Comparative Mitogenomic Analyses and New Insights into the Phylogeny of Thamnocephalidae (Branchiopoda: Anostraca)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. PCR Amplification, Sequencing, Sequence Assembly, and Gene Annotation
2.3. Analysis of Sequence Divergence
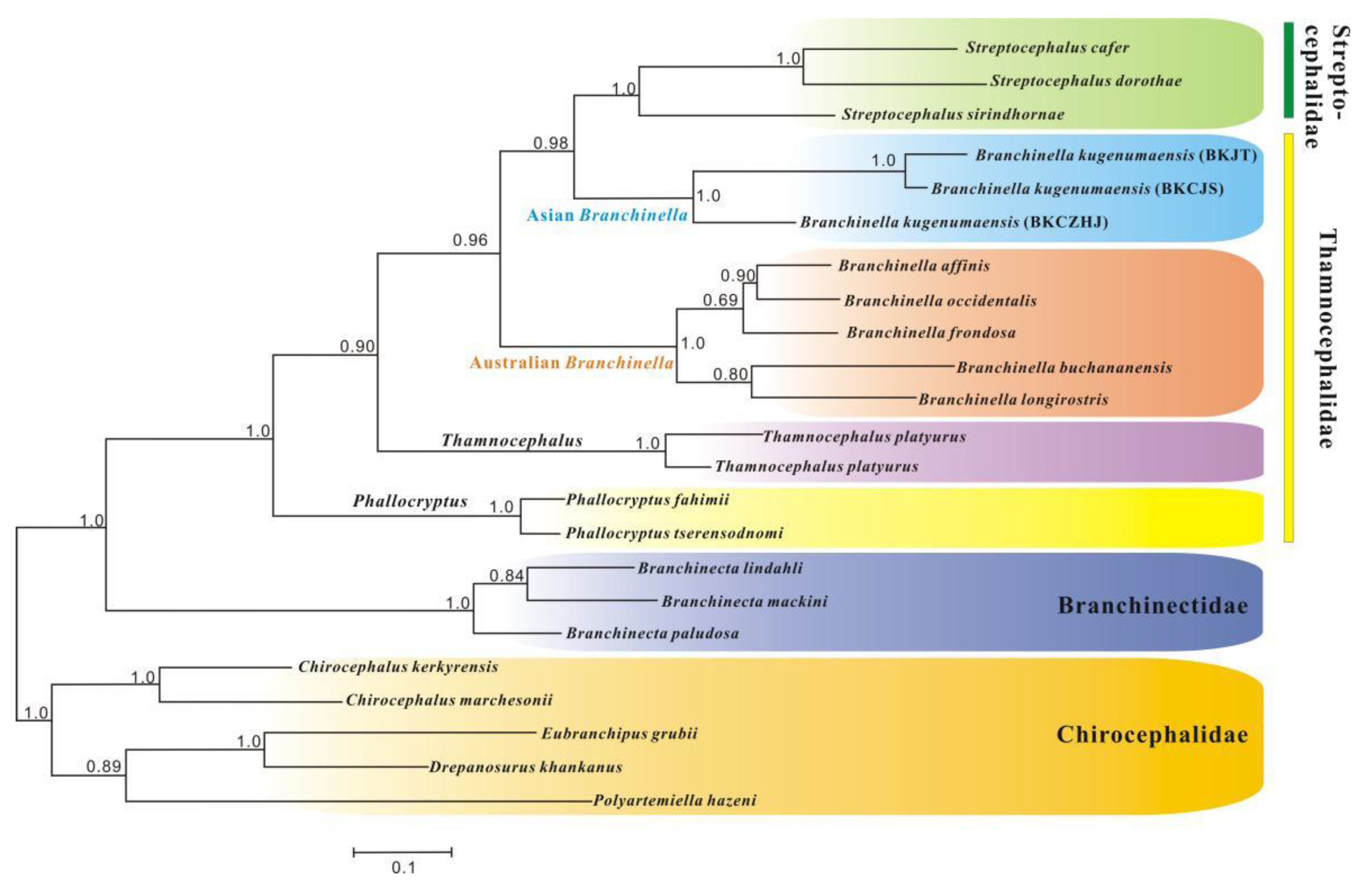
2.4. Phylogenetic Analysis
3. Results and Discussion
3.1. General Features of B. kugenumaensis Mitogenomes
3.2. Sequence Divergence within B. kugenumaensis Mitogenomes
3.3. Phylogenetic Analyses
3.3.1. Phylogenetic Position of B. kugenumaensis from Jinhua, Zhejiang Province (MN660045)
3.3.2. Paraphyly of Thamnocephalidae
3.3.3. Phylogenetic Relationships among Anostracan Families
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, D.C. Anostraca Catalogus (Crustacea: Branchiopoda). Raffles Bull. Zool. 2013, 61, 525–546. [Google Scholar]
- Belk, D. Patterns in anostracan distribution. In Vernal Pools and Intermittent Streams; Subnodh, J., Moyle, P., Eds.; University of California: Davis, CA, USA, 1984; pp. 168–172. [Google Scholar]
- Brendonck, L.; Rogers, D.C.; Olesen, J.; Weeks, S.; Hoeh, W.R. Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater. Hydrobiologia 2008, 595, 167–176. [Google Scholar] [CrossRef]
- Lindholm, M.; Angles d’Auriac, M.A.D.; Thaulow, J.; Hobæk, A. Dancing around the pole: Holarctic phylogeography of the Arctic fairy shrimp Branchinecta paludosa (Anostraca, Branchiopoda). Hydrobiologia 2016, 772, 189–205. [Google Scholar] [CrossRef]
- Rogers, D.C.; Kotov, A.A.; Sinev, A.Y.; Glagolev, S.M.; Korovchinsky, N.M.; Smirnov, N.N.; Bekker, E.I. Chapter 16.2—Arthropoda: Class Branchiopoda. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Rogers, D.C., Thorp, J.H., Eds.; Academic Press: London, UK, 2019. [Google Scholar] [CrossRef]
- Trusova, E.K. First find of Mesozoic members of the order Anostraca (Crustacea). Paleontol. J. 1971, 1971, 481–485. [Google Scholar]
- Van Houte, E.; Hegna, T.A.; Butler, A.D. A new genus and species of ?parthenogenic anostracan (Pancrustacea, Branchiopoda, ?Thamnocephalidae) from the Lower Cretaceous Koonwarra Fossil Bed in Australia. Alcheringa Australas. J. Palaeontol. 2022, 46, 180–187. [Google Scholar] [CrossRef]
- Timms, B. A review of the biology of Australian halophilic anostracans (Branchiopoda: Anostraca). J. Biol. Res.-Thessalon. 2014, 21, 21. [Google Scholar] [CrossRef]
