Molecular Cloning and Expression Responses of Jarid2b to High-Temperature Treatment in Nile Tilapia (Oreochromis niloticus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Fish Reproduction
2.3. Larvae Culturing and Sampling
2.4. MT and AI Treatments
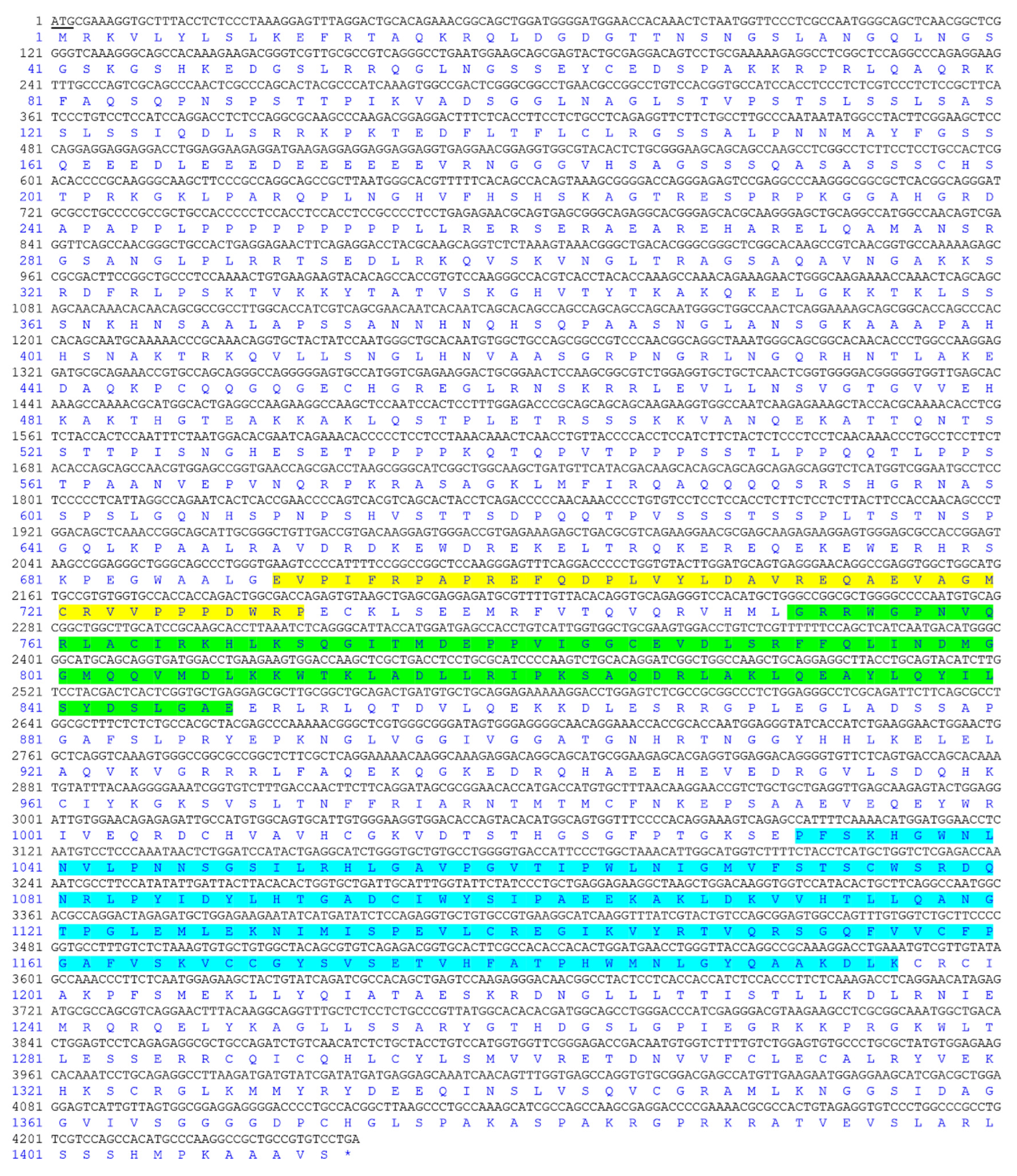
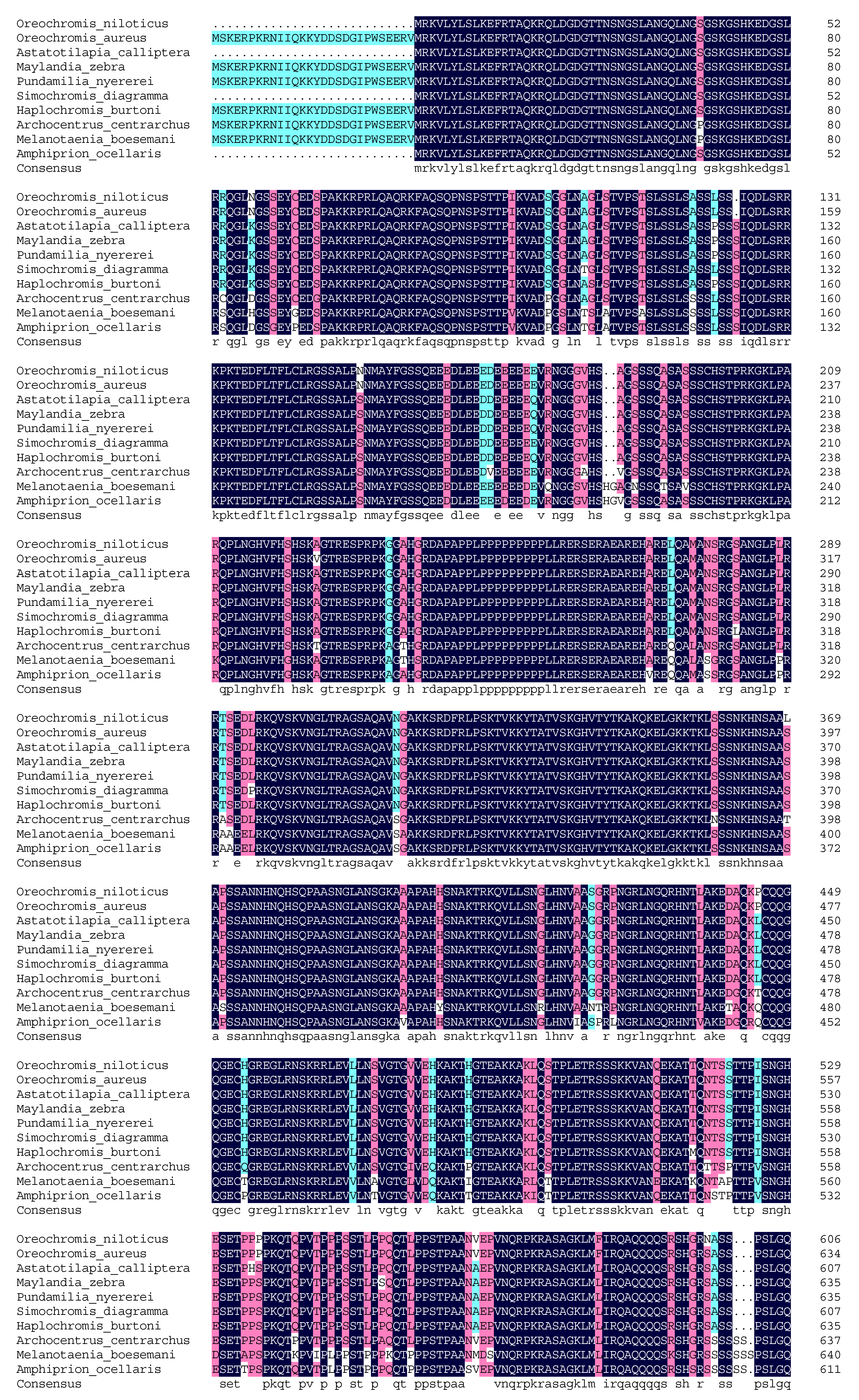
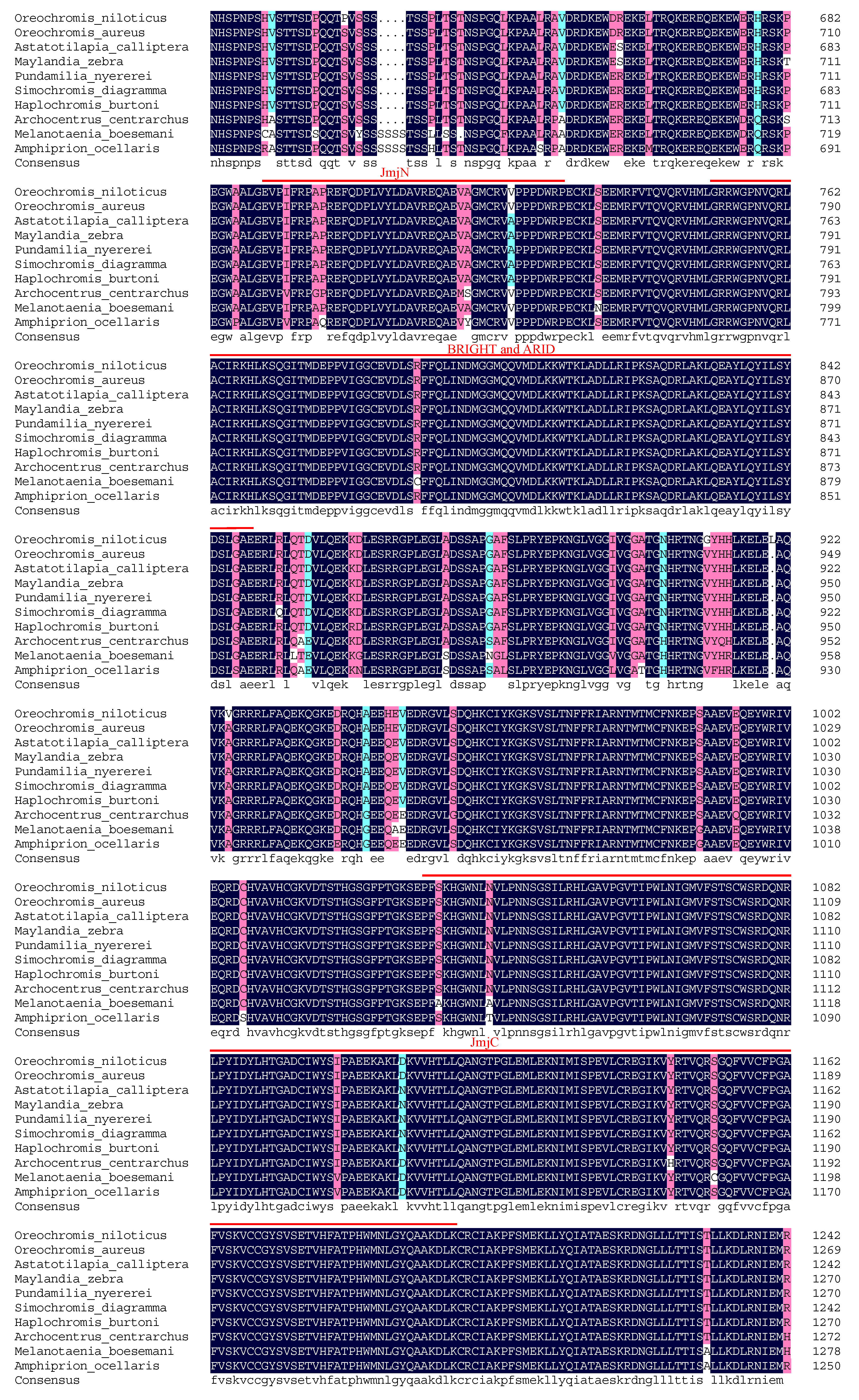
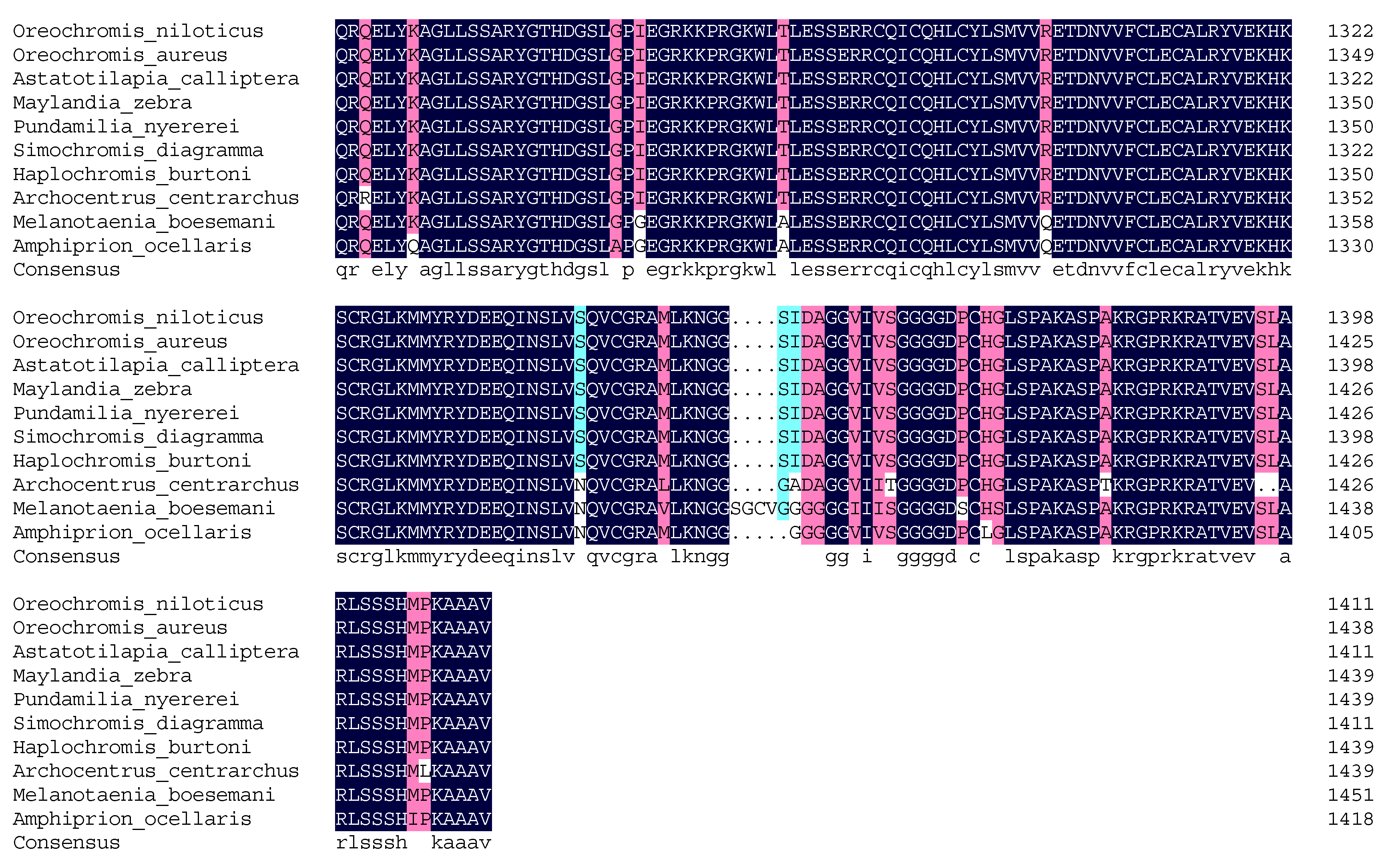
2.5. Molecular Cloning and Bioinformatics Analysis of Tilapia Jarid2b
2.6. qRT-PCR Analysis
2.7. Western Blot Analysis
2.8. Immunohistochemistry Analysis
2.9. Statistical Analysis
3. Results
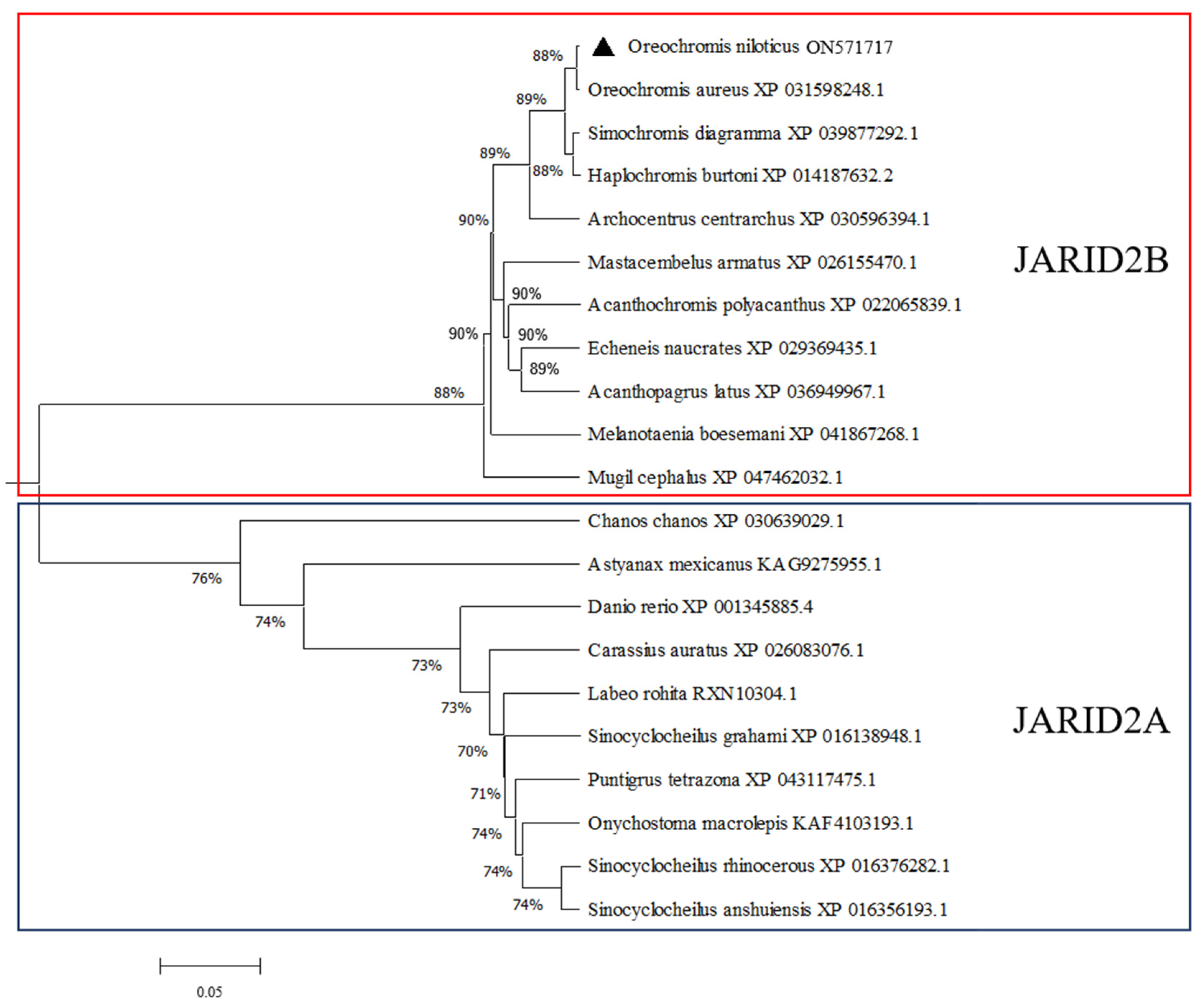
3.1. Molecular Cloning and Bioinformatic Analysis of Jarid2b
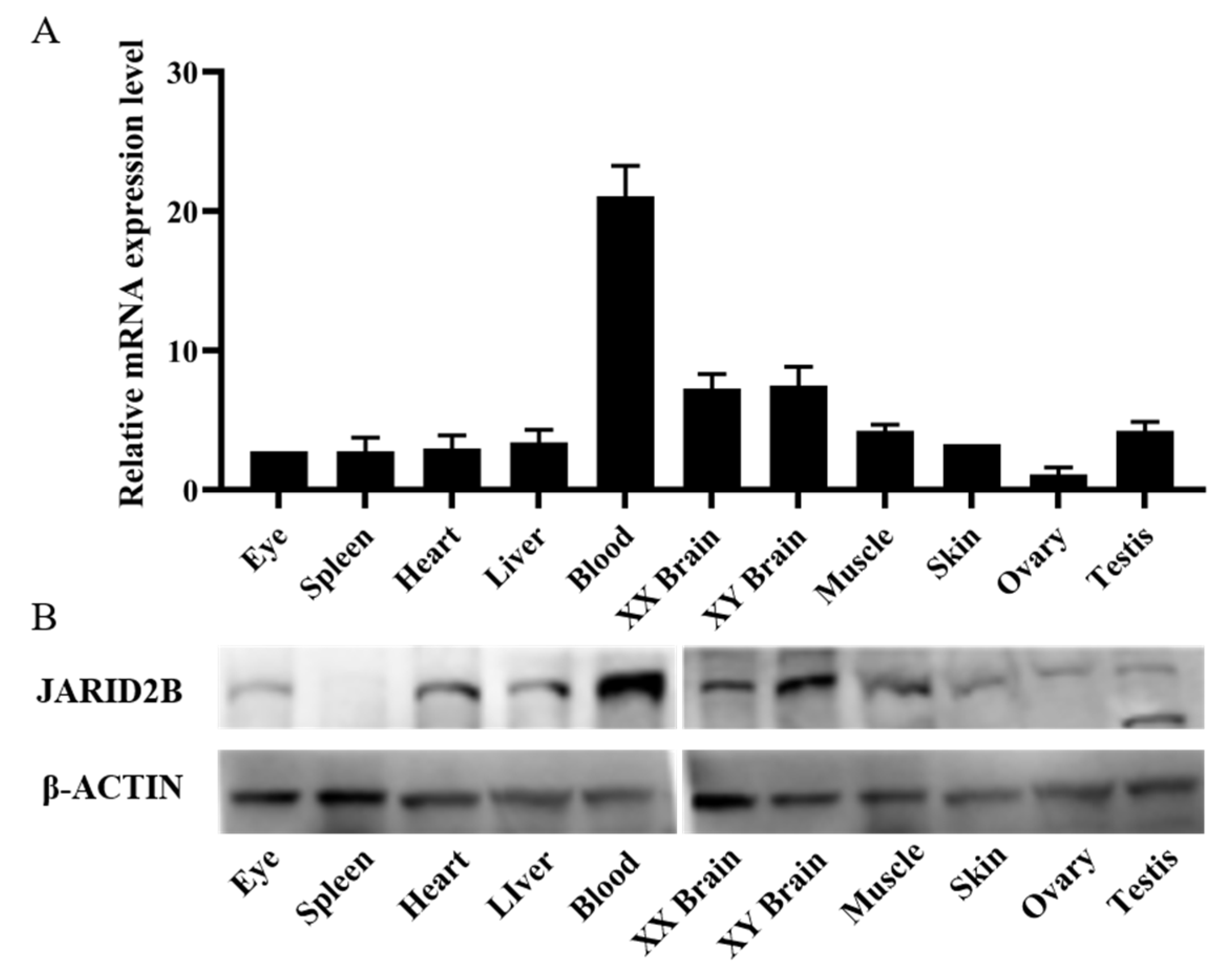
3.2. Tissue Expression Distribution of Jarid2b
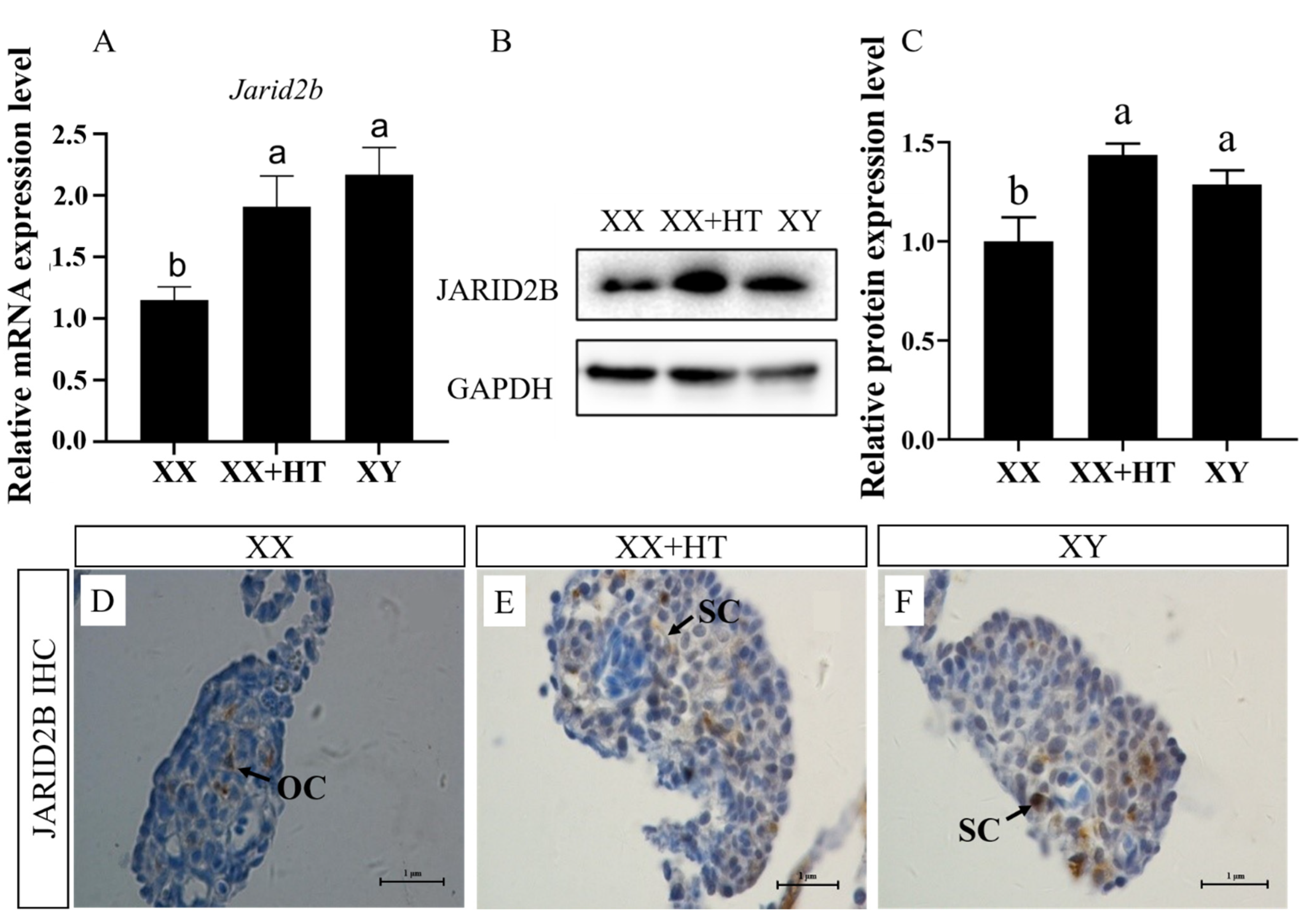
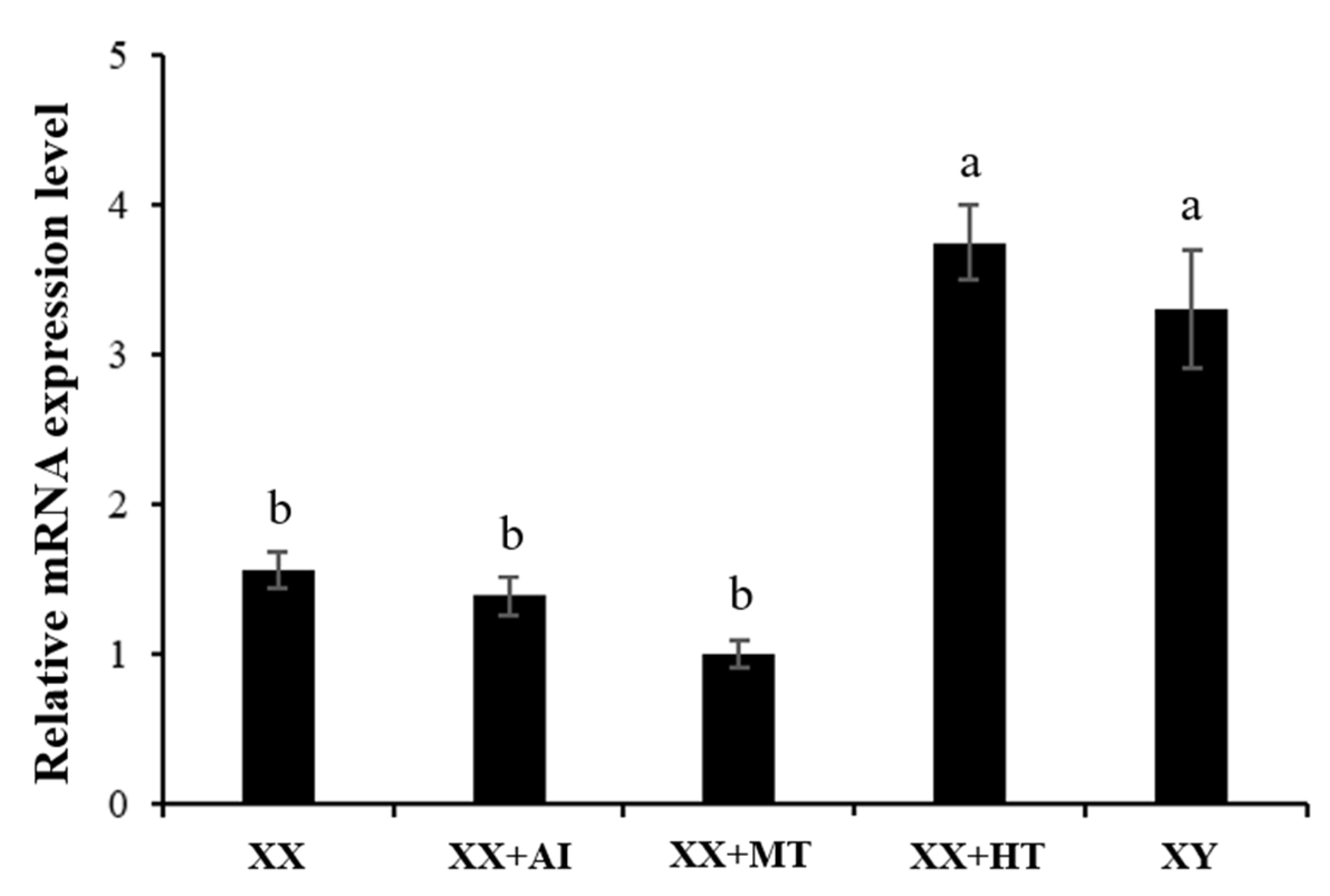
3.3. High-Temperature, but Not MT/letrozole Treatment, Upregulates Nile Tilapia Gonadal Jarid2b mRNA and Protein Expression
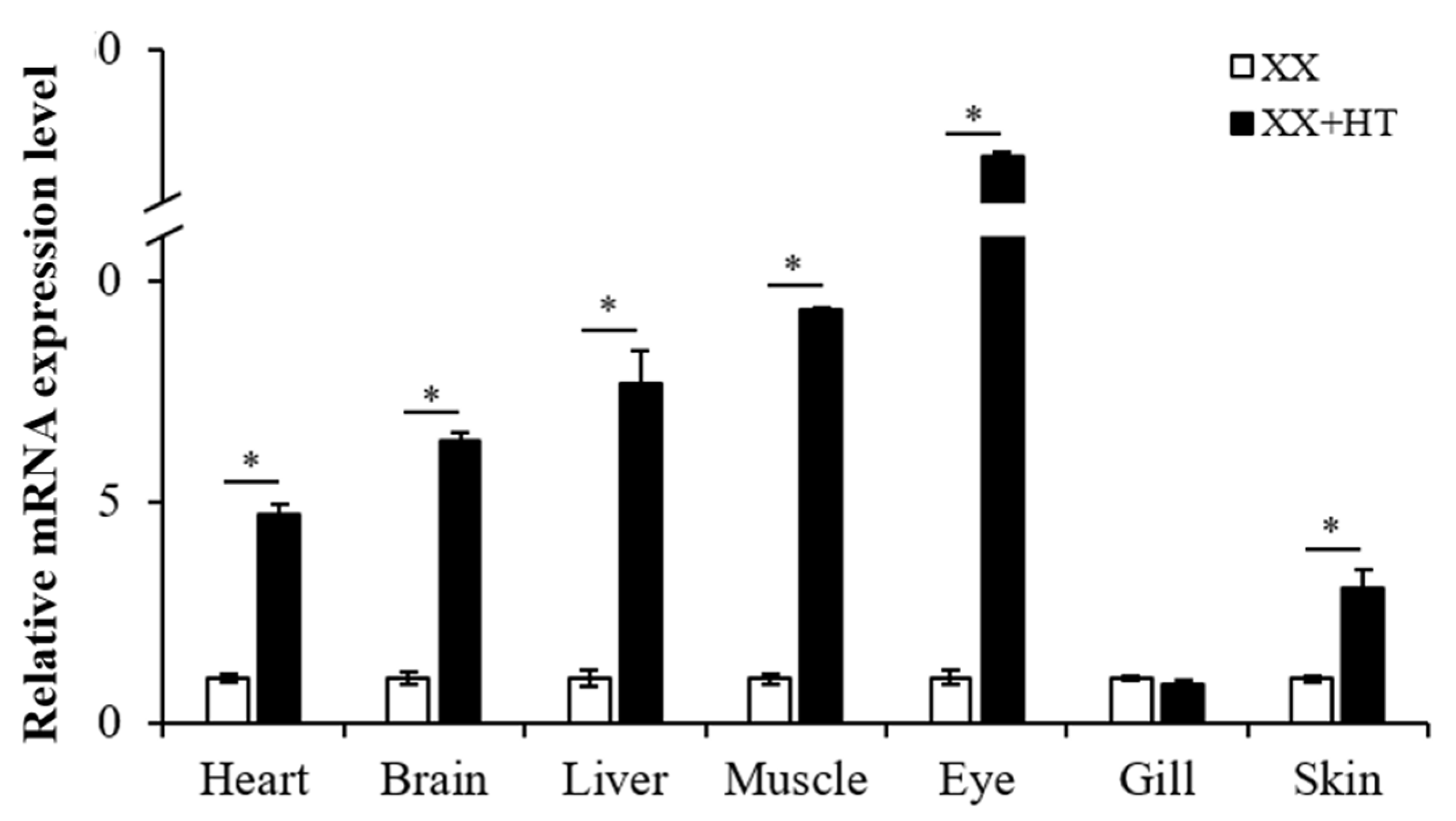
3.4. High-Temperature Treatment Also Upregulates Jarid2b Expression in Other Tissues in Nile Tilapia
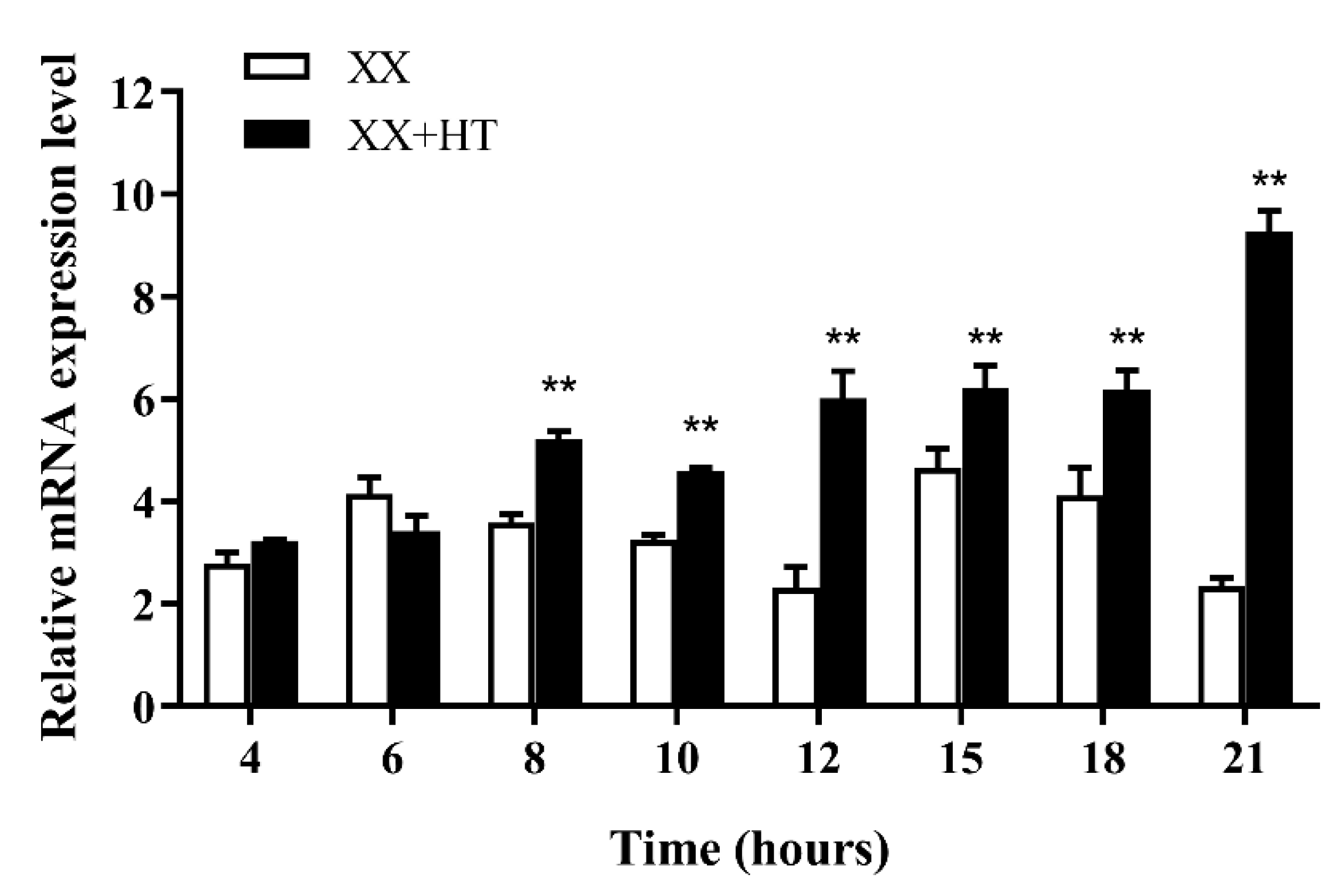
3.5. Jarid2b Expression Responds Early to High-Temperature Treatment
4. Discussion
4.1. The Identification and Tissue Distribution of Jarid2b
4.2. High-Temperature, but Not MT/letrozole Treatment, Affects Jarid2b Expression
4.3. Jarid2b Expression Was Affected Early by High-Temperature Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capel, B. Vertebrate sex determination: Evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 2017, 18, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, M.; Wang, D. Role of sex steroids in fish sex determination and differentiation as revealed by gene editing. Gen. Comp. Endocrinol. 2021, 313, 113893. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Alvarez, N.; Piferrer, F. Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 2008, 3, e2837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mei, Y.; Chen, H.J.; Zhang, L.T.; Wang, H.; Ji, X.S. Profiling expression changes of genes associated with temperature and sex during high temperature-induced masculinization in the Nile tilapia brain. Physiol. Genom. 2019, 51, 159–168. [Google Scholar] [CrossRef]
