Genetic Risk Profiling Associated with Recurrent Unprovoked Venous Thromboembolism
Abstract
:1. Introduction
2. Patients and Methods
2.1. Blood Collection, DNA Extraction and Genotyping
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansson, P.O.; Sörbo, J.; Eriksson, H. Recurrent venous thromboembolism after deep vein thrombosis: Incidence and risk factors. Arch. Intern. Med. 2000, 160, 769–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prandoni, P.; Noventa, F.; Ghirarduzzi, A.; Pengo, V.; Bernardi, E.; Pesavento, R.; Iotti, M.; Tormene, D.; Simioni, P.; Pagnan, A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. Haematologica 2007, 92, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Rosendaal, F.R. Venous thrombosis: A multicausal disease. Lancet 1999, 353, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Gemmati, D.; Serino, M.L.; Moratelli, S.; Mari, R.; Ballerini, G.; Scapoli, G.L. Coexistence of antithrombin deficiency, factor V Leiden and hyperhomocysteinemia in a thrombotic family. Blood Coagul. Fibrinolysis 1998, 9, 173–176. [Google Scholar] [CrossRef]
- Gemmati, D.; Serino, M.L.; Moratelli, S.; Tognazzo, S.; Ongaro, A.; Scapoli, G.L. Coexistence of Factor V G1691A and Factor II G20210A Gene Mutations in a Thrombotic Family Is Associated with Recurrence and Early Onset of Venous Thrombosis. Haemostasis 2001, 31, 99–105. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Reitsma, P.H. Genetics of venous thrombosis. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 301–304. [Google Scholar] [CrossRef]
- Boutitie, F.; Pinede, L.; Schulman, S.; Agnelli, G.; Raskob, G.; Julian, J.; Hirsh, J.; Kearon, C. Influence of preceding length of antico-agulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: Analysis of individual participants’ data from seven trials. BMJ 2011, 342, d3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palareti, G.; Leali, N.; Coccheri, S.; Poggi, M.; Manotti, C.; D’Angelo, A.; Pengo, V.; Erba, N.; Moia, M.; Ciavarella, N.; et al. Bleeding complications of oral anticoagulant treatment: An inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996, 348, 423–428. [Google Scholar] [CrossRef]
- Kearon, C.; Gent, M.; Hirsh, J.; Weitz, J.; Kovacs, M.J.; Anderson, D.R.; Turpie, A.G.; Green, D.; Ginsberg, J.S.; Wells, P.; et al. A Comparison of Three Months of Anticoagulation with Extended Anticoagulation for a First Episode of Idiopathic Venous Thromboembolism. N. Engl. J. Med. 1999, 340, 901–907. [Google Scholar] [CrossRef]
- Fahrni, J.; Husmann, M.; Gretener, S.B.; Keo, H.H. Assessing the risk of recurrent venous thromboembolism—A practical approach. Vasc. Health Risk Manag. 2015, 11, 451–459. [Google Scholar]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3080. [Google Scholar]