- Linder, F. Contributions to the morphology and the taxonomy of the Branchiopoda Anostraca. Zool. Bidr. Från Upps. 1941, 20, 101–303. [Google Scholar]
- Belk, D.; Brtek, J. Checklist of the Anostraca. Hydrobiologia 1995, 298, 315–353. [Google Scholar] [CrossRef]
- Belk, D.; Brtek, J. Supplement to ‘Checklist of the Anostraca’. Hydrobiologia 1997, 359, 243–245. [Google Scholar] [CrossRef]
- Brtek, J.; Mura, G. Revised key to the families and genera of the Anostraca with notes on their geographical distribution. Crustaceana 2000, 73, 1037–1088. [Google Scholar] [CrossRef]
- Rogers, D.C. A morphological re-evaluation of the anostracan families Linderiellidae and Polyartemiidae, with a redescription of the linderiellid Dexteria floridana (Dexter 1956) (Crustacea: Branchiopoda). Hydrobiologia 2002, 486, 57–61. [Google Scholar] [CrossRef]
- Remigio, E.A.; Hebert, P.D. Affinities among anostracan (Crustacea: Branchiopoda) families inferred from phylogenetic analyses of multiple gene sequences. Mol. Phylogenet. Evol. 2000, 17, 117–128. [Google Scholar] [CrossRef]
- Spears, T.; Abele, L.G. Branchiopod monophyly and interordinal phylogeny inferred from 18S ribosomal DNA. J. Crustac. Biol. 2000, 20, 1–24. [Google Scholar] [CrossRef]
- Weekers, P.H.; Murugan, G.; Vanfleteren, J.R.; Belk, D.; Dumont, H.J. Phylogenetic analysis of anostracans (Branchiopoda: Anostraca) inferred from nuclear 18S ribosomal DNA (18S rDNA) sequences. Mol. Phylogenet. Evol. 2002, 25, 535–544. [Google Scholar] [CrossRef]
- Richter, S.; Olesen, J.; Wheeler, W.C. Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci. Cladistics 2007, 23, 301–336. [Google Scholar] [CrossRef]
- Olesen, J. Monophyly and phylogeny of Branchiopoda, with focus on morphology and homologies of branchiopod phyllopodous limbs. J. Crustac. Biol. 2007, 27, 165–183. [Google Scholar] [CrossRef]
- Olesen, J. Phylogeny of Branchiopoda (Crustacea)—character evolution and contribution of uniquely preserved fossils. Arthropod Syst. Phylogeny 2009, 67, 3–39. [Google Scholar]
- Kappas, I.; Mura, G.; Synefiaridou, D.; Marrone, F.; Alfonso, G.; Alonso, M.; Abatzopoulos, T.J. Molecular and morphological data suggest weak phylogeographic structure in the fairy shrimp Streptocephalus torvicornis (Branchiopoda, Anostraca). Hydrobiologia 2017, 801, 21–32. [Google Scholar] [CrossRef]