- Kitano, T.; Takamune, K.; Kobayashi, T.; Nagahama, Y.; Abe, S.I. Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at a high water temperature during a period of sex differentiation in the Japanese flounder (Paralichthys olivaceus). J. Mol. Endocrinol. 1999, 23, 167–176. [Google Scholar] [CrossRef]
- Palaiokostas, C.; Bekaert, M.; Khan, M.G.Q.; Taggart, J.B.; Gharbi, K.; McAndrew, B.J.; Penman, D.J. Mapping and validation of the major sex-determining region in Nile tilapia (Oreochromis niloticus L.) using RAD sequencing. PLoS ONE 2013, 8, e68389. [Google Scholar]
- Wang, Y.Y.; Sun, L.X.; Zhu, J.J.; Zhao, Y.; Wang, H.; Liu, H.J.; Ji, X.S. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization. J. Therm. Biol. 2017, 69, 76–84. [Google Scholar] [CrossRef]
- Baroiller, J.F.; Chourrout, D.; Fostier, A.; Jalabert, B. Temperature and sex-chromosomes govern sex-ratios of the mouthbrooding cichlid fish Oreochromis niloticus. J. Exp. Zool. 1995, 273, 216–223. [Google Scholar] [CrossRef]
- Abucay, J.S.; Mair, G.C.; Skibinski, D.O.F.; Beardmore, J.A. Environmental sex determination: The effect of temperature and salinity on sex ratio in Oreochromis niloticus L. Aquaculture 1999, 173, 219–234. [Google Scholar] [CrossRef]
- Rougeot, C.; Prignon, C.; Kengne, C.N.; Melard, C. Effect of high temperature during embryogenesis on the sex differentiation process in the Nile tilapia, Oreochromis niloticus. Aquaculture 2008, 276, 205–208. [Google Scholar] [CrossRef]
- Eshel, O.; Shirak, A.; Weller, J.I.; Slossman, T.; Hulata, G.; Cnaani, A.; Ron, M. Finemapping of a locus on linkage group 23 for sex determination in Nile tilapia (Oreochromis niloticus). Anim. Genet. 2011, 42, 222–224. [Google Scholar] [CrossRef]
- Eshel, O.; Shirak, A.; Weller, J.I.; Hulata, G.; Ron, M. Linkage and physical mapping of sex region on LG23 of Nile tilapia (Oreochromis niloticus). G3 2012, 2, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K. A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.C.; Valouev, A.; Swigut, T.; Zhang, J.; Zhao, Y.; Sidow, A.; Wysocka, J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 2009, 139, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhu, Z.; Han, G.; Lin, H.; Xu, L.; Chen, C.D. JMJD3 is a histone H3K27 demethylase. Cell Res. 2007, 17, 850–857. [Google Scholar] [CrossRef]
