Conservation in the Iron Responsive Element Family
Abstract
1. Introduction
1.1. IRE Function
1.2. IRE Family
2. Background
2.1. Two Applied Criteria
2.2. Classic IRE Model
2.3. IRE-Like” Sequences
3. Discussion
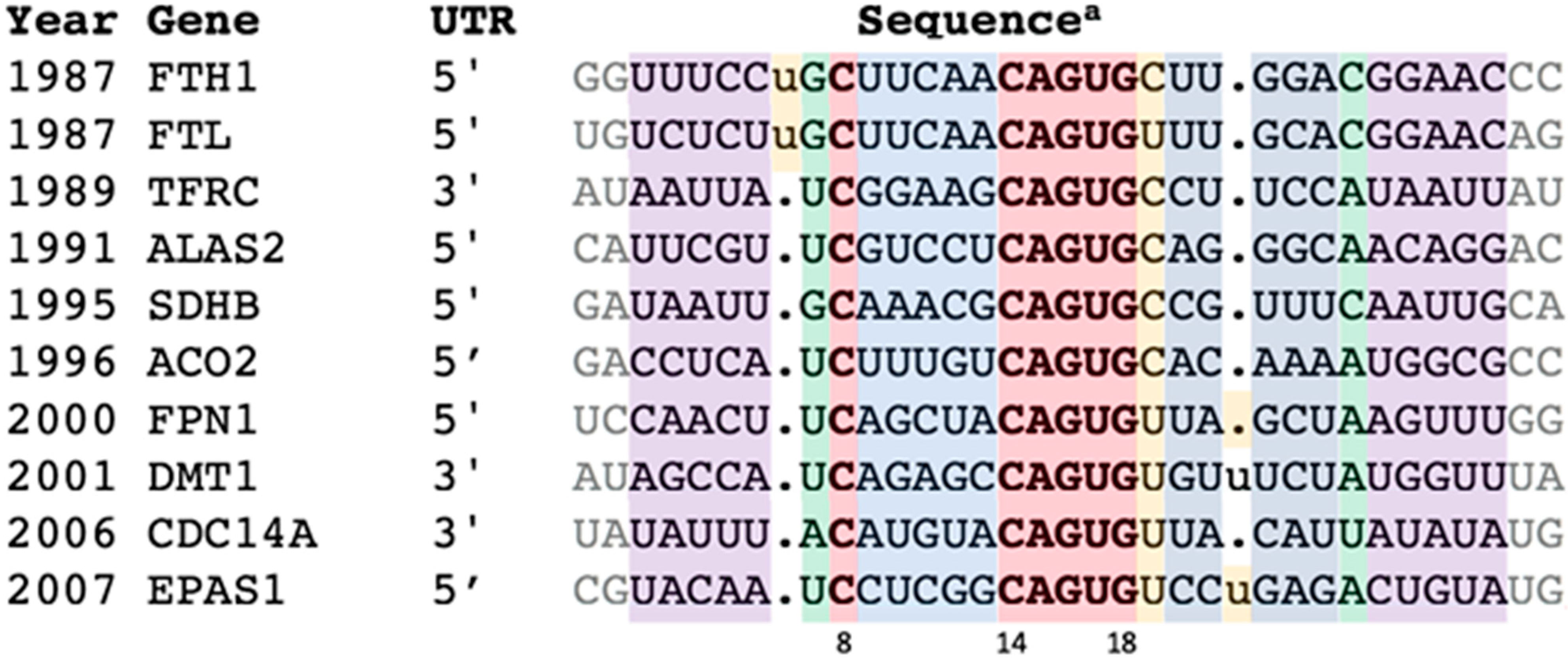
3.1. Scoring Results
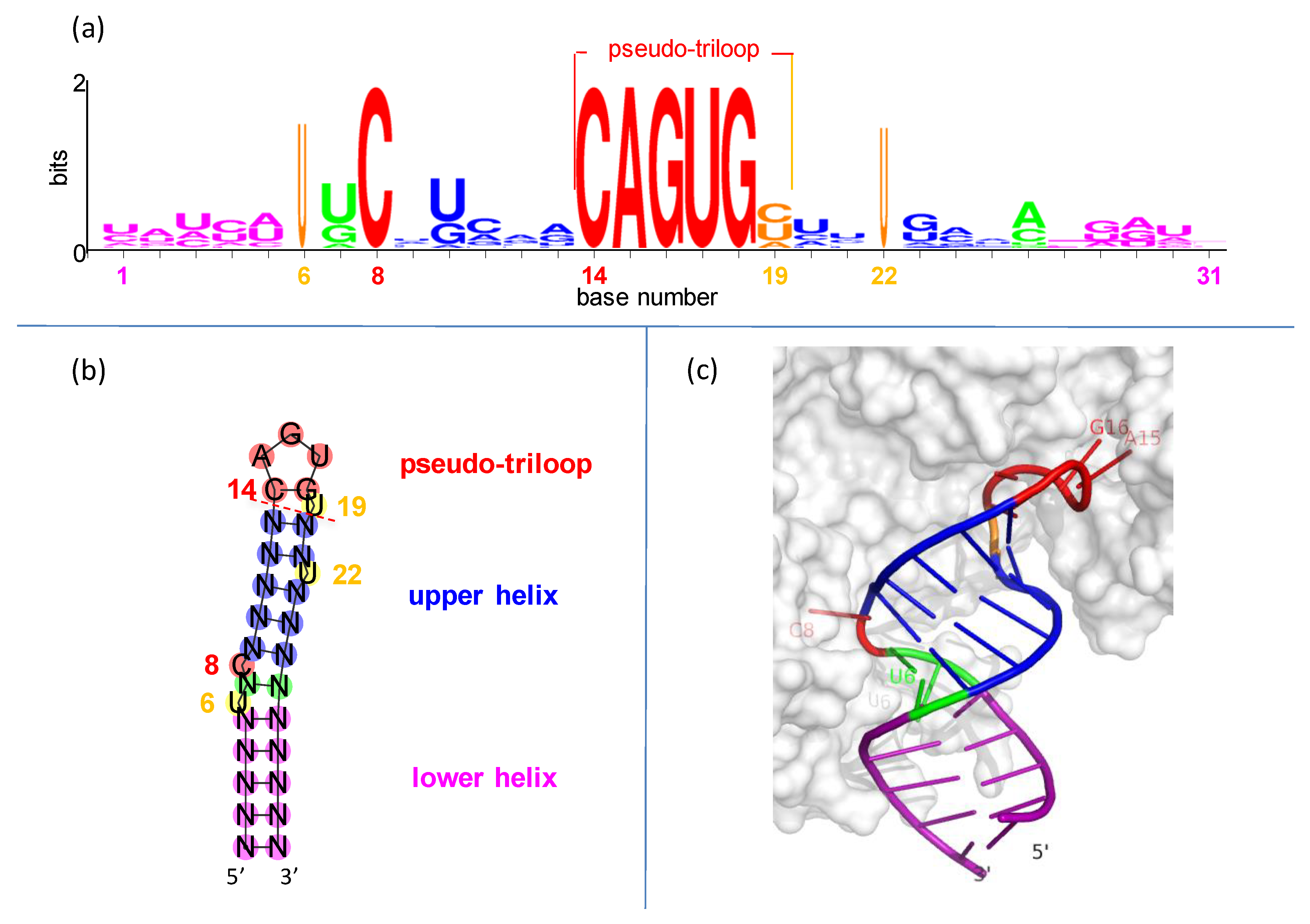
3.2. IRE Stem-Loops and the PTL Motif
3.3. One Type of IRE PTL Cross-Loop
3.4. Three IRE PTL Recognition Bases
3.5. A Critical IRE PTL Scaffold Position
3.6. A Specific IRE Stem Recognition Base
3.7. Precise Three-Dimensional Spacing
3.8. Additional Criteria for IRE Identification
4. Conclusions
IRE Family Reassessment
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muckenthaler, M.; Galy, B.; Hentze, M. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (ire/irp) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Maio, N.; Zhang, D.-L.; Ghosh, M.C.; Jain, A.; SantaMaria, A.M.; Rouault, T.A. Mechanisms of cellular iron sensing, regulation of erythropoiesis and mitochondrial iron utilization. Semin. Hematol. 2021, 58, 161–174. [Google Scholar] [CrossRef]
- Leibold, E.A.; Munro, H.N. Characterization and evolution of the expressed rat ferritin light subunit gene and its pseudogene family. Conservation of sequences within noncoding regions of ferritin genes. J. Biol. Chem. 1987, 262, 7335–7341. [Google Scholar] [CrossRef]
- Hentze, M.; Caughman, S.; Rouault, T.; Barriocanal, J.; Dancis, A.; Harford, J.; Klausner, R. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science 1987, 238, 1570–1573. [Google Scholar] [CrossRef]
- Dandekar, T.; Stripecke, R.; Gray, N.; Goossen, B.; Constable, A.; Johansson, H.; Hentze, M. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991, 10, 1903–1909. [Google Scholar] [CrossRef]
- Pesole, G.; Liuni, S.; D’Souza, M. PatSearch: A pattern matcher software that finds functional elements in nucleotide and protein sequences and assesses their statistical significance. Bioinformatics 2000, 16, 439–450. [Google Scholar] [CrossRef]
- Piccinelli, P.; Samuelsson, T. Evolution of the iron-responsive element. RNA 2007, 13, 952–966. [Google Scholar] [CrossRef]
- Campillos, M.; Cases, I.; Hentze, M.; Sanchez, M. SIREs: Searching for iron-responsive elements. Nucleic Acids Res. 2010, 38, W360–W367. [Google Scholar] [CrossRef]
- Stevens, S.G.; Gardner, P.P.; Brown, C. Two covariance models for iron-responsive elements. RNA Biol. 2011, 8, 792–801. [Google Scholar] [CrossRef]
- Stevens, S.G.; Brown, C.M. Bioinformatic methods to discover cis-regulatory elements in mRNAs. In Springer Handbook of Bio-/Neuroinformatics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 10, pp. 151–169. [Google Scholar]
