Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Japanese Black Cattle
2.3. RNA Preparation from Fat Tissue
2.4. Preparation of Sequence Library
2.5. RNA-seq Analysis
2.6. Gene Expression Analysis
2.7. qPCR Analysis
2.8. Tissue Staining with Paraffin-Embedded Sections
2.9. Statistical Analysis
3. Results
3.1. RNA-seq Analysis of Subcutaneous Fat and Intramuscular Fat
3.2. Verification of Characteristic Genes Expressed in Intramuscular Fat
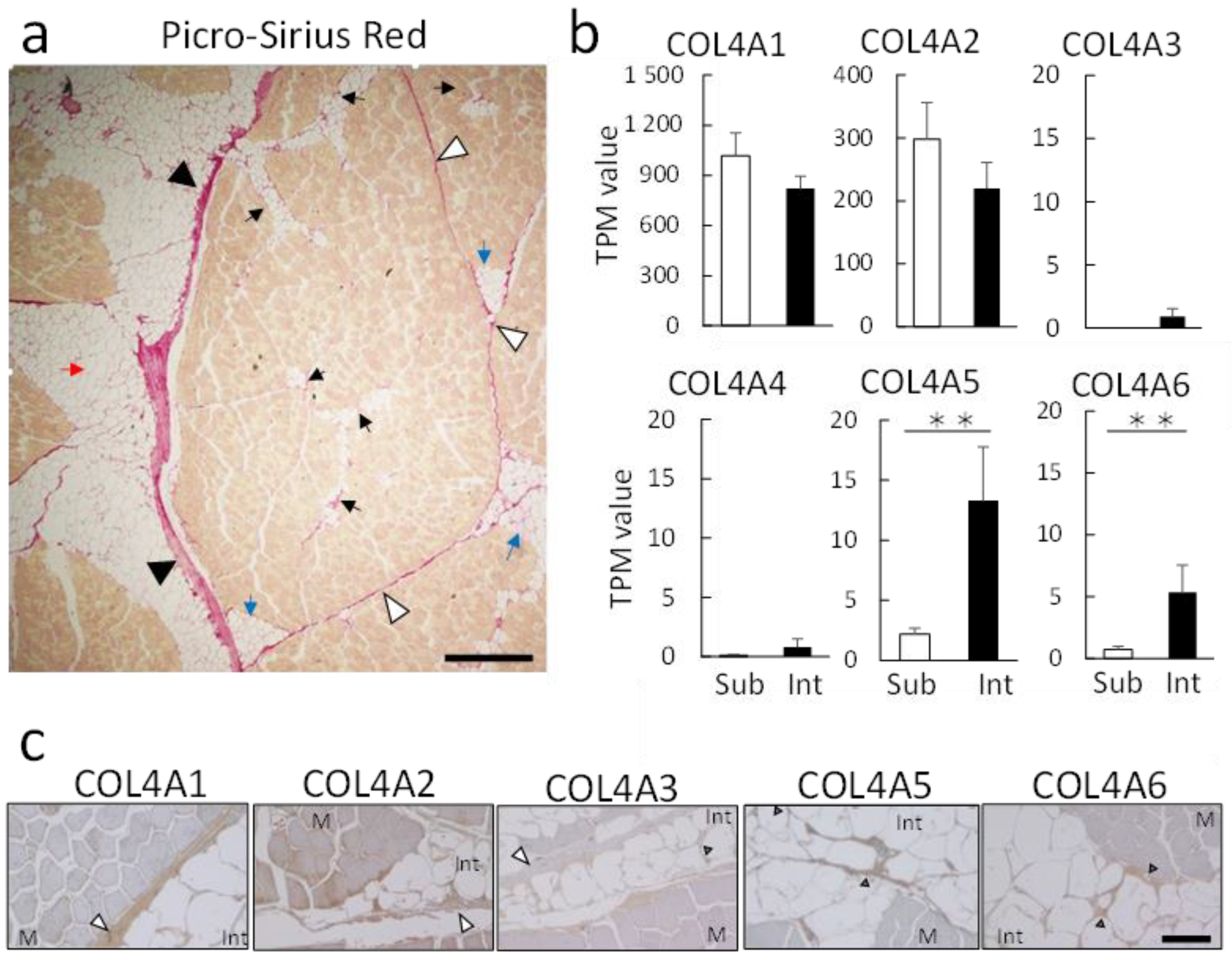
3.3. Expression Analysis of the COL4 Isoform
3.4. Pathway Analysis of Differentially Expressed Genes
3.5. Analysis of COL4-Related Cellular Signals in Intramuscular Fat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueda, S.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry. Metabolites 2021, 11, 56. [Google Scholar] [CrossRef]
- Matsuishi, M.; Fujimori, M.; Okitani, A. Wagyu Beef Aroma in Wagyu (Japanese Black Cattle) Beef Preferred by the Japanese over Imported Beef. Nihon Chikusan Gakkaiho 2001, 72, 498–504. [Google Scholar] [CrossRef]
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef] [Green Version]
- Bergen, W.G.; Brandebourg, T.D. Regulation of lipid deposition in farm animals: Parallels between agriculture and human physiology. Exp. Biol. Med. 2016, 241, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Shan, T. Factors inducing transdifferentiation of myoblasts into adipocytes. J. Cell. Physiol. 2021, 236, 2276–2289. [Google Scholar] [CrossRef]