- Sanulli, S.; Justin, N.; Teissandier, A.; Ancelin, K.; Portoso, M.; Caron, M.; Michaud, A.; Lombard, B.; da Rocha, S.T.; Offer, J.; et al. Jarid2 Methylation via the PRC2 Complex Regulates H3K27me3 Deposition during Cell Differentiation. Mol. Cell 2015, 57, 769–783. [Google Scholar] [CrossRef]
- Li, G.; Margueron, R.; Ku, M.; Chambon, P.; Bernstein, B.E.; Reinberg, D. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010, 24, 368–380. [Google Scholar] [CrossRef]
- Pasini, D.; Cloos, P.A.; Walfridsson, J.; Olsson, L.; Bukowski, J.P.; Johansen, J.V.; Bak, M.; Tommerup, N.; Rappsilber, J.; Helin, K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 2010, 464, 306–310. [Google Scholar] [CrossRef]
- Mysliwiec, M.R.; Carlson, C.D.; Tietjen, J.; Hung, H.; Ansari, A.Z.; Lee, Y. Jarid2 (Jumonji, AT rich interactive domain 2) regulates NOTCH1 expression via histone modification in the developing heart. J. Biol. Chem. 2012, 287, 1235–1241. [Google Scholar] [CrossRef]
- Kasinath, V.; Beck, C.; Sauer, P.; Poepsel, S.; Kosmatka, J.; Faini, M.; Toso, D.; Aebersold, R.; Nogales, E. JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications. Science 2021, 371, eabc3393. [Google Scholar] [CrossRef]
- Takeuchi, T.; Watanabe, Y.; Takano-Shimizu, T.; Kondo, S. Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev. Dyn. 2006, 235, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Wang, H. Polycomb repressive complex 2 in embryonic stem cells: An overview. Protein Cell 2010, 1, 1056–1062. [Google Scholar] [CrossRef]
- Vizán, P.; Beringer, M.; Ballaré, C.; Di Croce, L. Role of PRC2-associated factors in stem cells and disease. FEBS J. 2015, 282, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Xu, J.F.; Chang, R.M.; Fang, F.; Zuo, C.H.; Yang, L.Y. JARID2 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial-mesenchymal transition through PTEN/AKT signaling. Oncotarget 2016, 7, 40266–40284. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Mysliwiec, M.R.; Carlson, C.D.; Ansari, A.; Schwartz, R.J.; Lee, Y. Cardiac-specific developmental and epigenetic functions of Jarid2 during embryonic development. J. Biol. Chem. 2018, 293, 11659–11673. [Google Scholar] [CrossRef]