- Kalvari, I.; Nawrocki, E.P.; Argasinska, J.; Quinones-Olvera, N.; Finn, R.; Bateman, A.; Petrov, A.I. Non-Coding RNA analysis using the rFam database. Curr. Protoc. Bioinform. 2018, 62, e51. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 2013, 29, 2933–2935. [Google Scholar] [CrossRef]
- Aziz, N.; Munro, H.N. Iron regulates ferritin mRNA translation through a segment of its 5′ untranslated region. Proc. Natl. Acad. Sci. USA 1987, 84, 8478–8482. [Google Scholar] [CrossRef]
- Leibold, E.A.; Munro, H.N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc. Natl. Acad. Sci. USA 1988, 85, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Walden, W.E.; Daniels-McQueen, S.; Brown, P.H.; Gaffield, L.; Russell, D.A.; Bielser, D.; Bailey, L.C.; Thach, R.E. Translational repression in eukaryotes: Partial purification and characterization of a repressor of ferritin mRNA translation. Proc. Natl. Acad. Sci. USA 1988, 85, 9503–9507. [Google Scholar] [CrossRef] [PubMed]
- Koeller, D.M.; Casey, J.; Hentze, M.; Gerhardt, E.M.; Chan, L.N.; Klausner, R.D.; Harford, J.B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc. Natl. Acad. Sci. USA 1989, 86, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.; Bawden, M.; Martin, A.; May, B. Human erythroid 5-aminolevulinate synthase: Promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991, 10, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.A.; Galloway, J.L.; Barut, B.; Foott, H.; Fraenkel, P.; Axe, J.L.; Weber, G.J.; Dooley, K.; Davidson, A.J.; Schmidt, B.; et al. Deficiency of glutaredoxin 5 reveals Fe–S clusters are required for vertebrate haem synthesis. Nat. Cell Biol. 2005, 436, 1035–1039. [Google Scholar] [CrossRef]
- Kohler, S.A.; Henderson, B.R.; Kühn, L.C. Succinate dehydrogenase b mRNA of drosophila melanogaster has a functional iron-responsive element in its 5′-Untranslated region. J. Biol. Chem. 1995, 270, 30781–30786. [Google Scholar] [CrossRef]
- Melefors, Ö. Translational Regulationin Vivoof the Drosophila Melanogaster mRNA Encoding Succinate Dehydrogenase Iron Protein via Iron Responsive Elements. Biochem. Biophys. Res. Commun. 1996, 221, 437–441. [Google Scholar] [CrossRef]
- Gray, N.; Pantopoulos, K.; Dandekar, T.; Ackrell, B.A.; Hentze, M. Translational regulation of mammalian and Drosophila citric acid cycle enzymes via iron-responsive elements. Proc. Natl. Acad. Sci. USA 1996, 93, 4925–4930. [Google Scholar] [CrossRef]
- Kim, H.Y.; LaVaute, T.; Iwai, K.; Klausner, R.D.; Rouault, T.A. Identification of a Conserved and Functional Iron-Responsive Element in the 5′-Untranslated Region of Mammalian Mitochondrial Aconitase. J. Biol. Chem. 1996, 271, 24226–24230. [Google Scholar] [CrossRef]
