A Decade in Review after Idiopathic Scoliosis Was First Called a Complex Trait—A Tribute to the Late Dr. Yves Cotrel for His Support in Studies of Etiology of Scoliosis
Abstract
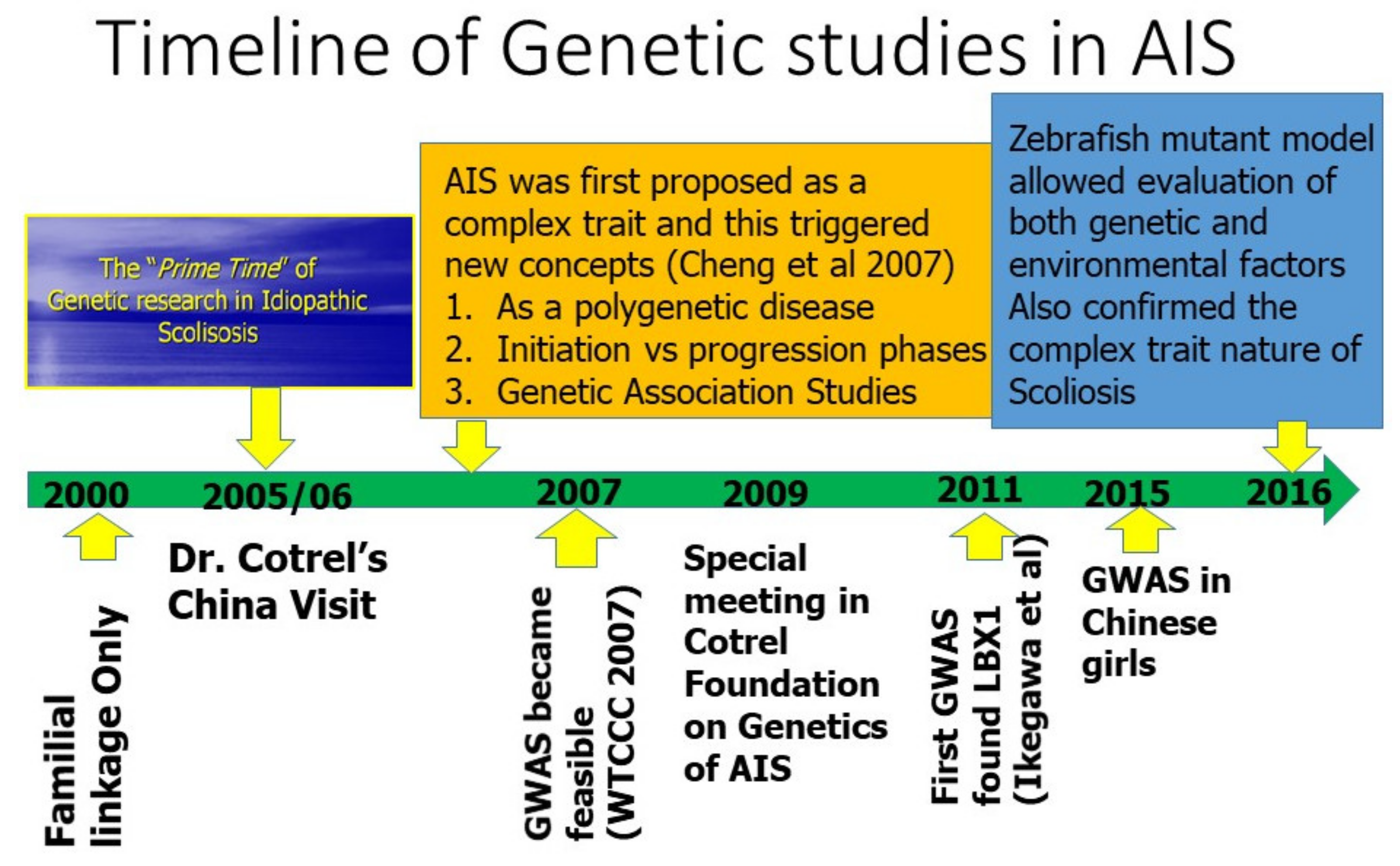
:1. Setting the Scene
2. AIS Was First Considered as a Complex Trait
3. A High Heritability of Liability to AIS
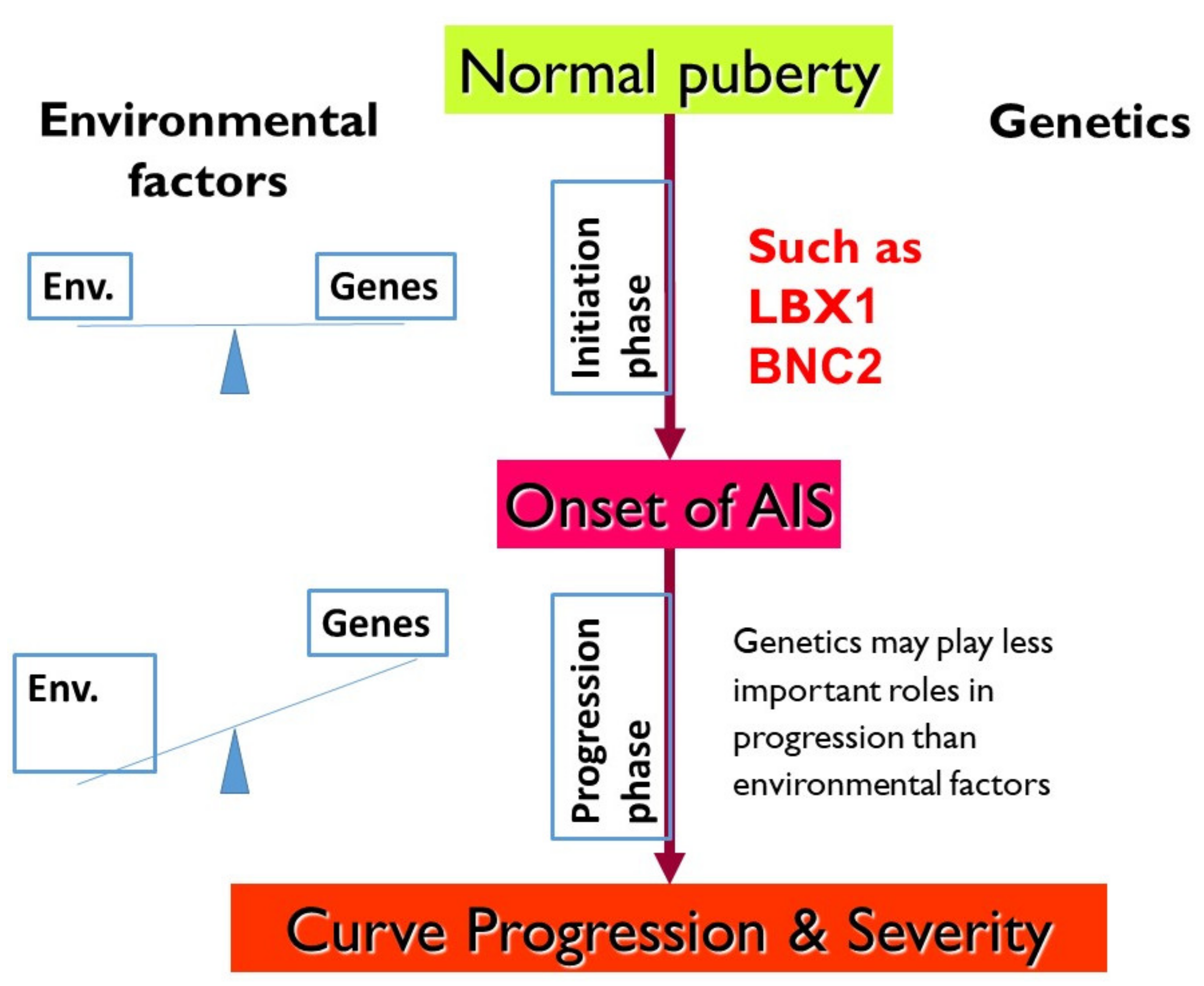