- Souto, J.C.; Almasy, L.; Borrell, M.; Blanco-Vaca, F.; Mateo, J.; Soria, J.M.; Coll, I.; Felices, R.; Stone, W.; Fontcuberta, J.; et al. Genetic Susceptibility to Thrombosis and Its Relationship to Physiological Risk Factors: The GAIT Study. Genetic analysis of idiopathic thrombophilia. Am. J. Hum. Genet. 2000, 67, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoller, B.; Ohlsson, H.; Sundquist, J.; Sundquist, K. A sibling based design to quantify genetic and shared environmental effects of venous thromboembolism in Sweden. Thromb. Res. 2017, 149, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Coppens, M.; Reijnders, J.H.; Middeldorp, S.; Doggen, C.J.M.; Rosendaal, F.R. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J. Thromb. Haemost. 2008, 6, 1474–1477. [Google Scholar] [CrossRef]
- Zee, R.Y.; Bubes, V.; Shrivastava, S.; Ridker, P.M.; Glynn, R.J. Genetic risk factors in recurrent venous thromboembolism: A multilocus, population-based, prospective approach. Clin. Chim. Acta 2009, 402, 189–192. [Google Scholar] [CrossRef] [Green Version]
- van Hylckama Vlieg, A.; Baglin, C.A.; Bare, L.A.; Rosendaal, F.R.; Baglin, T.P. Proof of principle of potential clinical utility of multiple SNP analysis for prediction of recurrent venous thrombosis. J. Thromb. Haemost. 2008, 6, 751–754. [Google Scholar] [CrossRef]
- van Hylckama Vlieg, A.; Flinterman, L.E.; Bare, L.A.; Cannegieter, S.C.; Reitsma, P.H.; Arellano, A.R.; Tong, C.H.; Devlin, J.J.; Rosendaal, F.R. Genetic variations associated with recurrent venous thrombosis. Circ. Cardiovasc. Genet. 2014, 7, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Baglin, T.; Luddington, R.; Brown, K.; Baglin, C. Incidence of recurrent venous thromboembolism in relation to clinical and throm-bophilic risk factors: Prospective cohort study. Lancet 2003, 362, 523–526. [Google Scholar] [CrossRef]
- Piran, S.; Schulman, S. Treatment of bleeding complications in patients on anticoagulant therapy. Blood 2019, 133, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearon, C. A conceptual framework for two phases of anticoagulant treatment of venous thromboembolism. J. Thromb. Haemost. 2012, 10, 507–511. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014, 123, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.A.; Bentley, D.P.; Prescott, R.J.; Routledge, P.A.; Shetty, H.G.M.; Williamson, I.J. Anticoagulation for three versus six months in patients with deep vein thrombosis or pulmonary embolism, or both: Randomised trial. BMJ 2007, 334, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnelli, G.; Prandoni, P.; Becattini, C.; Silingardi, M.; Taliani, M.R.; Miccio, M.; Imberti, D.; Poggio, R.; Ageno, W.; Pogliani, E.M.; et al. Extended Oral Anticoagulant Therapy after a First Episode of Pulmonary Embolism. Ann. Intern. Med. 2003, 139, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.N.; Hirsh, J.; Gent, M.; Turpie, A.G.; Weitz, J.; Ginsberg, J.; Geerts, W.; Leclerc, J.; Neemeh, J.; Powers, P.; et al. Optimal Duration of Oral Anticoagulant Therapy: A Randomized Trial Comparing Four Weeks with Three Months of Warfarin in Patients with Proximal Deep Vein Thrombosis. Thromb. Haemost. 