- Abboud, S.; Haile, D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Lymboussaki, A.; Pignatti, E.; Montosi, G.; Garuti, C.; Haile, D.J.; Pietrangelo, A. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J. Hepatol. 2003, 39, 710–715. [Google Scholar] [CrossRef]
- Gunshin, H.; Allerson, C.R.; Polycarpou-Schwarz, M.; Rofts, A.; Rogers, J.T.; Kishi, F.; Hentze, M.W.; Rouault, T.A.; Andrews, N.C.; Hediger, M.A. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001, 509, 309–316. [Google Scholar] [CrossRef]
- Sanchez, M.; Galy, B.; Dandekar, T.; Bengert, P.; Vainshtein, Y.; Stolte, J.; Muckenthaler, M.; Hentze, M. Iron regulation and the cell cycle: Identification of an iron-responsive element in the 3′-untranslated region of human cell division cycle 14A mRNA by a refined microarray-based screening strategy. J. Biol. Chem. 2006, 281, 22865–22874. [Google Scholar] [CrossRef]
- Sanchez, M.; Galy, B.; Muckenthaler, M.; Hentze, M. Iron-regulatory proteins limit hypoxia-inducible factor-2α expression in iron deficiency. Nat. Struct. Mol. Biol. 2007, 14, 420–426. [Google Scholar] [CrossRef]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef]
- Barquist, L.; Burge, S.W.; Gardner, P. Studying RNA homology and conservation with infernal: From single sequences to RNA families. Curr. Protoc. Bioinform. 2016, 54, 12–13. [Google Scholar] [CrossRef]
- Hendrix, D.K.; Brenner, S.E.; Holbrook, S.R. RNA structural motifs: Building blocks of a modular biomolecule. Q. Rev. Biophys. 2005, 38, 221–243. [Google Scholar] [CrossRef]
- Svoboda, P.; Cara, A.D. Hairpin RNA: A secondary structure of primary importance. Cell. Mol. Life Sci. 2006, 63, 901–908. [Google Scholar] [CrossRef]
- Bevilacqua, P.C.; Blose, J. Structures, kinetics, thermodynamics, and biological functions of RNA hairpins. Annu. Rev. Phys. Chem. 2008, 59, 79–103. [Google Scholar] [CrossRef]
- Gehring, N.H.; Wahle, E.; Fischer, U. Deciphering the mRNP Code: RNA-Bound determinants of post-transcriptional gene regulation. Trends Biochem. Sci. 2017, 42, 369–382. [Google Scholar] [CrossRef]
- Hentze, M.; Castello, A.; Schwarzl, T.; Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Stephens, R.M. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990, 18, 6097–6100. [Google Scholar] [CrossRef]
- Walden, W.E.; Selezneva, A.I.; Dupuy, J.; Volbeda, A.; Fontecilla-Camps, J.C.; Theil, E.C.; Volz, K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science 2006, 314, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.R.; Menotti, E.; Bonnard, C.; Kühn, L.C. Optimal sequence and structure of iron-responsive elements. selection of RNA stem-loops with high affinity for iron regulatory factor. J. Biol. Chem. 1994, 269, 17481–17489. [Google Scholar] [CrossRef]