- Campos, C.F.; Duarte, M.S.; Guimarães, S.E.; Verardo, L.L.; Wei, S.; Du, M.; Jiang, Z.; Bergen, W.G.; Hausman, G.J.; Fernyhough-Culver, M.; et al. Review: Animal model and the current understanding of molecule dynamics of adipogenesis. Animal 2016, 10, 927–932. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Zhang, T.; Liu, Y.; Wang, Z.; Xu, L.; Zhu, B.; Gao, X.; Zhang, L.; Gao, H.; Liu, G.E.; et al. Genome-Wide Assessment of Runs of Homozygosity in Chinese Wagyu Beef Cattle. Animals 2020, 10, 1425. [Google Scholar] [CrossRef]
- Sasazaki, S.; Kawaguchi, F.; Nakajima, A.; Yamamoto, R.; Akiyama, T.; Kohama, N.; Yoshida, E.; Kobayashi, E.; Honda, T.; Oyama, K.; et al. Detection of candidate polymorphisms around the QTL for fat area ratio to rib eye area on BTA7 using whole-genome resequencing in Japanese Black cattle. Anim. Sci. J. 2020, 91, e13335. [Google Scholar] [CrossRef]
- Fonseca, L.F.S.; dos Santos Silva, D.B.; Gimenez, D.F.J.; Baldi, F.; Ferro, J.A.; Chardulo, L.A.L.; de Albuquerque, L.G. Gene expression profiling and identification of hub genes in Nellore cattle with different marbling score levels. Genomics 2020, 112, 873–879. [Google Scholar] [CrossRef]
- Huang, W.; Guo, Y.; Du, W.; Zhang, X.; Li, A.; Miao, X. Global transcriptome analysis identifies differentially expressed genes related to lipid metabolism in Wagyu and Holstein cattle. Sci. Rep. 2017, 7, 5278. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.B.S.; Fonseca, L.F.S.; Pinheiro, D.G.; Magalhães, A.F.B.; Muniz, M.M.M.; Ferro, J.A.; Baldi, F.; Chardulo, L.A.L.; Schnabel, R.D.; Taylor, J.F.; et al. Spliced genes in muscle from Nelore Cattle and their association with carcass and meat quality. Sci. Rep. 2020, 10, 14701. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Li, Q.; Gao, Y.; Li, Q.; Li, J.; Cao, Y. Transcriptome profiling of longissimus lumborum in Holstein bulls and steers with different beef qualities. PLoS ONE 2020, 15, e0235218. [Google Scholar] [CrossRef]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography–mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef]