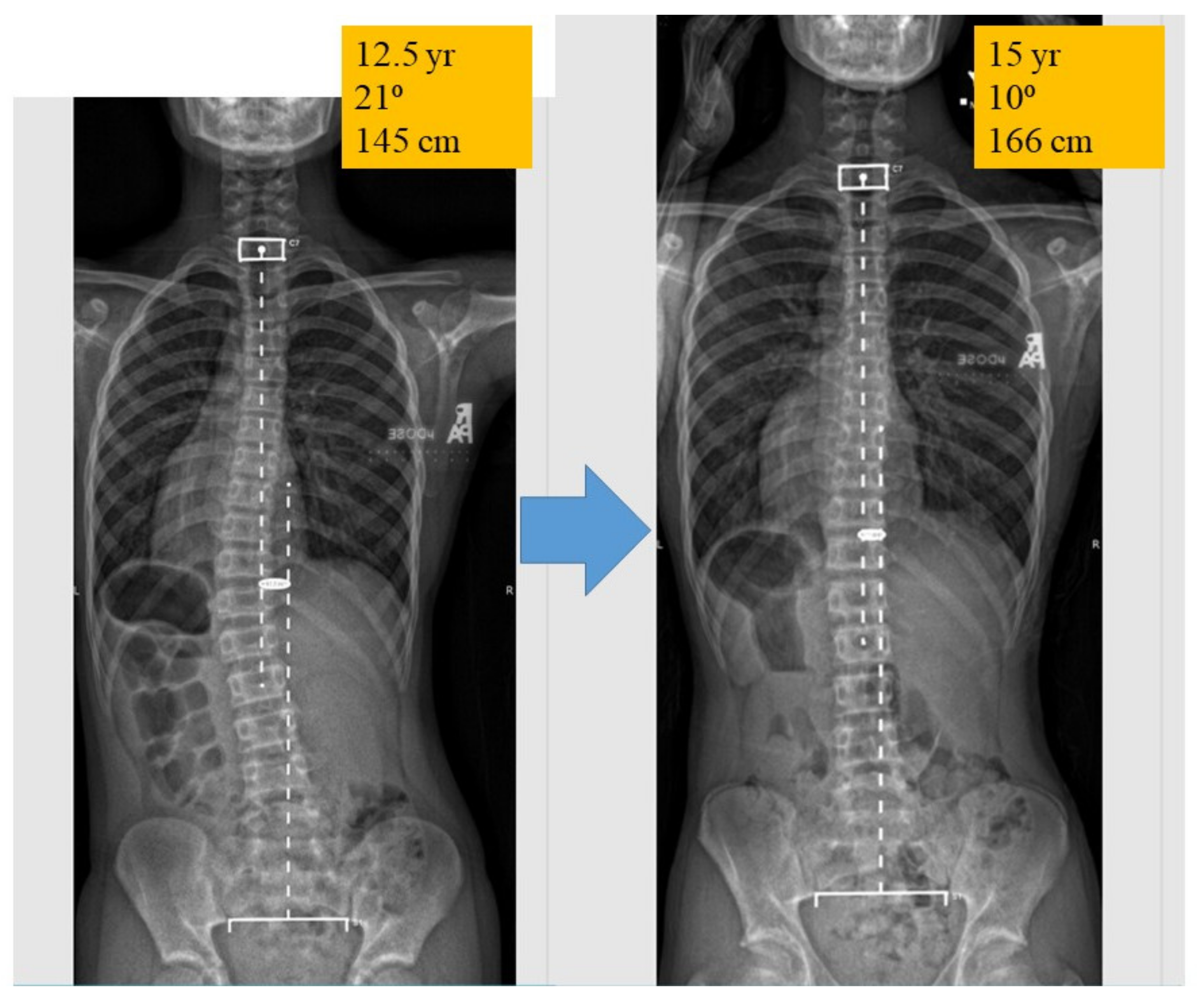
4. AIS Delineated into 2 Phases: Initiation and Progression
5. Genetic Association Study in AIS and GWAS
6. LBX1 as the First Confirmed AIS Genetic Predisposition Locus
7. BNC2 Is Another Replicated AIS Locus Related to Muscle Development
8. Extracellular Matrix and Fibers
9. Fish Studies Confirmed Scoliosis as a Complex Trait
10. Discussion and Looking Forward
10.1. What Genetic Studies Inform about the Etiology of Scoliosis?
10.2. What Should Be the Future Research Direction?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Génétique—Les projets soutenus. Fond. Yves Cotrel. Inst. Fr. Available online: https://www.fondationcotrel.org/genetique-les-projets-soutenus/ (accessed on 4 May 2021).