- Sierzputowska-Gracz, H.; McKenzie, R.A.; Theil, E.C. The importance of a single G in the hairpin loop of the iron responsive element (IRE) in ferritin mRNA for structure: An NMR spectroscopy study. Nucleic Acids Res. 1995, 23, 146–153. [Google Scholar] [CrossRef][Green Version]
- Lee, J.C.; Cannone, J.J.; Gutell, R.R. The lonepair triloop: A new motif in RNA structure. J. Mol. Biol. 2003, 325, 65–83. [Google Scholar] [CrossRef]
- Lisi, V.; Major, F. A comparative analysis of the triloops in all high-resolution RNA structures reveals sequence structure relationships. RNA 2007, 13, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, P.C.J.; Olsthoorn, R.C.L.; Bol, J.F. The brome mosaic virus subgenomic promoter hairpin is structurally similar to the iron-responsive element and functionally equivalent to the minus-strand core promoter stem-loop C. RNA 2002, 8, 110–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Werf, R.; Wijmenga, S.S.; Heus, H.; Olsthoorn, R.C.L. Structural and thermodynamic signatures that define pseudotriloop RNA hairpins. RNA 2013, 19, 1833–1839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laing, L.G.; Hall, K.B. A model of the iron responsive element RNA hairpin loop structure determined from NMR and thermodynamic data. Biochemistry 1996, 35, 13586–13596. [Google Scholar] [CrossRef]
- Dale, T.; Smith, R.; Serra, M.J. A test of the model to predict unusually stable RNA hairpin loop stability. RNA 2000, 6, 608–615. [Google Scholar] [CrossRef]
- Blose, J.M.; Proctor, D.J.; Veeraraghavan, N.; Misra, V.; Bevilacqua, P. Contribution of the closing base pair to exceptional stability in RNA tetraloops: Roles for molecular mimicry and electrostatic factors. J. Am. Chem. Soc. 2009, 131, 8474–8484. [Google Scholar] [CrossRef]
- Goforth, J.B.; Anderson, S.A.; Nizzi, C.P.; Eisenstein, R.S. Multiple determinants within iron-responsive elements dictate iron regulatory protein binding and regulatory hierarchy. RNA 2009, 16, 154–169. [Google Scholar] [CrossRef]
- Jaffrey, S.; Haile, D.J.; Klausner, R.D.; Harford, J.B. The interaction between the iron-responsive element binding protein and its cognate RNA is highly dependent upon both RNA sequence and structure. Nucleic Acids Res. 1993, 21, 4627–4631. [Google Scholar] [CrossRef]
- Henderson, B.R.; Menotti, E.; Kühn, L.C. Iron regulatory proteins 1 and 2 bind distinct sets of RNA target sequences. J. Biol. Chem. 1996, 271, 4900–4908. [Google Scholar] [CrossRef]
- Butt, J.; Kim, H.Y.; Basilion, J.P.; Cohen, S.; Iwai, K.; Philpott, C.C.; Altschul, S.; Klausner, R.D.; Rouault, T.A. Differences in the RNA binding sites of iron regulatory proteins and potential target diversity. Proc. Natl. Acad. Sci. USA 1996, 93, 4345–4349. [Google Scholar] [CrossRef]