- Ueda, S.; Tu-Sekine, B.; Yamanoue, M.; Raben, D.M.; Shirai, Y. The expression of diacylglycerol kinase theta during the organogenesis of mouse embryos. BMC Dev. Biol. 2013, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Sasaki, R.; Nakabayashi, R.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites 2021, 11, 203. [Google Scholar] [CrossRef]
- Nishimura, T. The role of intramuscular connective tissue in meat texture. Anim. Sci. J. 2010, 81, 21–27. [Google Scholar] [CrossRef]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian collagen IV. Microsc. Res. Technol. 2008, 71, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouellette, J.N.; Drifka, C.R.; Pointer, K.B.; Liu, Y.; Lieberthal, T.J.; Kao, W.J.; Kuo, J.S.; Loeffler, A.G.; Eliceiri, K.W. Navigating the Collagen Jungle: The Biomedical Potential of Fiber Organization in Cancer. Bioengineering 2021, 8, 17. [Google Scholar] [CrossRef]
- Reggio, S.; Rouault, C.; Poitou, C.; Bichet, J.-C.; Prifti, E.; Bouillot, J.-L.; Rizkalla, S.; Lacasa, D.; Tordjman, J.; Clément, K. Increased Basement Membrane Components in Adipose Tissue During Obesity: Links With TGFβ and Metabolic Phenotypes. J. Clin. Endocrinol. Metab. 2016, 101, 2578–2587. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; DeYoung, S.M.; Zhang, M.; Zhang, M.; Cheng, A.; Saltiel, A.R. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005, 2, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Raftopoulou, M.; Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, E.; Gotoh, T.; Ebara, F.; Xu, J.X.; Viergutz, T.; Nürnberg, G.; Maak, S.; Wegner, J. Cellular conditions for intramuscular fat deposition in Japanese Black and Holstein steers. Meat Sci. 2011, 89, 13–20. