google Scholar] [CrossRef]
- Wright, S. Evolution and the Genetics of Populations; University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Guo, S.W.; Thompson, E.A. Performing the Exact Test of Hardy-Weinberg Proportion for Multiple Alleles. Biometrics 1992, 48, 361. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Kimberly, A.H. Statistical analysis of STR data. Profiles DNA 1998, 1, 14–15. [Google Scholar]
- Paetkau, D.; Strobeck, C. Microsatellite analysis of genetic variation in black bear population. Mol. Ecol. 1994, 3, 189–195. [Google Scholar] [CrossRef]
- Jamieson, A.; Taylor, S.C.S. Comparisons of three probability formulae for parentage exclusion. Anim. Genet. 1997, 28, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Leroy, G. Genetic diversity, inbreeding and breeding practices in dogs: Results from pedigree analyses. Veter. J. 2011, 189, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Libiger, O.; Nievergelt, C.M.; Schork, N.J. Comparison of Genetic Distance Measures Using Human SNP Genotype Data. Hum. Biol. 2009, 81, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Veterinary Genetics Laboratory, UC Davis, in Collaboration with Dr. Niels C. Pedersen and Staff. Genetic Diversity Testing for Biewer. Available online: https://vgl.ucdavis.edu/canine-genetic-diversity/biewer (accessed on 1 January 2019).
- Buldog Francuski. Available online: http://www.piesporadnik.pl/title,pid,45,oid,47,-%20cid,176.html (accessed on 1 January 2020).
- Wictum, E.; Kun, T.; Lindquist, C.; Malvick, J.; Vankan, D.; Sacks, B. Developmental validation of DogFiler, a novel multiplex for canine DNA profiling in forensic casework. Forensic Sci. Int. Genet. 2013, 7, 82–91. [Google Scholar] [CrossRef]
- Tahir, M.S.; Hussain, T.; Babar, M.E.; Nadeem, A.; Naseer, M.; Ullah, Z.; Intizar, M.; Hussain, S.M. A panel of microsatellite markers for genetic diversity and parentage analysis of dog breeds in Pakistan. J. Anim. Plant Sci. 2015, 25, 351–356. [Google Scholar]
- Veterinary Genetics Laboratory, UC Davis, in Collaboration with Dr. Niels C. Pedersen and Staff. Davice Genetic Diversity Testing for Irish Wolfhounds. Available online: https://vgl.ucdavis.edu/canine-genetic-diversity/irish-wolfhound (accessed on 19 August 2019).
- Veterinary Genetics Laboratory, UC Davis. Statistics and Breed-Wide Allele Frequency—Irish Wolfhound. Report Issued August 19. Available online: https://vgl.ucdavis.edu/canine-genetic-diversity/irish-wolfhound/stats (accessed on 19 August 2019).