- Bengert, P. A software tool-box for analysis of regulatory RNA elements. Nucleic Acids Res. 2003, 31, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Connell, G.J.; Danial, J.S.; Haastruthers, C.X. Evaluation of the iron regulatory protein-1 interactome. BioMetals 2018, 31, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pourcelot, E.; Lénon, M.; Charbonnier, P.; Louis, F.; Mossuz, P.; Moulis, J.-M. The iron regulatory proteins are defective in repressing translation via exogenous 5′ iron responsive elements despite their relative abundance in leukemic cellular models. Metallomics 2018, 10, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Allerson, C.R.; Cazzola, M.; Rouault, T.A. Clinical severity and thermodynamic effects of iron-responsive element mutations in hereditary hyperferritinemia-cataract syndrome. J. Biol. Chem. 1999, 274, 26439–26447. [Google Scholar] [CrossRef] [PubMed]
- Mumford, A.D.; Vulliamy, T.; Lindsay, J.; Watson, A. Hereditary hyperferritinemia-cataract syndrome: Two novel mutations in the l-ferritin iron-responsive element. Blood 1998, 91, 367–368. [Google Scholar] [CrossRef]
- Walden, W.E.; Selezneva, A.; Volz, K. Accommodating variety in iron-responsive elements: Crystal structure of transferrin receptor 1 B IRE bound to iron regulatory protein 1. FEBS Lett. 2011, 586, 32–35. [Google Scholar] [CrossRef]
- Selezneva, A.I.; Walden, W.E.; Volz, K.W. Nucleotide-Specific recognition of iron-responsive elements by iron regulatory protein 1. J. Mol. Biol. 2013, 425, 3301–3310. [Google Scholar] [CrossRef]
- Beaumont, C.; Leneuve, P.; Devaux, I.; Scoazec, J.-Y.; Berthier, M.; Loiseau, M.-N.; Grandchamp, B.; Bonneau, D. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat. Genet. 1995, 11, 444–446. [Google Scholar] [CrossRef]
- Kato, J.; Fujikawa, K.; Kanda, M.; Fukuda, N.; Sasaki, K.; Takayama, T.; Kobune, M.; Takada, K.; Takimoto, R.; Hamada, H.; et al. A mutation, in the iron-responsive element of H Ferritin mRNA, causing autosomal dominant iron overload. Am. J. Hum. Genet. 2001, 69, 191–197. [Google Scholar] [CrossRef]
- Cao, W.; McMahon, M.; Wang, B.; O’Connor, R.; Clarkson, M. A case report of spontaneous mutation (C33>U) in the iron-responsive element of l-ferritin causing hyperferritinemia-cataract syndrome. Blood Cells Mol. Dis. 2010, 44, 22–27. [Google Scholar] [CrossRef]
- Allers, J.; Shamoo, Y. Structure-based analysis of protein-RNA interactions using the program ENTANGLE. J. Mol. Biol. 2001, 311, 75–86. [Google Scholar] [CrossRef]