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J. Clin. Endocrinol. Metab. 2012, 97, E1880–E1889. [Google Scholar] [CrossRef] [Green Version]
- Angel, I.; Pilo Kerman, O.; Rousso-Noori, L.; Friedmann-Morvinski, D. Tenascin C promotes cancer cell plasticity in mesenchymal glioblastoma. Oncogene 2020, 39, 6990–7004. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, S.; Zhan, Y.; He, A.; Fang, D.; Gong, Y.; Li, X.; Zhou, L. TGF-β-induced transgelin promotes bladder cancer metastasis by regulating epithelial-mesenchymal transition and invadopodia formation. EBioMedicine 2019, 47, 208–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsafadi, M.; Manikandan, M.; Dawud, R.A.; Alajez, N.M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Mahmood, A. Transgelin is a TGFβ-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016, 7, e2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.; Wu, H.-T.; Qin, X.-Y.; Lan, R. Dissecting carboxypeptidase E: Properties, functions and pathophysiological roles in disease. Endocr. Connect. 2017, 6, R18–R38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, T.S.; Baldi, F.; Sant’Anna, A.C.; Albuquerque, L.G.; Paranhos da Costa, M.J.R. Genome-Wide Association Study between Single Nucleotide Polymorphisms and Flight Speed in Nellore Cattle. PLoS ONE 2016, 11, e0156956. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.J.; Reverter, A.; Griffiths, W.J.; Yutuc, E.; Wang, Y.; Jeanes, A.; McWilliam, S.; Pethick, D.W.; Greenwood, P.L. Gene expression identifies metabolic and functional differences between intramuscular and subcutaneous adipocytes in cattle. BMC Genom. 