- Axenovich, T.I.; Zaidman, A.M.; Zorkoltseva, I.V.; Tregubova, I.L.; Borodin, P.M. Segregation analysis of idiopathic scoliosis: Demonstration of a major gene effect. Am. J. Med. Genet. 1999, 86, 389–394. [Google Scholar] [CrossRef]
- Ogilvie, J.W.; Braun, J.; Argyle, V.; Nelson, L.; Meade, M.; Ward, K. The search for idiopathic scoliosis genes. Spine 2006, 31, 679–681. [Google Scholar] [CrossRef]
- Cheng, J.C.Y.; Tang, N.L.S.; Yeung, H.-Y.; Miller, N. Genetic association of complex traits: Using idiopathic scoliosis as an example. Clin. Orthop. 2007, 462, 38–44. [Google Scholar] [CrossRef]
- Cheng, J.C.; Castelein, R.M.; Chu, W.C.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primer 2015, 1, 15068. [Google Scholar] [CrossRef]
- Miller, N.H.; Justice, C.M.; Marosy, B.; Doheny, K.F.; Pugh, E.; Zhang, J.; Dietz, H.C.; Wilson, A.F. Identification of candidate regions for familial idiopathic scoliosis. Spine 2005, 30, 1181–1187. [Google Scholar] [CrossRef]
- Justice, C.M.; Miller, N.H.; Marosy, B.; Zhang, J.; Wilson, A.F. Familial idiopathic scoliosis: Evidence of an X-linked susceptibility locus. Spine 2003, 28, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Gordon, D.; Zhang, D.; Browne, R.; Helms, C.; Gillum, J.; Weber, S.; Devroy, S.; Swaney, S.; Dobbs, M.; et al. CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. Am. J. Hum. Genet. 2007, 80, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Wise, C.A. The Genetic Architecture of Idiopathic Scoliosis. In Molecular Genetics of Pediatric Orthopaedic Disorders; Wise, C.A., Rios, J.J., Eds.; Springer: New York, NY, USA, 2015; pp. 71–89. ISBN 978-1-4939-2169-0. [Google Scholar]
- Claus, E.B.; Risch, N.; Thompson, W.D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am. J. Hum. Genet. 1991, 48, 232–242. [Google Scholar]
- Amir, E.; Freedman, O.C.; Seruga, B.; Evans, D.G. Assessing women at high risk of breast cancer: A review of risk assessment models. J. Natl. Cancer Inst. 2010, 102, 680–691. [Google Scholar] [CrossRef] [Green Version]
- Tenesa, A.; Haley, C.S. The heritability of human disease: Estimation, uses and abuses. Nat. Rev. Genet. 2013, 14, 139–149. [Google Scholar] [CrossRef]
- Grauers, A.; Rahman, I.; Gerdhem, P. Heritability of scoliosis. Eur. Spine J. 2012, 21, 1069–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silventoinen, K.; Sammalisto, S.; Perola, M.; Boomsma, D.I.; Cornes, B.K.; Davis, C.; Dunkel, L.; De Lange, M.; Harris, J.R.; Hjelmborg, J.V.B.; et al. Heritability of adult body height: A comparative study of twin cohorts in eight countries. Twin Res. Off. J. Int. Soc. Twin Stud. 2003, 6, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.L.S.; Yeung, H.-Y.; Hung, V.W.Y.; Di Liao, C.; Lam, T.-P.; Yeung, H.-M.; Lee, K.-M.; Ng, B.K.-W.; Cheng, J.C.-Y. Genetic epidemiology and heritability of AIS: A study of 415 Chinese female patients. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 1464–1469. [Google Scholar] [CrossRef]
- Khanshour, A.M.; Wise, C.A. The Genetic Architecture of Adolescent Idiopathic Scoliosis. In Pathogenesis of Idiopathic Scoliosis; Machida, M., Weinstein, S.L., Dubousset, J., Eds.; Springer: Tokyo, Japan, 2018; pp. 51–74. ISBN 978-4-431-56541-3. [Google Scholar]
- Ward, K.; Ogilvie, J.; Argyle, V.; Nelson, L.; Meade, M.; Braun, J.; Chettier, R. Polygenic inheritance of adolescent idiopathic scoliosis: A study of extended families in Utah. Am. J. Med. Genet. A. 2010, 152A, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Aulisa, A.G.; Guzzanti, V.; Galli, M.; Bottaro, G.; Vitelli, O.; Ferrara, P.; Logroscino, G. The familiarity of idiopathic scoliosis: Statistical analysis and clinical considerations. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2013, 23, 781–784. [Google Scholar] [CrossRef]