1995, 74, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Rhedin, A.-S.; Lindmarker, P.; Carlsson, A.; Lärfars, G.; Nicol, P.; Loogna, E.; Svensson, E.; Ljungberg, B.; Walter, H.; et al. A Comparison of Six Weeks with Six Months of Oral Anticoagulant Therapy after a First Episode of Venous Thromboembolism. N. Engl. J. Med. 1995, 332, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Ginsberg, J.S.; Anderson, D.R.; Kovacs, M.J.; Wells, P.; Julian, J.A.; MacKinnon, B.; Demers, C.; Douketis, J.; Turpie, A.G.; et al. Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J. Thromb. Haemost. 2004, 2, 743–749. [Google Scholar] [CrossRef]
- de Haan, H.G.; Bezemer, I.D.; Doggen, C.J.; Le Cessie, S.; Reitsma, P.H.; Arellano, A.R.; Tong, C.H.; Devlin, J.J.; Bare, L.A.; Rosendaal, F.R.; et al. Multiple SNP testing improves risk prediction of first venous thrombosis. Blood 2012, 120, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Emmerich, J.; Rosendaal, F.R.; Cattaneo, M.; Margaglione, M.; De Stefano, V.; Cumming, T.; Arruda, V.; Hillarp, A.; Reny, J.L. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism–pooled analysis of 8 casecontrol studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb. Haemost. 2001, 86, 809–816. [Google Scholar]
- Souto, J.C.; Almasy, L.; Muniz-Diaz, E.; Soria, J.M.; Borrell, M.; Bayén, L.; Mateo, J.; Madoz, P.; Stone, W.; Blangero, J.; et al. Func-tional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial throm-boplastin time. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2024–2028. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.H.; Haff, E.; Platt, S.J.; Rawlins, P.; Drews, C.D.; Dilley, A.B.; Evatt, B. Measurement of von Willebrand factor activity: Relative effects of ABO blood type and race. J. Thromb. Haemost. 2003, 1, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Salomon, O.; Steinberg, D.M.; Zucker, M.; Varon, D.; Zivelin, A.; Seligsohn, U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb. Haemost. 2011, 105, 269–273. [Google Scholar] [CrossRef]
- Meijers, J.C.; Tekelenburg, W.L.; Bouma, B.N.; Bertina, R.M.; Rosendaal, F.R. High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. N. Engl. J. Med. 2000, 342, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Uitte de Willige, S.; de Visser, M.C.H.; Houwing-Duistermaat, J.J.; Rosendaal, F.R.; Vos, H.L.; Bertina, R.M. Genetic variation in the fi-brinogen gamma gene increases the risk for deep venous thrombosis by reducing plasma fibrinogen gamma’ levels. Blood 2005, 106, 4176–4183. [Google Scholar] [CrossRef]
- Ahmad, A.; Sundquist, K.; Palmér, K.; Svensson, P.J.; Sundquist, J.; Memon, A.A. Risk prediction of recurrent venous throm-boembolism: A multiple genetic risk model. J. Thromb. Thrombolysis 2019, 47, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stefano, V.; Martinelli, I.; Mannucci, P.M.; Paciaroni, K.; Chiusolo, P.; Casorelli, I.; Rossi, E.; Leone, G. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N. Engl. J. Med. 1999, 341, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Simioni, P.; Prandoni, P.; Lensing, A.W.A.; Manfrin, D.; Tormene, D.; Gavasso, S.; Girolami, B.; Sardella, C.; Prins, M.; Girolami, A. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000, 96, 3329–3333. [Google Scholar]