2020, 21, 77. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.; Borkham-Kamphorst, E.; Haas, U.; Van de Leur, E.; Fraga, M.F.; Esteller, M.; Gressner, A.M.; Weiskirchen, R. The expression of CSRP2 encoding the LIM domain protein CRP2 is mediated by TGF-β in smooth muscle and hepatic stellate cells. Biochem. Biophys. Res. Commun. 2006, 345, 1526–1535. [Google Scholar] [CrossRef]
- Hattori, Y.; Sentani, K.; Shinmei, S.; Oo, H.Z.; Hattori, T.; Imai, T.; Sekino, Y.; Sakamoto, N.; Oue, N.; Niitsu, H.; et al. Clinicopathological significance of RCAN2 production in gastric carcinoma. Histopathology 2019, 74, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tao, T.; Zhao, W.; Wei, L.; She, F.; Wang, P.; Li, Y.; Zheng, Y.; Chen, X.; Wang, W.; et al. CPI-17-mediated contraction of vascular smooth muscle is essential for the development of hypertension in obese mice. J. Genet. Genom. 2019, 46, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kang, Y.; Im, N.R.; Kim, B.; Kwon, T.K.; Jung, K.Y.; Baek, S.K. Actin-Associated Gene Expression is Associated with Early Regional Metastasis of Tongue Cancer. Laryngoscope 2021, 131, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Tanjore, H.; Kalluri, R. The role of type IV collagen and basement membranes in cancer progression and metastasis. Am. J. Pathol. 2006, 168, 715–717. [Google Scholar] [CrossRef] [Green Version]
- Angel, P.M.; Spruill, L.; Jefferson, M.; Bethard, J.R.; Ball, L.E.; Hughes-Halbert, C.; Drake, R.R. Zonal regulation of collagen-type proteins and posttranslational modifications in prostatic benign and cancer tissues by imaging mass spectrometry. Prostate 2020, 80, 1071–1086. [Google Scholar] [CrossRef]