- Morozova, N.; Allers, J.; Myers, J.; Shamoo, Y. Protein-RNA interactions: Exploring binding patterns with a three-dimensional superposition analysis of high resolution structures. Bioinformatics 2006, 22, 2746–2752. [Google Scholar] [CrossRef]
- Girelli, D.; Corrocher, R.; Bisceglia, L.; Olivieri, O.; De Franceschi, L.; Zelante, L.; Gasparini, P. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: A mutation in the iron-responsive element of ferritin l-subunit gene (the “Verona Mutation”). Blood 1995, 86, 4050–4053. [Google Scholar] [CrossRef]
- Casey, J.; Hentze, M.; Koeller, D.; Caughman, S.; Rouault, T.; Klausner, R.; Harford, J. Iron-responsive elements: Regulatory RNA sequences that control mRNA levels and translation. Science 1988, 240, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Rupani, D.N.; Connell, G.J. Transferrin receptor mRNA interactions contributing to iron homeostasis. RNA 2016, 22, 1271–1282. [Google Scholar] [CrossRef]
- Draper, D.E. Themes in RNA-protein recognition. J. Mol. Biol. 1999, 293, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Showalter, S.; Baker, N.; Tang, C.; Hall, K.B. Iron responsive element RNA flexibility described by NMR and Isotropic reorientational eigenmode dynamics. J. Biomol. NMR 2005, 32, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Flodell, S.; Schleucher, J.; Cromsigt, J.; Ippel, H.; Kidd-Ljunggren, K.; Wijmenga, S. The apical stem-loop of the hepatitis B virus encapsidation signal folds into a stable tri-loop with two underlying pyrimidine bulges. Nucleic Acids Res. 2002, 30, 4803–4811. [Google Scholar] [CrossRef]
- Flodell, S.; Petersen, M.; Girard, F.; Zdunek, J.; Kidd-Ljunggren, K.; Schleucher, J.U.; Wijmenga, S. Solution structure of the apical stem–loop of the human hepatitis B virus encapsidation signal. Nucleic Acids Res. 2006, 34, 4449–4457. [Google Scholar] [CrossRef]
- Haasnoot, P.C.J.; Bol, J.F.; Olsthoorn, R.C.L. A plant virus replication system to assay the formation of RNA pseudotriloop motifs in RNA-protein interactions. Proc. Natl. Acad. Sci. USA 2003, 100, 12596–12600. [Google Scholar] [CrossRef]
- Skov, J.; Gaudin, M.; Podbevšek, P.; Olsthoorn, R.C.L.; Petersen, M. The subgenomic promoter of brome mosaic virus folds into a stem–loop structure capped by a pseudo-triloop that is structurally similar to the triloop of the genomic promoter. RNA 2012, 18, 992–1000. [Google Scholar] [CrossRef][Green Version]
- Jeong, B.R.; Chung, S.-M.; Baek, N.J.; Koo, K.B.; Baik, H.S.; Joo, H.-S.; Chang, C.-S.; Choi, J.W. Characterization, cloning and expression of the ferritin gene from the Korean polychaete, Periserrula leucophryna. J. Microbiol. 2006, 44, 54–63. [Google Scholar]