- Rogers, D.C. A genus level revision of the Thamnocephalidae (Crustacea: Branchiopoda: Anostraca). Zootaxa 2006, 1260, 1–25. [Google Scholar] [CrossRef]
- Rogers, D.C.; Shu, S.; Yang, J. The identity of Branchinella yunnanensis, with a brief review of the subgenus Branchinellites (Branchiopoda: Anostraca: Thamnocephalidae). J. Crustac. Biol. 2013, 33, 576–581. [Google Scholar] [CrossRef]
- Xu, S.; Han, B.; Martínez, A.; Schwentner, M.; Fontaneto, D.; Dumont, H.J.; Kotov, A.A. Mitogenomics of Cladocera (Branchiopoda): Marked gene order rearrangements and independent predation roots. Mol. Phylogenet. Evol. 2021, 164, 107275. [Google Scholar] [CrossRef]
- Kitano, T.; Sato, H.; Takahashi, N.; Igarashi, S.; Hatanaka, Y.; Igarashi, K.; Umetsu, K. Complete mitochondrial genomes of three fairy shrimps from snowmelt pools in Japan. BMC Zool. 2022, 7, 11. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Tian, X.; Liu, W.; Wei, T.; Wang, W.; Dong, Q.; Wang, B.; Meng, Y.; Zhang, R.; Gleason, M.L.; et al. Comparative analysis of the mitochondrial genomes of Colletotrichum gloeosporioides sensu lato: Insights into the evolution of a fungal species complex interacting with diverse plants. BMC Genom. 2017, 18, 171. [Google Scholar] [CrossRef]
- Drosopoulou, E.; Syllas, A.; Goutakoli, P.; Zisiadis, G.; Konstantinou, T.; Pangea, D.; Sentis, G.; van Sauers-Muller, A.; Wee, S.; Augustinos, A.A.; et al. The complete mitochondrial genome of Bactrocera carambolae (Diptera: Tephritidae): Genome description and phylogenetic implications. Insects 2019, 10, 429. [Google Scholar] [CrossRef]
- Sun, X. Divergence across the mitogenomes of Branchinella kugenumaensis (Anostraca: Thamnocephalidae) with implications for species delimitation. Mitochondrial DNA Part B 2021, 6, 631–633. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, J. Characterization of the complete mitochondrial genome of Chinese Triops granarius and implications for species delimitation. Int. J. Biol. Macromol. 2019, 135, 734–744. [Google Scholar] [CrossRef]
- Rogers, D.C. Revision of the Thamnocephalid genus Phallocryptus (Crustacea: Branchiopoda: Anostraca). Zootaxa 2003, 257, 1–14. [Google Scholar] [CrossRef]
- Xia, X. DAMBE6: New tools for microbial genomics, phylogenetics, and molecular evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. Tracer v1.5. 2009. Available online: http://beast.bio.ed. ac.uk/Tracer (accessed on 12 May 2013).