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in regulation of the tumor microenvironment and drug delivery (Review). Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Katoh, D.; Kozuka, Y.; Noro, A.; Ogawa, T.; Imanaka-Yoshida, K.; Yoshida, T. Tenascin-C Induces Phenotypic Changes in Fibroblasts to Myofibroblasts with High Contractility through the Integrin αvβ1/Transforming Growth Factor β/SMAD Signaling Axis in Human Breast Cancer. Am. J. Pathol. 2020, 190, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, S.; Kataoka, T.; Satoh, T. Role of the Sec14-like domain of Dbl family exchange factors in the regulation of Rho family GTPases in different subcellular sites. Cell. Signal. 2004, 16, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, S.; Zhan, H.; Yan, Z.; Zhang, G. Transcriptome sequencing identifies key pathways and genes involved in gastric adenocarcinoma. Mol. Med. Rep. 2018, 18, 3673–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goicoechea, S.M.; Zinn, A.; Awadia, S.S.; Snyder, K.; Garcia-Mata, R. A RhoG-mediated signaling pathway that modulates invadopodia dynamics in breast cancer cells. J. Cell Sci. 2017, 130, 1064–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Shen, S.; Wang, M.; Ding, F.; Xiao, H.; Li, G.; Hu, F. Rho GTPase Activating Protein 24 (ARHGAP24) Silencing Promotes Lung Cancer Cell Migration and Invasion by Activating β-Catenin Signaling. Med. Sci. Monit. 2019, 25, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.C.; Olayioye, M.A. Rho regulation: DLC proteins in space and time. Cell Signal. 2015, 27, 1643–1651. [Google Scholar] [CrossRef]