- Radko, A.; Słota, E. Application of 19 microsatellite DNA markers for parentage control in Borzoi dogs. Pol. J. Vet. Sci. 2009, 12, 113–117. [Google Scholar] [PubMed]
- Ciampolini, R.; Cecchi, F.; Bramante, A.; Casetti, F.; Presciuttini, S. Genetic variability of the Bracco Italiano dog breed based on microsatellite polymorphism. Ital. J. Anim. Sci. 2011, 10, 267–270. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Takagi, K.; Tsumagari, S.; Ishihama, K.; Morita, M. Test in based on microsatellite polymorphisms. J. Vet. Med. Sci. 2001, 63, 1209–1213. [Google Scholar] [CrossRef]
- Kang, B.-T.; Kim, K.-S.; Min, M.-S.; Chae, Y.-J.; Kang, J.-W.; Yoon, J.; Choi, J.; Seong, J.-K.; Park, H.-C.; An, J.; et al. Microsatellite loci analysis for the genetic variability and the parentage test of five dog breeds in South Korea. Genes Genet. Syst. 2009, 84, 245–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eichmann, C.; Berger, B.; Parson, W. Relevant aspects for forensic STR analysis of canine DNA: Repeat-based nomenclature and sensitive PCR multiplexes. Int. Congr. Ser. 2006, 1288, 813–815. [Google Scholar] [CrossRef]
- Boonderm, N.; Suriyanratakorn, D.; Sangpueng, S.; Onthong, N.; Nettakul, A.; Waiyawuth, W. Population genetic data of 21 STR markers in Thais of southern border provinces of Thailand. For. Sci. Int. Genet. 2017, 6, 523–525. [Google Scholar] [CrossRef][Green Version]
- Waits, L.P.; Luikart, G.; Taberlet, P. Estimating the probability of identity among genotypes in natural populations: Cautions and guidelines. Mol. Ecol. 2001, 10, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, C.; Berger, B.; Steinlechner, M.; Parson, W. Estimating the probability of identity in a random dog population using 15 highly polymorphic canine STR markers. Forensic Sci. Int. 2005, 151, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Radko, A.; Słota, E.; Kościelny, M. Polymorphism of 10 microsatellites and their usefulness for paternity control in dogs. In Biotechnology, Agriculture and the Food Industry; Zaikov, G.E., Ed.; Nova Scie. Publishers: New York, NY, USA, 2006; pp. 141–144. [Google Scholar]
- Dodd, J.N.; Morris, B.G.; Oliveira, D.A.A.; Bernoco, D. DNA testing for parentage verification and individual identification in seven breeds of dogs. Rev. Bras. Reprod. Anim. 2001, 25, 35–41. [Google Scholar]

| Breed | FB | GR | LR | M | GS | IW | YT | BYT |
|---|---|---|---|---|---|---|---|---|
| BF | 0.000 | |||||||
| GR | 0.540 | 0.000 | ||||||
| LR | 0.469 | 0.577 | 0.000 | |||||
| M | 0.409 | 0.501 | 0.459 | 0.000 | ||||