- Huang, T.-S.; Melefors, Ö.; Lind, M.I.; Söderhäll, K. An atypical Iron-Responsive Element (IRE) within crayfish ferritin mRNA and an Iron Regulatory Protein 1 (IRP1)-like protein from crayfish hepatopancreas. Insect Biochem. Mol. Biol. 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Chiu, Y.-C.; Kuo, C.-M. Molecular cloning and tissue distribution of ferritin in Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish. Immunol. 2006, 21, 279–283. [Google Scholar] [CrossRef]
- Meehan, H.A.; Connell, G.J. The hairpin loop but not the bulged C of the iron responsive element is essential for high affinity binding to iron regulatory Protein-1. J. Biol. Chem. 2001, 276, 14791–14796. [Google Scholar] [CrossRef] [PubMed]
- Barton, H.A.; Eisenstein, R.S.; Bomford, A.; Munro, H.N. Determinants of the interaction between the iron-responsive el-ement-binding protein and its binding site in rat L-Ferritin mRNA. J. Biol. Chem. 1990, 265, 7000–7008. [Google Scholar] [CrossRef]
- Kikinis, Z.; Eisenstein, R.S.; Bettany, A.J.E.; Munro, H.N. Role of RNA secondary structure of the iron-responsive element in translational regulation of ferritin synthesis. Nucleic Acids Res. 1995, 23, 4190–4195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giansily, M.; Beaumont, C.; Desveaux, C.; Hetet, G.; Schved, J.-F.; Aguilar-Martinez, P. Denaturing gradient gel electrophoresis screening for mutations in the hereditary hyperferritinaemia cataract syndrome. Br. J. Haematol. 2001, 112, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Balas, A.; Aviles, M.J.; Garcia-Sanchez, F.; Vicario, J.L. Description of a new mutation in the L-Ferrin iron-responsive element associated with hereditary hyperferritinemia-cataract syndrome in a Spanish family. Blood 1999, 93, 4020–4021. [Google Scholar] [CrossRef]
- Millonig, G.; Muckenthaler, M.; Mueller, S. Hyperferritinaemia-cataract syndrome: Worldwide mutations and phenotype of an increasingly diagnosed genetic disorder. Hum. Genom. 2010, 4, 250–262. [Google Scholar] [CrossRef]
- Leibold, E.A.; Laudano, A.; Yu, Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990, 18, 1819–1824. [Google Scholar] [CrossRef]
- Luscieti, S.; Tolle, G.; Aranda, J.; Campos, C.B.; Risse, F.; Morán, É.; Muckenthaler, M.U.; Sánchez, M. Novel mutations in the ferritin-L iron-responsive element that only mildly impair IRP binding cause hereditary hyperferritinaemia cataract syndrome. Orphanet J. Rare Dis. 2013, 8, 30. [Google Scholar] [CrossRef]
- Bettany, A.; Eisenstein, R.; Munro, H. Mutagenesis of the iron-regulatory element further defines a role for RNA secondary structure in the regulation of ferritin and transferrin receptor expression. J. Biol. Chem. 1992, 267, 16531–16537. [Google Scholar] [CrossRef]