- Grauers, A.; Danielsson, A.; Karlsson, M.; Ohlin, A.; Gerdhem, P. Family history and its association to curve size and treatment in 1463 patients with idiopathic scoliosis. Eur. Spine J. 2013, 22, 2421–2426. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Minami, S.; Kitahara, H.; Otsuka, Y.; Nakata, Y.; Takaso, M.; Moriya, H. Idiopathic scoliosis in twins studied by DNA fingerprinting: The incidence and type of scoliosis. J. Bone Jt. Surg. Br. 1998, 80, 212–217. [Google Scholar] [CrossRef]
- Simony, A.; Carreon, L.Y.; Jmark, K.H.; Kyvik, K.O.; Andersen, M.Ø. Concordance Rates of Adolescent Idiopathic Scoliosis in a Danish Twin Population. Spine 2016, 41, 1503–1507. [Google Scholar] [CrossRef]
- Schlösser, T.P.C.; Simony, A.; Gerdhem, P.; Andersen, M.Ø.; Castelein, R.M.; Kempen, D.H.R. The heritability of coronal and sagittal phenotype in idiopathic scoliosis: A report of 12 monozygotic twin pairs. Spine Deform. 2021, 9, 51–55. [Google Scholar] [CrossRef]
- Ward, K.; Ogilvie, J.W.; Singleton, M.V.; Chettier, R.; Engler, G.; Nelson, L.M. Validation of DNA-based prognostic testing to predict spinal curve progression in adolescent idiopathic scoliosis. Spine 2010, 35, E1455–E1464. [Google Scholar] [CrossRef]
- Dobbs, M.B.; Gurnett, C.A. Re: Ward, K.; Ogilvie, J.W.; Singleton, M.V.; et al. Validation of DNA-based prognostic testing to predict spinal curve progression in adolescent idiopathic scoliosis. Spine 2010; 35:E1455-64. Spine 2011, 36, 1257, author reply 1257. [Google Scholar] [CrossRef]
- Ogura, Y.; Takahashi, Y.; Kou, I.; Nakajima, M.; Kono, K.; Kawakami, N.; Uno, K.; Ito, M.; Minami, S.; Yanagida, H.; et al. A Replication Study for Association of 53 Single Nucleotide Polymorphisms in a Scoliosis Prognostic Test with Progression of Adolescent Idiopathic Scoliosis in Japanese. Spine 2013, 38, 1375–1379. [Google Scholar] [CrossRef]
- Xu, L.; Qin, X.; Sun, W.; Qiao, J.; Qiu, Y.; Zhu, Z. Replication of Association between 53 Single-Nucleotide Polymorphisms in a DNA-Based Diagnostic Test and AIS Progression in Chinese Han Population. Spine 2016, 41, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.L.; Julien, C.; Eveleigh, R.; Bourque, G.; Franco, A.; Labelle, H.; Grimard, G.; Parent, S.; Ouellet, J.; Mac-Thiong, J.-M.; et al. A Replication Study for Association of 53 Single Nucleotide Polymorphisms in ScoliScore Test with Adolescent Idiopathic Scoliosis in French-Canadian Population. Spine 2015, 40, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Roye, B.D.; Wright, M.L.; Matsumoto, H.; Yorgova, P.; McCalla, D.; Hyman, J.E.; Roye, D.P.; Shah, S.A.; Vitale, M.G. An Independent Evaluation of the Validity of a DNA-Based Prognostic Test for Adolescent Idiopathic Scoliosis. J. Bone Jt. Surg. Am. 2015, 97, 1994–1998. [Google Scholar] [CrossRef]
- Rudnick, S.B.; Zabriskie, H.; Ho, J.; Gurnett, C.A.; Dobbs, M.B. Scoliosis severity does not impact the risk of scoliosis in family members. J. Pediatr. Orthop. Part. B 2018, 27, 147–151. [Google Scholar] [CrossRef]
- Yip, B.H.K.; Yu, F.W.P.; Wang, Z.; Hung, V.W.Y.; Lam, T.P.; Ng, B.K.W.; Zhu, F.; Cheng, J.C.Y. Prognostic Value of Bone Mineral Density on Curve Progression: A Longitudinal Cohort Study of 513 Girls with Adolescent Idiopathic Scoliosis. Sci. Rep. 2016, 6, 39220. [Google Scholar] [CrossRef] [Green Version]
- Vergari, C.; Skalli, W.; Abelin-Genevois, K.; Bernard, J.C.; Hu, Z.; Cheng, J.C.Y.; Chu, W.C.W.; Assi, A.; Karam, M.; Ghanem, I.; et al. Effect of curve location on the severity index for adolescent idiopathic scoliosis: A longitudinal cohort study. Eur. Radiol. 2021. [Google Scholar] [CrossRef]