- Lindmarker, P.; Schulman, S.; Sten-Linder, M.; Wiman, B.; Egberg, N.; Johnsson, H. DURAC (Duration of Anticoagulation) Trial Study Group. The Risk of Recurrent Venous Thromboembolism in Carriers and Non-carriers of the G1691A Allele in the Coagulation Factor V Gene and the G20210A Allele in the Prothrombin Gene. Thromb. Haemost. 1999, 81, 684–689. [Google Scholar]
- Santamaria, M.G.; Agnelli, G.; Taliani, M.R.; Prandoni, P.; Moia, M.; Bazzan, M.; Guazzaloca, G.; Ageno, W.; Bertoldi, A.; Silingardi, M.; et al. Thrombophilic abnormalities and recurrence of venous thromboembolism in patients treated with standardized anticoagulant treatment. Thromb. Res. 2005, 116, 301–306. [Google Scholar] [CrossRef]
- Lijfering, W.M.; Middeldorp, S.; Veeger, N.J.G.M.; Hamulyák, K.; Prins, M.H.; Büller, H.R.; van der Meer, J. Risk of Recurrent Venous Thrombosis in Homozygous Carriers and Double Heterozygous Carriers of Factor V Leiden and Prothrombin G20210A. Circulation 2010, 20, 1706–1712. [Google Scholar] [CrossRef] [Green Version]

| Total (n = 224) | Recurrence (n = 58) | Non Recurrence (n = 166) | Test of Sig. | p | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 108 (48.2%) | 28 (48.3%) | 80 (48.2%) | χ2 = 0.0 | 0.991 |
| Female | 116 (51.8%) | 30 (51.7%) | 86 (51.8%) | ||
| Age (years) | |||||
| Mean ± SD. | 51.7 ± 6 | 52.1 ± 6.3 | 51.6 ± 6 | t = 0.455 | 0.650 |
| Median (Min.–Max.) | 50 (41–66) | 51 (41–66) | 50 (42–66) | ||
| Total (n = 224) | Recurrence (n = 58) | Non Recurrence (n = 166) | χ2 | p | |
|---|---|---|---|---|---|
| ABO | |||||
| O | 71 (31.7%) | 15 (25.9%) | 56 (33.7%) | χ2 = 24.189 * | <0.001 * |
| A | 67 (29.9%) | 15 (25.9%) | 52 (31.3%) | ||
| B | 60 (26.8%) | 11 (19%) | 49 (29.5%) | ||
| AB | 26 (11.6%) | 17 (29.3%) | 9 (5.4%) | ||
| Non O | 153 (68.3%) | 43 (74.1%) | 110(66.3%) | χ2 = 1.231 | 0.267 |
| FVR506 Q | |||||
| RR | 163 (72.8%) | 35 (60.3%) | 128 (77.1%) | 7.684 * | MCp = 0.011 * |
| RQ | 60 (26.8%) | 22 (37.9%) | 38 (22.9%) | ||
| 1 (0.4%) | 1 (1.7%) | 0 (0%) | |||
| RQ + QQ | 61(27.2%) | 23(39.7%) | 38 (22.9%) | 6.095 * | 0.014 * |
| FIIG20210 A | |||||
| GG | 199 (88.8%) | 49 (84.5%) | 150 (90.4%) | 1.498 | 0.221 |
| GA | 25 (11.2%) | 9 (15.5%) | 16 (9.6%) | ||
| AA | 0(0%) | 0(0%) | 0(0%) | ||
| GA + AA | 25 (11.2%) | 9 (15.5%) | 16 (9.6%) | 1.498 | 0.221 |
| FG G | |||||
| CC | 134 (59.8%) | 30 (51.7%) | 104 (62.7%) | 5.257 | 0.072 |
| CT | 68 (30.4%) | 18 (31%) | 50 (30.1%) | ||
| TT | 22 (9.8%) | 10 (17.2%) | 12 (7.2%) | ||
| CT + TT | 90(40.2%) | 28(48.3%) | 62(37.3%) | 2.135 | 0.144 |
| FX I | |||||
| TT | 112 (50%) | 26 (44.8%) | 86 (51.8%) | 8.473 * | 0.014 * |
| CT | 91 (40.6%) | 21 (36.2%) | 70 (42.2%) | ||
| CC | 21 (9.4%) | 11 (19%) | 10 (6%) | ||