- Landeira, D.; Bagci, H.; Malinowski, A.R.; Brown, K.E.; Soza-Ried, J.; Feytout, A.; Webster, Z.; Ndjetehe, E.; Cantone, I.; Asenjo, H.G.; et al. Jarid2 Coordinates Nanog Expression and PCP/Wnt Signaling Required for Efficient ESC Differentiation and Early Embryo Development. Cell Rep. 2015, 12, 573–586. [Google Scholar] [CrossRef]
- Senthilkumaran, B.; Sudhakumari, C.C.; Mamta, S.K.; Raghuveer, K.; Swapna, I.; Murugananthkumar, R. “Brain sex differentiation” in teleosts: Emerging concepts with potential biomarkers. Gen. Comp. Endocrinol. 2015, 220, 33–40. [Google Scholar] [CrossRef]
- Castañeda-Cortés, D.C.; Fernandino, J.I. Stress and sex determination in fish: From brain to gonads. Int. J. Dev. Biol. 2021, 65, 207–214. [Google Scholar] [CrossRef]
- Deveson, I.W.; Holleley, C.E.; Blackburn, J.; Marshall Graves, J.A.; Mattick, J.S.; Waters, P.D.; Georges, A. Differential intron retention in Jumonji chromatin modifier genes is implicated in reptile temperature-dependent sex determination. Sci. Adv. 2017, 3, e1700731. [Google Scholar] [CrossRef]
- Whiteley, S.L.; McCuaig, R.D.; Holleley, C.E.; Rao, S.; Georges, A. Dynamics of epigenetic modifiers and environmentally sensitive proteins in a reptile with temperature induced sex reversal. Biol. Reprod. 2022, 106, 132–144. [Google Scholar] [CrossRef]
- Whiteley, S.L.; Wagner, S.; Holleley, C.E.; Deveson, I.W.; Marshall Graves, J.A.; Georges, A. Truncated jarid2 and kdm6b transcripts are associated with temperature-induced sex reversal during development in a dragon lizard. Sci. Adv. 2022, 8, eabk0275. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Guner, Y.; Celik, P. Effect of orally-administered 17α-methyltestosterone at different doses on the sex reversal of the Nile Tilapia (Oreochromis niloticus, Linneaus 1758). J. Anim. Vet. Adv. 2011, 10, 853–857. [Google Scholar] [CrossRef]
- Sun, L.X.; Teng, J.; Zhao, Y.; Li, N.; Wang, H.; Ji, X.S. Gonad transcriptome analysis of high temperature treated-females and high temperature-induced sex-reversal neomales in Nile tilapia. Int. J. Mol. Sci. 2018, 19, 689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.J.; Wang, Y.Y.; Mei, Y.; Huang, L.B.; Wang, H.; Ji, X.S. Gonad development examination of high-temperature-treated genetically female Nile tilapia. Aquaculture 2020, 515, 734535. [Google Scholar] [CrossRef]
- Wang, J.Y.; Ma, Y.X.; Hu, Q.M.; Peng, F.; Zhou, M.; Ji, X.S.; Zhao, Y. All-male Nile tilapia larvae production using high-temperature and low dose of MT combination treatment. Aquaculture 2022, 546, 737311. [Google Scholar] [CrossRef]