| GS | 0.519 | 0.596 | 0.549 | 0.459 | 0.000 | |||
| IW | 0.574 | 0.606 | 0.588 | 0.539 | 0.634 | 0.000 | ||
| YT | 0.430 | 0.503 | 0.465 | 0.364 | 0.484 | 0.500 | 0.000 | |
| BYT | 0.435 | 0.511 | 0.485 | 0.372 | 0.511 | 0.510 | 0.260 | 0.0000 |
| Locus | GS | M | IW | BYT | YT | GR | LR | FB | N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Ae | A | Ae | A | Ae | A | Ae | A | Ae | A | Ae | A | Ae | A | Ae | ||
| AHT121 | 7 | 1.8 | 9 | 4.9 | 5 | 3.1 | 9 | 4.5 | 8 | 5.2 | 6 | 2.4 | 7 | 2.4 | 8 | 4.6 | 11 |
| AHT137 | 7 | 2.0 | 6 | 4.5 | 2 | 1.2 | 9 | 4.1 | 7 | 3.4 | 5 | 4.5 | 9 | 3.2 | 7 | 4.0 | 12 |
| AHTh171 | 7 | 2.7 | 9 | 2.5 | 5 | 2.4 | 5 | 1.6 | 9 | 4.7 | 3 | 2.7 | 7 | 3.8 | 4 | 2.4 | 11 |
| AHTh260 | 8 | 2.6 | 6 | 4.0 | 3 | 1.6 | 8 | 3.5 | 6 | 3.7 | 4 | 1.4 | 9 | 3.1 | 6 | 2.5 | 10 |
| AHTk211 | 4 | 2.8 | 5 | 3.4 | 3 | 1.9 | 6 | 2.9 | 5 | 1.6 | 4 | 2.0 | 6 | 2.1 | 4 | 2.0 | 6 |
| AHTk253 | 6 | 1.5 | 5 | 2.5 | 3 | 1.9 | 6 | 2.8 | 5 | 3.3 | 3 | 1.8 | 5 | 2.0 | 4 | 3.2 | 7 |
| CXX279 | 5 | 2.8 | 4 | 3.4 | 5 | 2.2 | 8 | 3.5 | 8 | 3.5 | 4 | 1.3 | 8 | 3.1 | 5 | 2.6 | 8 |
| FH2054 | 6 | 3.3 | 8 | 5.3 | 5 | 2.4 | 6 | 3.9 | 6 | 3.2 | 6 | 3.4 | 7 | 2.7 | 8 | 4.0 | 8 |
| FH2848 | 6 | 2.2 | 6 | 2.2 | 3 | 2.2 | 7 | 3.1 | 5 | 3.3 | 3 | 1.8 | 4 | 3.9 | 5 | 2.8 | 8 |
| INRA21 | 6 | 3.0 | 5 | 3.8 | 4 | 2.9 | 6 | 3.8 | 6 | 3.5 | 4 | 2.6 | 5 | 3.1 | 5 | 1.4 | 8 |
| INU005 | 4 | 2.3 | 5 | 1.5 | 3 | 2.4 | 6 | 1.3 | 8 | 3.2 | 5 | 3.5 | 5 | 2.0 | 5 | 2.8 | 9 |
| INU030 | 5 | 2.1 | 6 | 2.6 | 2 | 1.7 | 6 | 2.5 | 7 | 3.2 | 3 | 2.7 | 4 | 1.8 | 3 | 1.8 | 7 |
| INU055 | 5 | 3.0 | 5 | 3.8 | 4 | 1.7 | 6 | 2.6 | 6 | 2.6 | 4 | 2.3 | 5 | 1.9 | 2 | 2.0 | 7 |
| REN162C04 | 5 | 2.4 | 6 | 1.9 | 3 | 2.1 | 7 | 3.4 | 7 | 3.4 | 4 | 1.6 | 5 | 2.1 | 3 | 1.7 | 8 |
| REN169D01 | 3 | 1.9 | 8 | 3.7 | 2 | 1.1 | 5 | 2.9 | 6 | 3.1 | 4 | 3.6 | 7 | 3.3 | 4 | 3.0 | 8 |
| REN169O18 | 10 | 4.3 | 5 | 2.4 | 4 | 2.1 | 6 | 2.4 | 5 | 3.2 | 6 | 3.3 | 5 | 2.3 | 5 | 3.1 | 12 |
| REN247M23 | 4 | 1.5 | 5 | 3.3 | 3 | 2.2 | 4 | 3.4 | 4 | 3.4 | 5 | 1.6 | 3 | 1.3 | 4 | 2.7 | 7 |
| REN54P11 | 5 | 2.1 | 8 | 5.4 | 3 | 1.9 | 7 | 4.4 | 6 | 3.8 | 7 | 3.7 | 6 | 2.9 | 6 | 3.0 | 9 |
| AHTh130 | 7 | 3.8 | 10 | 5.1 | 2 | 1.3 | 8 | 3.5 | 5 | 3.0 | 3 | 2.6 | 6 | 3.9 | 8 | 3.6 | 10 |
| REN105L03 | 8 | 2.3 | 7 | 4.7 | 4 | 2.6 | 7 | 3.9 | 5 | 4.0 | 5 | 4.0 | 4 | 1.5 | 5 | 3.0 | 10 |
| REN64E19 | 7 | 1.2 | 7 | 2.2 | 4 | 2.8 | 6 | 3.2 | 7 | 3.7 | 4 | 2.7 | 5 | 3.2 | 5 | 4.2 | 9 |
| N | 125 | 135 | 72 | 138 | 131 | 92 | 122 | 106 | 185 | ||||||||
| A | 5.9 | 6.4 | 3.4 | 6.6 | 6.2 | 4.4 | 5.8 | 5.1 | |||||||||