- Haller, G.; Zabriskie, H.; Spehar, S.; Kuensting, T.; Bledsoe, X.; Syed, A.; Gurnett, C.A.; Dobbs, M.B. Lack of joint hypermobility increases the risk of surgery in adolescent idiopathic scoliosis. J. Pediatr. Orthop. Part. B 2018, 27, 152–158. [Google Scholar] [CrossRef]
- Noshchenko, A.; Hoffecker, L.; Lindley, E.M.; Burger, E.L.; Cain, C.M.; Patel, V.V.; Bradford, A.P. Predictors of spine deformity progression in adolescent idiopathic scoliosis: A systematic review with meta-analysis. World J. Orthop. 2015, 6, 537–558. [Google Scholar] [CrossRef]
- Lenz, M.; Oikonomidis, S.; Harland, A.; Fürnstahl, P.; Farshad, M.; Bredow, J.; Eysel, P.; Scheyerer, M.J. Scoliosis and Prognosis-a systematic review regarding patient-specific and radiological predictive factors for curve progression. Eur. Spine J. 2021. [Google Scholar] [CrossRef]
- Altaf, F.; Gibson, A.; Dannawi, Z.; Noordeen, H. Adolescent idiopathic scoliosis. BMJ 2013, 346, f2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edery, P.; Margaritte-Jeannin, P.; Biot, B.; Labalme, A.; Bernard, J.-C.; Chastang, J.; Kassai, B.; Plais, M.-H.; Moldovan, F.; Clerget-Darpoux, F. New disease gene location and high genetic heterogeneity in idiopathic scoliosis. Eur. J. Hum. Genet. 2011, 19, 865–869. [Google Scholar] [CrossRef]
- Patten, S.A.; Margaritte-Jeannin, P.; Bernard, J.-C.; Alix, E.; Labalme, A.; Besson, A.; Girard, S.L.; Fendri, K.; Fraisse, N.; Biot, B.; et al. Functional variants of POC5 identified in patients with idiopathic scoliosis. J. Clin. Invest. 2015, 125, 1124–1128. [Google Scholar] [CrossRef]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Kou, I.; Takahashi, A.; Johnson, T.A.; Kono, K.; Kawakami, N.; Uno, K.; Ito, M.; Minami, S.; Yanagida, H.; et al. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat. Genet. 2011, 43, 1237–1240. [Google Scholar] [CrossRef]
- Sharma, S.; Gao, X.; Londono, D.; Devroy, S.E.; Mauldin, K.N.; Frankel, J.T.; Brandon, J.M.; Zhang, D.; Li, Q.-Z.; Dobbs, M.B.; et al. Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum. Mol. Genet. 2011, 20, 1456–1466. [Google Scholar] [CrossRef]
- Londono, D.; Kou, I.; Johnson, T.A.; Sharma, S.; Ogura, Y.; Tsunoda, T.; Takahashi, A.; Matsumoto, M.; Herring, J.A.; Lam, T.-P.; et al. A meta-analysis identifies adolescent idiopathic scoliosis association with LBX1 locus in multiple ethnic groups. J. Med. Genet. 2014, 51, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tang, N.L.-S.; Xu, L.; Qin, X.; Mao, S.; Song, Y.; Liu, L.; Li, F.; Liu, P.; Yi, L.; et al. Genome-wide association study identifies new susceptibility loci for adolescent idiopathic scoliosis in Chinese girls. Nat. Commun. 2015, 6, 8355. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Xu, L.; Leung-Sang Tang, N.; Qin, X.; Feng, Z.; Sun, W.; Zhu, W.; Shi, B.; Liu, P.; Mao, S.; et al. Genome-wide association study identifies novel susceptible loci and highlights Wnt/beta-catenin pathway in the development of adolescent idiopathic scoliosis. Hum. Mol. Genet. 2017, 26, 1577–1583. [Google Scholar] [CrossRef] [Green Version]
- Ott, J. Association of genetic loci: Replication or not, that is the question. Neurology 2004, 63, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.E. Examining the current standards for genetic discovery and replication in the era of mega-biobanks. Nat. Commun. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Gross, M.K.; Moran-Rivard, L.; Velasquez, T.; Nakatsu, M.N.; Jagla, K.; Goulding, M. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Dev. Camb. Engl. 2000, 127, 413–424. [Google Scholar]