- Teng, J.; Chen, H.J.; Xu, G.P.; Wang, Y.Y.; Ji, X.S. Quantitative comparative analysis uncovered the role of E2 in Nile tilapia GSD + TE. Aquaculture 2020, 529, 735656. [Google Scholar] [CrossRef]
- SMS2. Available online: http://www.detaibio.com/sms2/protein_iep.html (accessed on 2 May 2022).
- SignalP 3.0. Available online: http://www.cbs.dtu.dk/services/SignalP-3.0/ (accessed on 2 May 2022).
- SMART. Available online: http://smart.embl-heidelberg.de/ (accessed on 2 May 2022).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Takeuchi, T.; Yamazaki, Y.; Katoh-Fukui, Y.; Tsuchiya, R.; Kondo, S.; Motoyama, J.; Higashinakagawa, T. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995, 9, 1211–1222. [Google Scholar] [CrossRef]
- Balciunas, D.; Ronne, H. Evidence of domain swapping within the Jumonji family of transcription factors. Trends Biochem. Sci. 2000, 25, 274–276. [Google Scholar] [CrossRef]
- Clissold, P.M.; Ponting, C.P. JmjC: Cupin metalloenzyme-like domains in jumonji, hairless and phospholipase A2β. Trends Biochem. Sci. 2001, 26, 7–9. [Google Scholar] [CrossRef]
- Trewick, S.C.; McLaughlin, P.J.; Allshire, R.C. Methylation: Lost in hydroxylation? EMBO Rep. 2005, 6, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, Y.; Fang, J.; Erdjument-Bromage, H.; Warren, M.E.; Borchers, C.H.; Tempst, P.; Zhang, Y. Histone demethylation by a family of JmjC-domain-containing proteins. Nature 2006, 439, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Kallin, E.M.; Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 2006, 7, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Oliver, S.G.; Zhang, N. JmjN interacts with JmjC to ensure selective proteolysis of Gis1 by the proteasome. Microbiology. 2011, 157, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chandrasekharan, M.B.; Chen, Y.C.; Bhaskara, S.; Hiebert, S.W.; Sun, Z.W. The JmjN domain of Jhd2 is important for its protein stability, and the plant homeodomain (PHD) finger mediates its chromatin association independent of H3K4 methylation. J. Biol. Chem. 2010, 285, 24548–24561. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Z.; Yang, S.; Wang, F.; Gao, T.; Tao, W.; Zhou, L.; Wang, D.; Sun, L. Genome-wide identification, evolution of histone lysine demethylases (KDM) genes and their expression during gonadal development in Nile tilapia. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110674. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Tahara, N.; Kawakami, Y.; Das, S.; Koyano-Nakagawa, N.; Gong, W.; Garry, M.G.; Garry, D.J. Etv2-miR-130a-Jarid2 cascade regulates vascular patterning during embryogenesis. PLoS ONE 2017, 12, e0189010. [Google Scholar] [CrossRef]
- Fellous, A.; Favrel, P.; Guo, X.; Riviere, G. The Jumonji gene family in Crassostrea gigas suggests evolutionary conservation of Jmj-C histone demethylases orthologues in the oyster gametogenesis and development. Gene 2014, 538, 164–175. [Google Scholar] [CrossRef]
- Berge-Lefranc, J.L. Characterization of the human jumonji gene. Hum. Mol. Genet. 1996, 5, 1637–1641. [Google Scholar] [CrossRef]
- Jung, J.; Mysliwiec, M.R.; Lee, Y. Roles of JUMONJI in mouse embryonic development. Dev. Dyn. 2005, 232, 21–32. [Google Scholar] [CrossRef]
- Bovill, E. Induction by left ventricular overload and left ventricular failure of the human Jumonji gene (JARID2) encoding a protein that regulates transcription and reexpression of a protective fetal program. J. Thorac. Cardiovasc. Surg. 2008, 136, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Govoroun, M.; McMeel, O.M.; D’Cotta, H.; Ricordel, M.J.; Smith, T.; Fostier, A.; Guiguen, Y. Steroid enzyme gene expressions during natural and androgen-induced gonadal differentiation in the rainbow trout, Oncorhynchus mykiss. J. Exp. Zool. 2001, 290, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martín, L.; Blázquez, M.; Piferrer, F. Masculinization of the European sea bass (Dicentrarchus labrax) by treatment with an androgen or aromatase inhibitor involves different gene expression and has distinct lasting effects on maturation. Gen. Comp. Endocrinol. 2009, 160, 3–11. [Google Scholar] [CrossRef]
- Holbech, H.; Kinnberg, K.; Petersen, G.I.; Jackson, P.; Hylland, K.; Norrgren, L.; Bjerregaard, P. Detection of endocrine disrupters: Evaluation of a Fish Sexual Development Test (FSDT). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 57–66. [Google Scholar] [CrossRef]