- Ueda, S.; Kataoka, T.; Satoh, T. Activation of the small GTPase Rac1 by a specific guanine-nucleotide-exchange factor suffices to induce glucose uptake into skeletal-muscle cells. Biol. Cell 2008, 100, 645–657. [Google Scholar] [CrossRef] [PubMed]
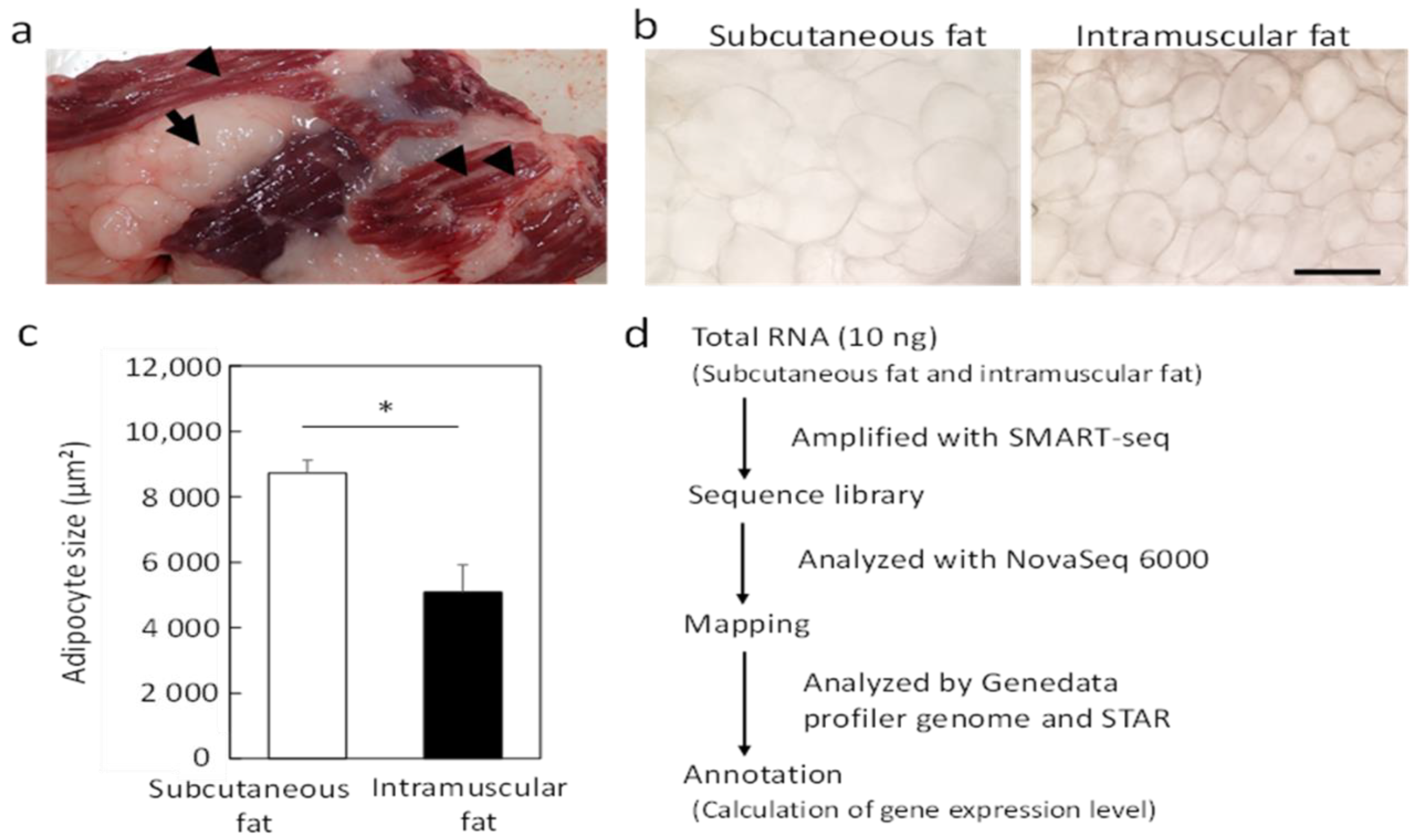
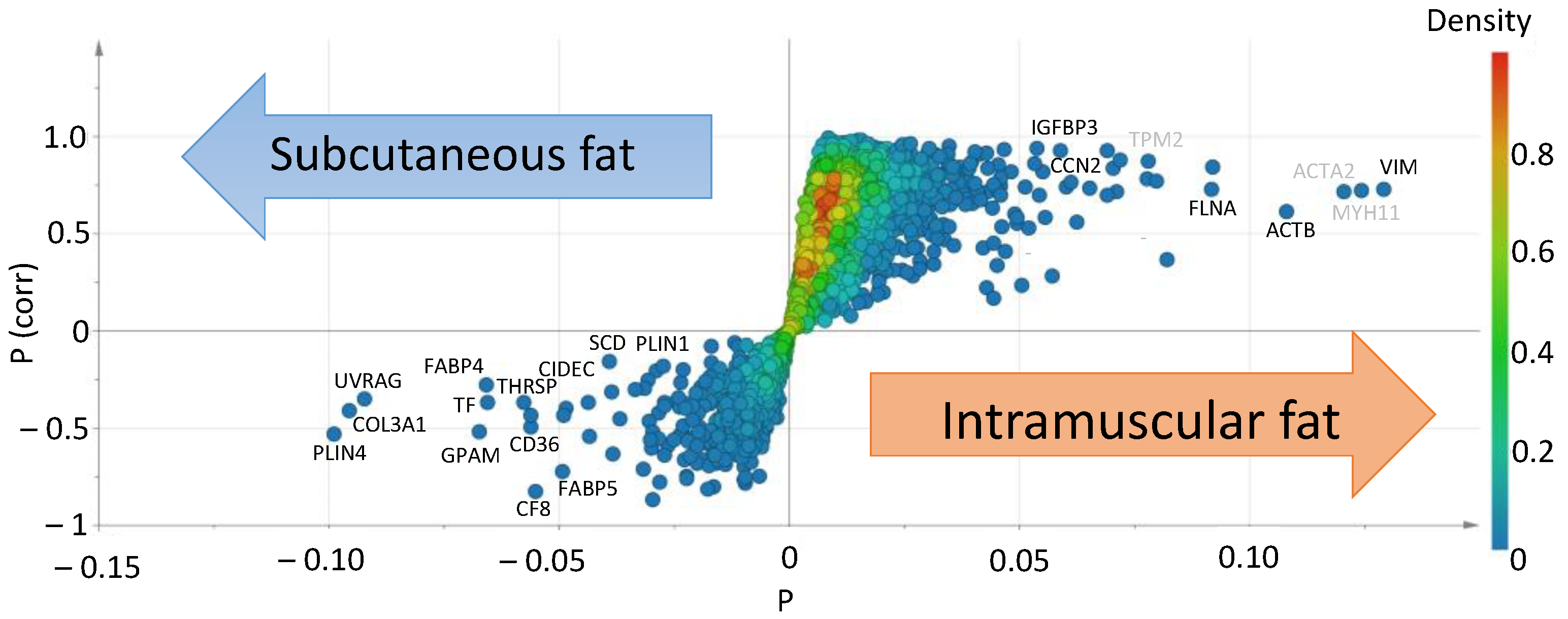
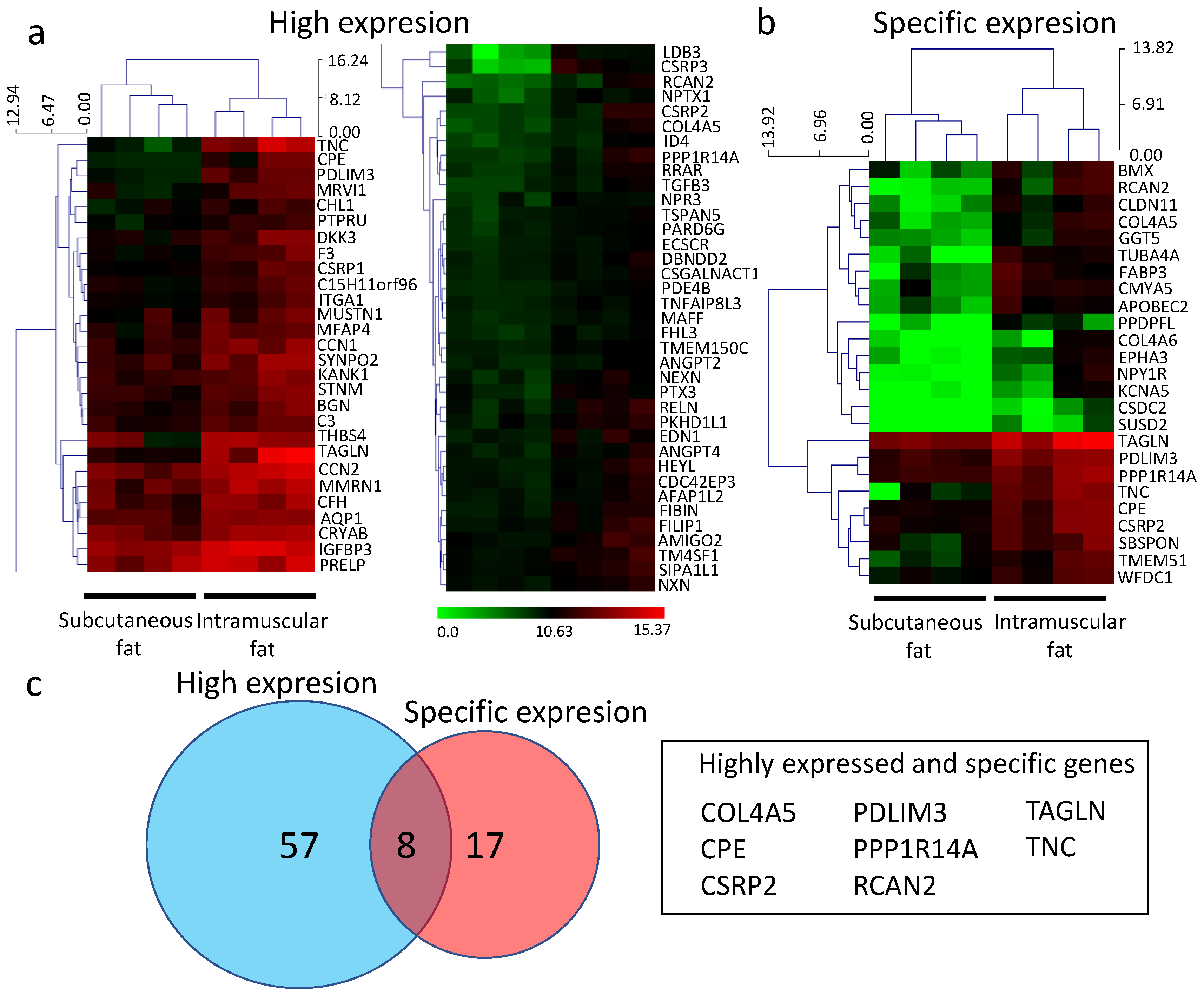
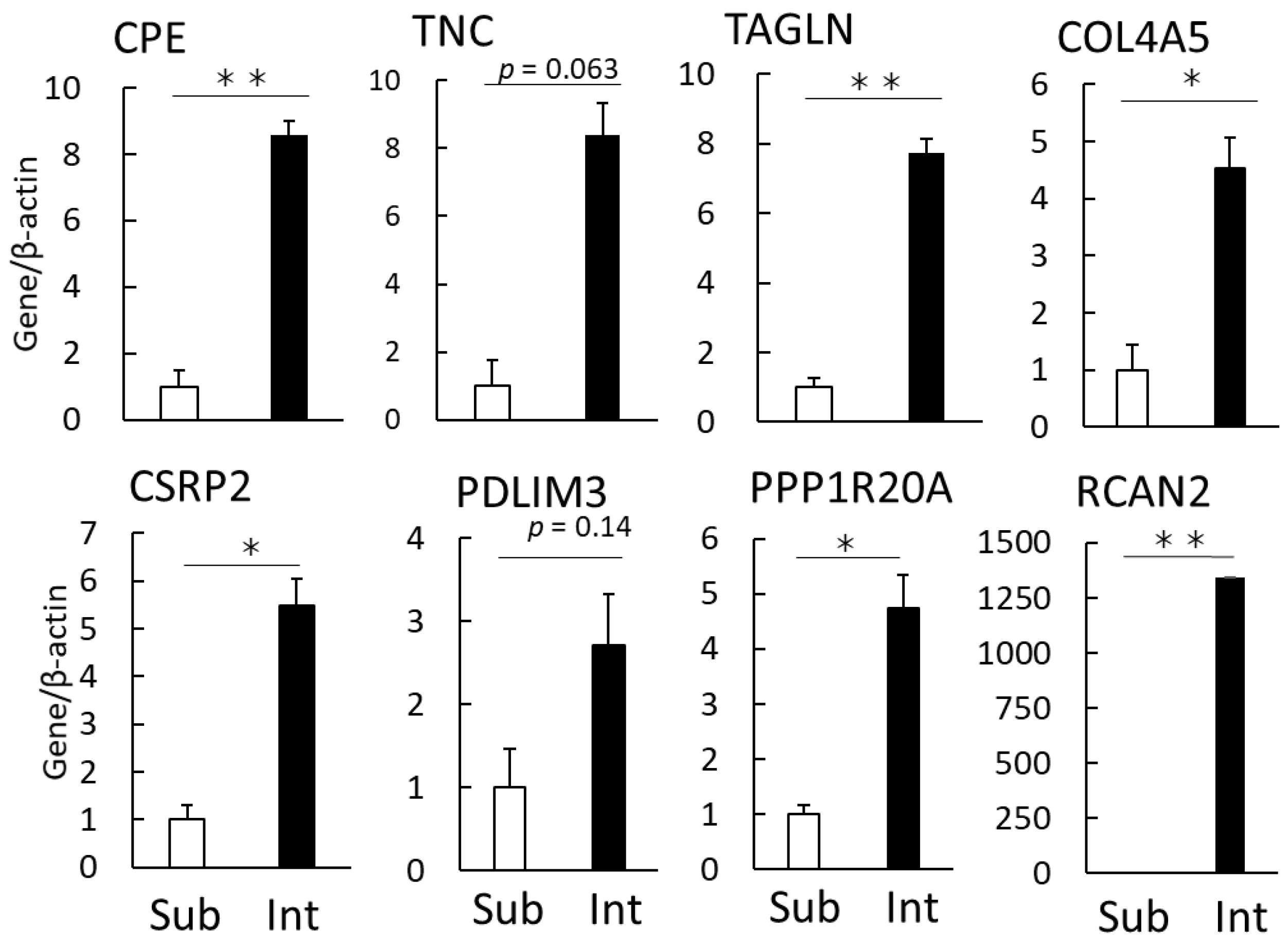


| No. | Gene | Name | Mean of a TPM Value | Ratio | t-Test | |
|---|---|---|---|---|---|---|
| Subcutaneous Fat | Intramuscular Fat | (Int/Sub) | (p Vales) | |||
| TGF-β signal | ||||||
| 1 | TGFB2 | Transforming growth factor beta 2 | 24.90 | 30.87 | 1.24 | 0.010 * |
| 2 | TGFB3 | Transforming growth factor beta 3 | 5.34 | 17.70 | 3.32 | 0.038 * |
| 3 | TGIF1 | TGFB induced factor homeobox | 26.85 | 36.77 | 1.37 | 0.016 * |
| 4 | SMAD7 | SMAD family member 7 | 3.69 | 6.27 | 1.70 | 0.011 * |
| 5 | CHRD | Chordin | 4.68 | 6.82 | 1.46 | 0.011 * |
| 6 | TGFBR1 | Transforming growth factor beta receptor 1 | 44.31 | 50.05 | 1.13 | 0.113 |
| 7 | TGFB1 | Transforming growth factor beta 1 | 98.20 | 61.95 | 0.63 | 0.056 |
| 8 | SMAD2 | SMAD family member 2 | 78.02 | 85.55 | 1.10 | 0.289 |
| 9 | SMAD3 | SMAD family member 3 | 15.73 | 16.63 | 1.06 | 0.826 |
| 10 | SMAD4 | SMAD family member 4 | 47.21 | 51.80 | 1.10 | 0.371 |
| 11 | SMAD6 | SMAD family member 6 | 6.05 | 8.37 | 1.38 | 0.209 |
| Integrin | ||||||
| 12 | ITGA2 | Integrin subunit alpha 2 | 0.56 | 1.32 | 2.36 | 0.044 * |
| 13 | ITGB1 | Integrin subunit beta 1 | 528.35 | 710.96 | 1.35 | 0.029 * |
| 14 | ITGB6 | Integrin subunit beta 6 | 0.02 | 0.45 | 25.52 | 0.017 * |
| 15 | ITGA6 | Integrin subunit alpha 6 | 211.64 | 186.69 | 0.88 | 0.521 |