- Mootoosamy, R.C.; Dietrich, S. Distinct regulatory cascades for head and trunk myogenesis. Development 2002, 129, 573–583. [Google Scholar] [CrossRef]
- Wotton, K.R.; Schubert, F.R.; Dietrich, S. Hypaxial muscle: Controversial classification and controversial data? Vertebr. Myogenesis Stem Cells Precursors 2015, 25–48. [Google Scholar] [CrossRef]
- Watanabe, S.; Kondo, S.; Hayasaka, M.; Hanaoka, K. Functional analysis of homeodomain-containing transcription factor Lbx1 in satellite cells of mouse skeletal muscle. J. Cell Sci. 2007, 120, 4178–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, G.C.-W.; Tang, N.L.-S.; Chan, T.F.; Lam, T.P.; Li, J.W.; Ng, B.K.-W.; Zhu, Z.; Qiu, Y.; Cheng, J.C.-Y. Replication Study for the Association of GWAS-associated Loci with Adolescent Idiopathic Scoliosis Susceptibility and Curve Progression in a Chinese Population. Spine 2019, 44, 464–471. [Google Scholar] [CrossRef]
- Ogura, Y.; Kou, I.; Miura, S.; Takahashi, A.; Xu, L.; Takeda, K.; Takahashi, Y.; Kono, K.; Kawakami, N.; Uno, K.; et al. A Functional SNP in BNC2 Is Associated with Adolescent Idiopathic Scoliosis. Am. J. Hum. Genet. 2015, 97, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Xia, C.; Qin, X.; Sun, W.; Tang, N.L.-S.; Qiu, Y.; Cheng, J.C.-Y.; Zhu, Z. Genetic variant of BNC2 gene is functionally associated with adolescent idiopathic scoliosis in Chinese population. Mol. Genet. Genom. 2017, 292, 789–794. [Google Scholar] [CrossRef]
- Kou, I.; Otomo, N.; Takeda, K.; Momozawa, Y.; Lu, H.-F.; Kubo, M.; Kamatani, Y.; Ogura, Y.; Takahashi, Y.; Nakajima, M.; et al. Genome-wide association study identifies 14 previously unreported susceptibility loci for adolescent idiopathic scoliosis in Japanese. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Dietz, H. Marfan Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Malfait, F.; Wenstrup, R.; De Paepe, A. Classic Ehlers-Danlos Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Buchan, J.G.; Alvarado, D.M.; Haller, G.E.; Cruchaga, C.; Harms, M.B.; Zhang, T.; Willing, M.C.; Grange, D.K.; Braverman, A.C.; Miller, N.H.; et al. Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum. Mol. Genet. 2014, 23, 5271–5282. [Google Scholar] [CrossRef] [Green Version]
- Haller, G.; Alvarado, D.; Mccall, K.; Yang, P.; Cruchaga, C.; Harms, M.; Goate, A.; Willing, M.; Morcuende, J.A.; Baschal, E.; et al. A polygenic burden of rare variants across extracellular matrix genes among individuals with adolescent idiopathic scoliosis. Hum. Mol. Genet. 2016, 25, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, D.T.; Boswell, C.W.; Morante, N.F.C.; Henkelman, R.M.; Burdine, R.D.; Ciruna, B. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 2016, 352, 1341–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boswell, C.W.; Ciruna, B. Understanding Idiopathic Scoliosis: A New Zebrafish School of Thought. Trends Genet. 2017, 33, 183–196. [Google Scholar] [CrossRef]
- Hayes, M.; Gao, X.; Yu, L.X.; Paria, N.; Henkelman, R.M.; Wise, C.A.; Ciruna, B. ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nat. Commun. 2014, 5, 4777. [Google Scholar] [CrossRef] [Green Version]
- Aw, W.Y.; Devenport, D. Planar cell polarity: Global inputs establishing cellular asymmetry. Curr. Opin. Cell Biol. 2017, 44, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; de Marco, P.; Capra, V.; Kibar, Z. Update on the Role of the Non-Canonical Wnt/Planar Cell Polarity Pathway in Neural Tube Defects. Cells 2019, 8, 1198. [Google Scholar] [CrossRef] [Green Version]