- Hu, Y.; Li, D.; Ma, X.; Liu, R.; Qi, Y.; Yuan, C.; Huang, D. Effects of 2,4-dichlorophenol exposure on zebrafish: Implications for the sex hormone synthesis. Aquat. Toxicol. 2021, 236, 105868. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Haghpanah, V.; Kogson-Hurtado, L.M.; McAndrew, B.J.; Penman, D.J. Masculinization of genetic female nile tilapia (Oreochromis niloticus) by dietary administration of an aromatase inhibitor during sexual differentiation. J. Exp. Zool. 2000, 287, 46–53. [Google Scholar] [CrossRef]
- Peng, G.; Zhao, W.; Shi, Z.; Chen, H.; Liu, Y.; Wei, J.; Gao, F. Cloning HSP70 and HSP90 genes of kaluga (Huso dauricus) and the effects of temperature and salinity stress on their gene expression. Cell Stress Chaperones 2016, 21, 349–359. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, A.; Zhang, J.; Yang, S.; Cui, W.; Xia, D.; Qu, J. Cloning and molecular characterization of PRL and PRLR from turbot (Scophthalmus maximus) and their expressions in response to short-term and long-term low salt stress. Fish Physiol. Biochem. 2020, 46, 501–517. [Google Scholar] [CrossRef]
- Su, C.L.; Deng, T.R.; Shang, Z.; Xiao, Y. JARID2 inhibits leukemia cell proliferation by regulating CCND1 expression. Int. J. Hematol. 2015, 102, 76–85. [Google Scholar] [CrossRef]
- Kuang, W.; Jiang, W.; Chen, Y.; Tian, Y.; Liu, Z. The function and mechanism of the JARID2/CCND1 axis in modulating glioma cell growth and sensitivity to temozolomide (TMZ). Cancer Biol. Ther. 2021, 22, 392–403. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, J.; Ji, A.; Zhang, Q.; Liao, G. Jarid2 enhances the progression of bladder cancer through regulating PTEN/AKT signaling. Life Sci. 2019, 230, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, L.; He, Y.; He, B.; Li, Z.; Zhang, Z.; Zhang, H.; Yuan, X.; Li, J. Activation of Steroidogenesis, Anti-Apoptotic Activity, and Proliferation in Porcine Granulosa Cells by RUNX1 Is Negatively Regulated by H3K27me3 Transcriptional Repression. Genes 2020, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Asada, H.; Kizuka, F.; Tamura, I.; Maekawa, R.; Taketani, T.; Sato, S.; Yamagata, Y.; Tamura, H.; Sugino, N. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology 2013, 154, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, S.A.; Lin, Y.T.; Futtner, C.R.; Salamone, I.M.; Capel, B.; Maatouk, D.M. CBX2 is required to stabilize the testis pathway by repressing Wnt signaling. PLoS Genet. 2019, 15, e1007895. [Google Scholar] [CrossRef]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef]