| Ae | 3.3 | 3.5 | 2.1 | 3.2 | 3.5 | 2.6 | 2.6 | 2.9 | |||||||||
| Locus | Allele (bp) | GS | M | BYT | YT | GR | LR | FB |
|---|---|---|---|---|---|---|---|---|
| ATH121 | 92 | 0.031 | ||||||
| ATH137 | 127 | 0.0130 | ||||||
| AHTH260 | 256 | 0.0243 | ||||||
| AHTK253 | 296 | 0.0354 | ||||||
| CXX279 | 128 | 0.1641 * | 0.1753 * | |||||
| FH2848 | 234 | 0.0208 | ||||||
| INRA21 | 109 | 0.0185 | ||||||
| INU005 | 134 | 0.0454 | ||||||
| REN162C04 | 212 | 0.1982 | ||||||
| REN169O18 | 156 | 0.1197 | ||||||
| 158 | 0.2168 | |||||||
| 176 | 0.0097 | |||||||
| 178 | 0.0044 | |||||||
| REN247M23 | 274 | 0.0128 | ||||||
| 276 | 0.0146 | |||||||
| REN54P11 | 240 | 0.0062 | ||||||
| AHTH130 | 117 | 0.0123 | ||||||
| REN105L03 | 229 | 0.2179 | ||||||
| 245 | 0.0079 | |||||||
| REN64E19 | 159 | 0.0115 |
| Breed | HO | HE | FIS | p-Value | PIC | CPD | CPID | CPE1 | CPE2 |
|---|---|---|---|---|---|---|---|---|---|
| GS | 0.5451 | 0.5541 | 0.0171 | 0.4840 | 0.4941 | 1 * | 1.80 × 10−13 | 0.985991 | 0.9997326 |
| M | 0.6855 | 0.6771 | −0.0127 | 0.4907 | 0.6398 | 1 * | 4.47 × 10−19 | 0.999443 | 0.9999987 |
| IW | 0.4911 | 0.4743 | −0.0373 | 0.3952 | 0.4139 | 1 * | 6.71 × 10−11 | 0.949242 | 0.997768 |
| BYT | 0.6608 | 0.6581 | −0.0041 | 0.3425 | 0.6166 | 1 * | 6.82 × 10−18 | 0.99875 | 0.999996 |
| YT | 0.6623 | 0.6981 | 0.0533 | 0.3150 | 0.6545 | 1 * | 2.34 × 10−19 | 0.999495 | 0.999999 |
| GR | 0.5922 | 0.5620 | −0.0490 | 0.4414 | 0.5135 | 1 * | 3.37 × 10−14 | 0.99010 | 0.999875 |
| LR | 0.5954 | 0.5886 | −0.0088 | 0.2813 | 0.5429 | 1 * | 4.38 × 10−15 | 0.99316 | 0.999941 |
| FB | 0.6077 | 0.6177 | 0.0173 | 0.5395 | 0.5602 | 1 * | 8.65 × 10−16 | 0.99601 | 0.999964 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radko, A.; Podbielska, A. Microsatellite DNA Analysis of Genetic Diversity and Parentage Testing in the Popular Dog Breeds in Poland. Genes 2021, 12, 485. https://doi.org/10.3390/genes12040485
Radko A, Podbielska A. Microsatellite DNA Analysis of Genetic Diversity and Parentage Testing in the Popular Dog Breeds in Poland. Genes. 2021; 12(4):485. https://doi.org/10.3390/genes12040485
Chicago/Turabian StyleRadko, Anna, and Angelika Podbielska. 2021. "Microsatellite DNA Analysis of Genetic Diversity and Parentage Testing in the Popular Dog Breeds in Poland" Genes 12, no. 4: 485. https://doi.org/10.3390/genes12040485
APA StyleRadko, A., & Podbielska, A. (2021). Microsatellite DNA Analysis of Genetic Diversity and Parentage Testing in the Popular Dog Breeds in Poland. Genes, 12(4), 485. https://doi.org/10.3390/genes12040485