| Cell migration factor | ||||||
| 16 | CXCL5 | Chemokine (C-X-C motif) ligand 5 | 0.52 | 2.19 | 4.23 | 0.046 * |
| 17 | CXCR4 | C-X-C motif chemokine receptor 4 | 9.56 | 22.76 | 2.38 | 0.087 |
| 18 | FGF9 | Fibroblast growth factor 9 | 1.89 | 3.11 | 1.65 | 0.036 * |
| 19 | FGF13 | Fibroblast growth factor 13 | 1.07 | 1.88 | 1.75 | 0.040 * |
| Regulators of small GTPase | ||||||
| 20 | RASGRP3 | Ras guanyl releasing protein 3 | 9.02 | 16.91 | 1.87 | 0.003 ** |
| 21 | ARHGEF26 | Rho guanine nucleotide exchange factor 26 | 3.96 | 6.18 | 1.56 | 0.022 * |
| 22 | ARHGAP10 | Rho GTPase activating protein 10 | 27.56 | 47.61 | 1.73 | 0.025 * |
| 23 | ARHGAP24 | Rho GTPase activating protein 24 | 10.02 | 14.49 | 1.45 | 0.046 * |
| 24 | DLC3 | Deleted in liver cancer 3 (STARD8) | 9.32 | 13.59 | 1.46 | 0.041 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, S.; Hosoda, M.; Yoshino, K.-i.; Yamanoue, M.; Shirai, Y. Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle. Genes 2021, 12, 1107. https://doi.org/10.3390/genes12081107
Ueda S, Hosoda M, Yoshino K-i, Yamanoue M, Shirai Y. Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle. Genes. 2021; 12(8):1107. https://doi.org/10.3390/genes12081107
Chicago/Turabian StyleUeda, Shuji, Mana Hosoda, Ken-ichi Yoshino, Minoru Yamanoue, and Yasuhito Shirai. 2021. "Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle" Genes 12, no. 8: 1107. https://doi.org/10.3390/genes12081107
APA StyleUeda, S., Hosoda, M., Yoshino, K.-i., Yamanoue, M., & Shirai, Y. (2021). Gene Expression Analysis Provides New Insights into the Mechanism of Intramuscular Fat Formation in Japanese Black Cattle. Genes, 12(8), 1107. https://doi.org/10.3390/genes12081107