| CT + CC | 112(50%) | 32(55.2%) | 80(48.2%) | 0.838 | 0.360 |
| Total (n = 224) | Recurrence (n = 58) | Non Recurrence (n = 166) | χ2 | p | |
|---|---|---|---|---|---|
| ABO | |||||
| Allele | |||||
| O | 269(60%) | 56(48.3%) | 213(64.2%) | χ2 = 9.116 * | 0.010 * |
| Non O | 179 (40%) | 60 (51.7%) | 119 (35.8%) | ||
| FVR506Q | |||||
| Allele | |||||
| R | 386(86.2%) | 92(79.3%) | 294(88.6%) | 6.160 * | 0.013 * |
| Q | 62(13.8%) | 24(20.7%) | 38(11.4%) | ||
| FIIG20210A | |||||
| Allele | |||||
| G | 423(94.4%) | 107(92.2%) | 316(95.2%) | 1.410 | 0.235 |
| A | 25(5.6%) | 9(7.8%) | 16(4.8%) | ||
| FGG | |||||
| Allele | |||||
| C | 336(75%) | 78(67.2%) | 258(77.7%) | 5.025 * | 0.025 * |
| T | 112(25%) | 38(32.8%) | 74(22.3%) | ||
| FXI | |||||
| Allele | |||||
| T | 315(70.3%) | 73(62.9%) | 242(72.9%) | 4.086 * | 0.043 * |
| C | 133(29.7%) | 43(37.1%) | 90(27.1%) | ||
| Total (n = 224) | Recurrence (n = 58) | Non Recurrence (n = 166) | Test of Sig. | p | |
|---|---|---|---|---|---|
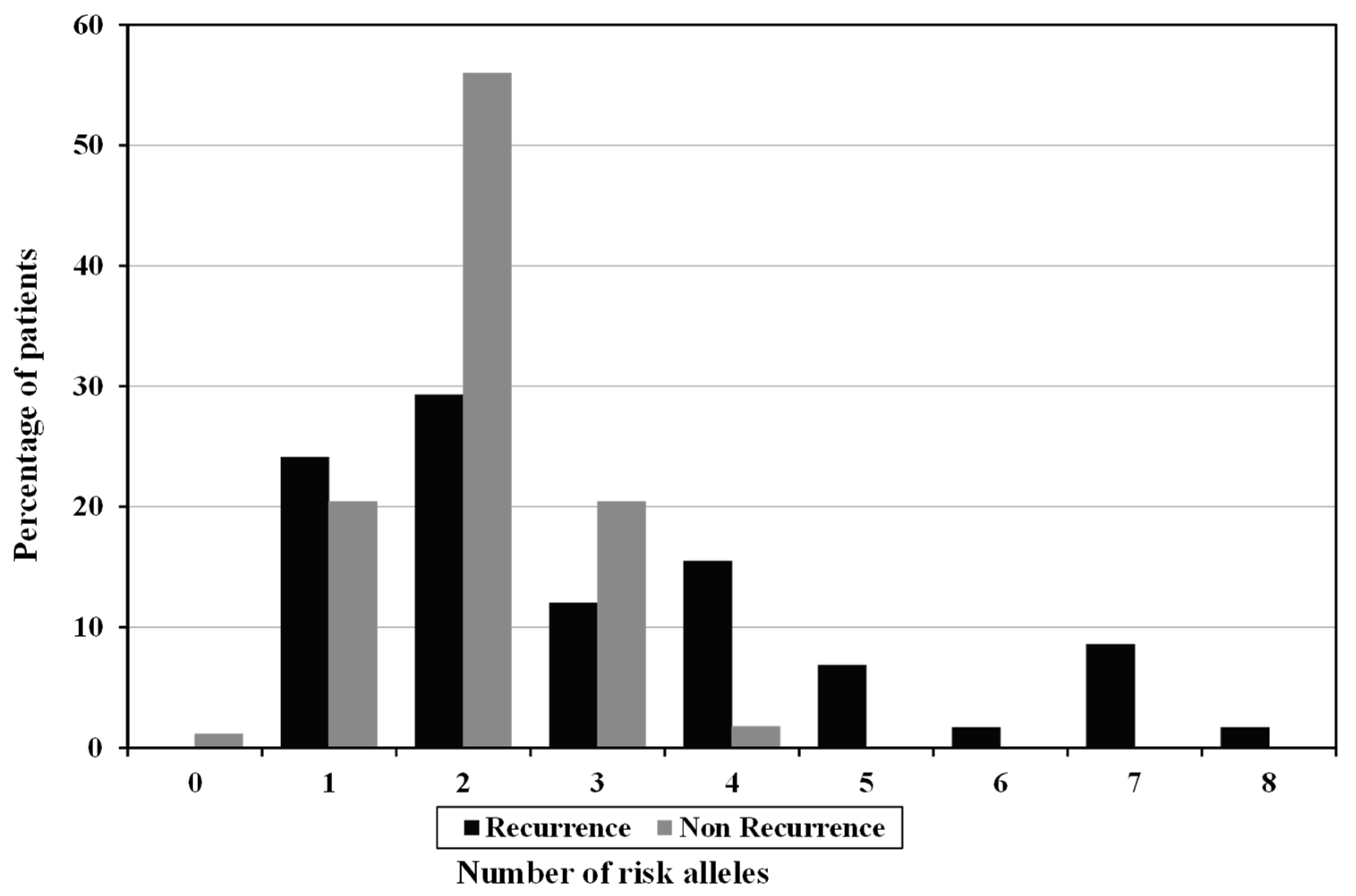
| No of risk Allele (GRS) | |||||
| Low risk (0–2) | 160(71.4%) | 31(53.4%) | 129(77.7%) | χ2 = 12.398 * | <0.001 * |
| High risk (3–8) | 64(28.6%) | 27(46.6%) | 37(22.3%) | ||
| Mean ± SD. | 2.3 ± 1.2 | 3 ± 1.9 | 2 ± 0.7 | U = 3631.0 * | 0.003 * |
| Median (Min.–Max.) | 2 (0–8) | 2 (1–8) | 2 (0–4) | ||
| SNP | p | HR (95% CI) | p | HR # (95% CI) | |
|---|---|---|---|---|---|
| FV | rs6025 | 0.019 * | 1.877(1.109–3.178) | 0.015 * | 1.951(1.141–3.338) |
| FII | rs1799963 | 0.198 | 1.595(0.784–3.248) | 0.207 | 1.593(0.773–3.284) |
| FGG | rs2066865 | 0.128 | 1.492(0.892–2.498) | 0.137 | 1.482(0.883–2.487) |
| ABO | rs8176719 | 0.239 | 1.424(0.791–2.563) | 0.232 | 1.431(0.795–2.577) |
| FXI | rs2036914 | 0.432 | 1.230(0.733–2.064) | 0.416 | 1.241(0.738–2.085) |
| GRS (≥3) | 0.001 * | 2.472(1.475–4.145) | 0.001 * | 2.503(1.486–4.217) |
| GRS | Mean | % | Log Rank | |
|---|---|---|---|---|
| χ2 | p | |||
| Low risk (0–2) | 52.163 | 80.6 | 12.657 * | <0.001 * |
| High risk (3–8) | 43.078 | 57.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodeib, H.; Youssef, A.; Allam, A.A.; Selim, A.; Tawfik, M.A.; Abosamak, M.F.; Esam, A.; Abd Elghafar, M.S.; Samir, S.; ELshora, O.A. Genetic Risk Profiling Associated with Recurrent Unprovoked Venous Thromboembolism. Genes 2021, 12, 874. https://doi.org/10.3390/genes12060874
Hodeib H, Youssef A, Allam AA, Selim A, Tawfik MA, Abosamak MF, Esam A, Abd Elghafar MS, Samir S, ELshora OA. Genetic Risk Profiling Associated with Recurrent Unprovoked Venous Thromboembolism. Genes. 2021; 12(6):874. https://doi.org/10.3390/genes12060874
Chicago/Turabian StyleHodeib, Hossam, Amira Youssef, Alzahraa A. Allam, Amal Selim, Mohamed A. Tawfik, Mohammed F. Abosamak, Ahmed Esam, Mohamed S. Abd Elghafar, Sameh Samir, and Ola A. ELshora. 2021. "Genetic Risk Profiling Associated with Recurrent Unprovoked Venous Thromboembolism" Genes 12, no. 6: 874. https://doi.org/10.3390/genes12060874