- Mizuno, T.; Nomoto, Y.; Oka, H.; Kitayama, M.; Takeuchi, A.; Tsubouchi, M.; Yamashino, T. Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 958–976. [Google Scholar] [CrossRef]
- Ford, B.; Deng, W.; Clausen, J.; Oliver, S.; Boden, S.; Hemming, M.; Trevaskis, B. Barley (Hordeum vulgare) circadian clock genes can respond rapidly to temperature in an EARLY FLOWERING 3-dependent manner. J. Exp. Bot. 2016, 67, 5517–5528. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.P.; Hua, X.T.; Liu, Y.F.; Zhang, Z.Q.; Li, X.H.; Liu, Y.; Liu, P.F. HSP70 gene expression responses to the temperature stress in pufferfish (Takifugu rubripes). Biosci. Biotechnol. Biochem. 2021, 85, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
| Primer Pairs | Primer Sequence (from 5′ to 3′) | Amplicon Length/bp | Purpose |
|---|---|---|---|
| Jarid2b-F1 | ATGCGAAAGGTGCTTTACCTCTCC | 4239 | Cloning |
| Jarid2b-R1 | TCAGGACACGGCAGCGGCCTTGGG | ||
| Jarid2b-F2 | GAAGGCATCAAGGTTTATCG | 221 | qRT-PCR |
| Jarid2b-R2 | GCGATCTGATACAGTAGCTT | ||
| β-actin-F | TGACCTCACAGACTACCTCATG | 224 | qRT-PCR |
| β-actin-R | GGCAACGGAACCTCTCATTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Yao, Z.; Zhao, M.; Fang, Q.; Ji, X.; Chen, H.; Zhao, Y. Molecular Cloning and Expression Responses of Jarid2b to High-Temperature Treatment in Nile Tilapia (Oreochromis niloticus). Genes 2022, 13, 1719. https://doi.org/10.3390/genes13101719
Zhou M, Yao Z, Zhao M, Fang Q, Ji X, Chen H, Zhao Y. Molecular Cloning and Expression Responses of Jarid2b to High-Temperature Treatment in Nile Tilapia (Oreochromis niloticus). Genes. 2022; 13(10):1719. https://doi.org/10.3390/genes13101719
Chicago/Turabian StyleZhou, Min, Zhilei Yao, Min Zhao, Qingfeng Fang, Xiangshan Ji, Hongju Chen, and Yan Zhao. 2022. "Molecular Cloning and Expression Responses of Jarid2b to High-Temperature Treatment in Nile Tilapia (Oreochromis niloticus)" Genes 13, no. 10: 1719. https://doi.org/10.3390/genes13101719
APA StyleZhou, M., Yao, Z., Zhao, M., Fang, Q., Ji, X., Chen, H., & Zhao, Y. (2022). Molecular Cloning and Expression Responses of Jarid2b to High-Temperature Treatment in Nile Tilapia (Oreochromis niloticus). Genes, 13(10), 1719. https://doi.org/10.3390/genes13101719