- Hirst, C.E.; Marcelle, C. The avian embryo as a model system for skeletal myogenesis. Results Probl. Cell Differ. 2015, 56, 99–122. [Google Scholar]
- Buchan, J.G.; Gray, R.S.; Gansner, J.M.; Alvarado, D.M.; Burgert, L.; Gitlin, J.D.; Gurnett, C.A.; Goldsmith, M.I. Kinesin family member 6 (kif6) is necessary for spine development in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2014, 243, 1646–1657. [Google Scholar] [CrossRef]
- Chu, W.C.W.; Man, G.C.W.; Lam, W.W.M.; Yeung, B.H.Y.; Chau, W.-W.; Ng, B.K.W.; Lam, T.-P.; Lee, K.-M.; Cheng, J.C.Y. A detailed morphologic and functional magnetic resonance imaging study of the craniocervical junction in adolescent idiopathic scoliosis. Spine 2007, 32, 1667–1674. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, S.; Chen, Z.; Chong, Y.L.; Xie, H.; Feng, D.; Wu, X.; Song, D.Z.; Roy, S.; Zhao, C. Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat. Genet. 2018, 50, 1666–1673. [Google Scholar] [CrossRef]
- Bearce, E.A.; Grimes, D.T. On being the right shape: Roles for motile cilia and cerebrospinal fluid flow in body and spine morphology. Semin. Cell Dev. Biol. 2021, 110, 104–112. [Google Scholar] [CrossRef]
- Ringers, C.; Olstad, E.W.; Jurisch-Yaksi, N. The role of motile cilia in the development and physiology of the nervous system. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Wu, J.; Jing, J.; Huang, P.; Li, Z.; Mei, J.; Gui, J.-F. Loss of stat3 function leads to spine malformation and immune disorder in zebrafish. Sci. Bull. 2017, 62, 185–196. [Google Scholar] [CrossRef]
- Whittle, J.; Antunes, L.; Harris, M.; Upshaw, Z.; Sepich, D.S.; Johnson, A.N.; Mokalled, M.; Solnica-Krezel, L.; Dobbs, M.B.; Gurnett, C.A. MYH3-associated distal arthrogryposis zebrafish model is normalized with para-aminoblebbistatin. EMBO Mol. Med. 2020, 12, e12356. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Scientific basis for the potential use of melatonin in bone diseases: Osteoporosis and adolescent idiopathic scoliosis. J. Osteoporos. 2010, 2010, 830231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gennip, J.L.M.; Boswell, C.W.; Ciruna, B. Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Sci. Adv. 2018, 4, eaav1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, N.L.S.; Dobbs, M.B.; Gurnett, C.A.; Qiu, Y.; Lam, T.P.; Cheng, J.C.Y.; Hadley-Miller, N. A Decade in Review after Idiopathic Scoliosis Was First Called a Complex Trait—A Tribute to the Late Dr. Yves Cotrel for His Support in Studies of Etiology of Scoliosis. Genes 2021, 12, 1033. https://doi.org/10.3390/genes12071033
Tang NLS, Dobbs MB, Gurnett CA, Qiu Y, Lam TP, Cheng JCY, Hadley-Miller N. A Decade in Review after Idiopathic Scoliosis Was First Called a Complex Trait—A Tribute to the Late Dr. Yves Cotrel for His Support in Studies of Etiology of Scoliosis. Genes. 2021; 12(7):1033. https://doi.org/10.3390/genes12071033
Chicago/Turabian StyleTang, Nelson L. S., Matthew B. Dobbs, Christina A. Gurnett, Yong Qiu, T. P. Lam, Jack C. Y. Cheng, and Nancy Hadley-Miller. 2021. "A Decade in Review after Idiopathic Scoliosis Was First Called a Complex Trait—A Tribute to the Late Dr. Yves Cotrel for His Support in Studies of Etiology of Scoliosis" Genes 12, no. 7: 1033. https://doi.org/10.3390/genes12071033
APA StyleTang, N. L. S., Dobbs, M. B., Gurnett, C. A., Qiu, Y., Lam, T. P., Cheng, J. C. Y., & Hadley-Miller, N. (2021). A Decade in Review after Idiopathic Scoliosis Was First Called a Complex Trait—A Tribute to the Late Dr. Yves Cotrel for His Support in Studies of Etiology of Scoliosis. Genes, 12(7), 1033. https://doi.org/10.3390/genes12071033