- Jencks, W.P. On the attribution and additivity of binding energies. Proc. Natl. Acad. Sci. USA 1981, 78, 4046–4050. [Google Scholar] [CrossRef]
- Helder, S.; Blythe, A.J.; Bond, C.S.; Mackay, J.P. Determinants of affinity and specificity in RNA-binding proteins. Curr. Opin. Struct. Biol. 2016, 38, 83–91. [Google Scholar] [CrossRef]
- Gunshin, H.; MacKenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nat. Cell Biol. 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Volz, K. The functional duality of iron regulatory protein 1. Curr. Opin. Struct. Biol. 2008, 18, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.R.; Clarke, S.L.; Ho, Y.-H.; Bruss, M.D.; Vasanthakumar, A.; Anderson, S.A.; Eisenstein, R.S. Differential translational control of 5′ ire-containing mRNA in response to dietary iron deficiency and acute iron overload. Metallomics 2020, 12, 2186–2198. [Google Scholar] [CrossRef]
- Mathews, D.H.; Moss, W.N.; Turner, D.H. Folding and finding RNA secondary structure. Cold Spring Harb. Perspect. Biol. 2010, 2, a003665. [Google Scholar] [CrossRef] [PubMed]
- Goossen, B.; Caughman, S.W.; Harford, J.B.; Klausner, R.D.; Hentze, M.W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990, 9, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Goossen, B.; Hentze, M.W. Position is the critical determinant for function of iron-responsive elements as translational regulators. Mol. Cell. Biol. 1992, 12, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Stripecke, R.; Oliveira, C.C.; McCarthy, J.E.; Hentze, M.W. Proteins binding to 5′ untranslated region sites: A general mechanism for translational regulation of mRNAs in human and yeast cells. Mol. Cell. Biol. 1994, 14, 5898–5909. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.; Gray, N.; Hentze, M.W. IRP-1 Binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol. Cell 1998, 2, 383–388. [Google Scholar] [CrossRef]
- Paraskeva, E.; Gray, N.K.; Schlaäger, B.; Wehr, K.; Hentze, M. Ribosomal pausing and scanning arrest as mechanisms of translational regulation from cap-distal iron-responsive elements. Mol. Cell. Biol. 1999, 19, 807–816. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volz, K. Conservation in the Iron Responsive Element Family. Genes 2021, 12, 1365. https://doi.org/10.3390/genes12091365
Volz K. Conservation in the Iron Responsive Element Family. Genes. 2021; 12(9):1365. https://doi.org/10.3390/genes12091365
Chicago/Turabian StyleVolz, Karl. 2021. "Conservation in the Iron Responsive Element Family" Genes 12, no. 9: 1365. https://doi.org/10.3390/genes12091365
APA StyleVolz, K. (2021). Conservation in the Iron Responsive Element Family. Genes, 12(9), 1365. https://doi.org/10.3390/genes12091365