- Yang, R.; Chen, Y. The complete mitochondrial genome of the freshwater fairy shrimp Branchinella kugenumaensis Ishikawa 1894 (Crustacea: Anostraca: Thamnocephalidae). Mitochondrial DNA Part B 2020, 5, 1048–1049. [Google Scholar] [CrossRef]
- Stothard, P.; Wishart, D.S. Circular genome visualization and exploration using CGView. Bioinformatics 2005, 21, 537–539. [Google Scholar] [CrossRef]
- Hamer, M.; Brendonck, L.; Coomans, A.; Appleton, C. A review of the African Streptocephalidae (Crustacea: Branchiopoda: Anostraca) Part 1: South of Zambezi and Kunene rivers. Arch. Hydrobiol. Suppl. 1994, 99, 235–277. [Google Scholar]
- Yang, M.; Zhang, H.; Song, L.; Shi, Y.; Liu, X. The complete mitochondrial genome of Mahanta tanyae compared with other zygaenoid moths (Lepidoptera: Zygaenoidea). J. Asia-Pac. Entomol. 2019, 22, 513–521. [Google Scholar] [CrossRef]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Gillespie, J.H. The Causes of Molecular Evolution; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Ohta, T. Synonymous and nonsynonymous substitutions in mammalian genes and the nearly neutral theory. J. Mol. Evol. 1995, 40, 56–63. [Google Scholar] [CrossRef]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef]
- Remigio, E.A.; Timms, B.V.; Hebert, P.D.N. Phylogenetic systematics of the Australian fairy shrimp genus Branchinella based on mitochondrial DNA sequences. J. Crustac. Biol. 2003, 23, 436–442. [Google Scholar] [CrossRef]
- Pinceel, T.; Vanschoenwinkel, B.; Waterkeyn, A.; Vanhove, M.P.M.; Pinder, A.; Timms, B.V.; Brendonck, L. Fairy shrimps in distress: A molecular taxonomic review of the diverse fairy shrimp genus Branchinella (Anostraca: Thamnocephalidae) in Australia in the light of ongoing environmental change. Hydrobiologia 2013, 700, 313–327. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, B.; Yang, J. The complete mitogenome of the fairy shrimp Phallocryptus tserensodnomi (Crustacea: Anostraca: Thamnocephalidae). Mitochondrial DNA Part A 2016, 27, 3113–3114. [Google Scholar] [CrossRef]
- Tladi, M.; Dalu, T.; Rogers DCNyamukondiwa, C.; Parbhu, S.P.; Teske, P.R.; Emami-Khoyi, A.; Wasserman, R.J. The complete mitogenome of the fairy shrimp Streptocephalus cafer (Lovén, 1847) (Crustacea: Branchiopoda: Anostraca) from an ephemeral pond in Botswana, southern Africa. Mitochondrial DNA Part B 2020, 5, 623–625. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Jermnak, U.; Yang, J. The complete mitogenome of the freshwater fairy shrimp Streptocephalus sirindhornae (Crustacea: Anostraca: Streptocephalidae). Mitochondrial DNA Part A 2016, 27, 3189–3191. [Google Scholar] [CrossRef]
- Daniels, S.R.; Hamer, M.; Rogers, D.C. Molecular evidence suggests an ancient radiation for the fairy shrimp genus Streptocephalus (Branchiopoda: Anostraca). Biol. J. Linn. Soc. 2004, 82, 313–327. [Google Scholar] [CrossRef]
- Perez, M.; Valverde, J.; Batuecas, B.; Amat, F.; Marco, R.; Garesse, R. Speciation in the Artemia genus: Mitochondrial DNA analysis of bisexual and parthenogenetic brine shrimps. J. Mol. Evol. 1994, 38, 156–168. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Q.; Sun, J.; Liu, F.; Wu, G.; Yu, J.; Wang, W. Mitochondrial genome sequences of Artemia tibetiana and Artemia urmiana: Assessing molecular changes for high plateau adaptation. Sci. China Life Sci. 2013, 56, 440–452. [Google Scholar] [CrossRef]
- Asem, A.; Li, W.; Wang, P.; Eimanifar, A.; Shen, C.; de Vos, S.; van Stappen, G. The complete mitochondrial genome of Artemia sinica Cai, 1989 (Crustacea: Anostraca) using next-generation sequencing. Mitochondrial DNA Part B 2019, 4, 746–747. [Google Scholar] [CrossRef]
- Deji, G.; Zhang, C.; Sui, L.; Han, X. The complete mitochondrial genome of Artemia salina Leach, 1819 (Crustacea: Anostraca). Mitochondrial DNA Part B 2021, 6, 3255–3256. [Google Scholar] [CrossRef]
- Han, X.; Tashi, L.; Sui, L.; Wang, G.; Deji, G.; Zhang, C. The complete mitochondrial genome of Artemia persimilis Piccinelli and Prosdocimi, 1968 (Crustacea: Anostraca). Mitochondrial DNA Part B 2022, 7, 464–465. [Google Scholar] [CrossRef]
- Bellec, L.; Debruyne, R.; Utge, J.; Rabet, N. The first complete mitochondrial genome of Limnadia lenticularis (Branchiopoda, Spinicaudata), with new insights on its phylogeography and on the taxonomy of the genus. Hydrobiologia 2019, 826, 145–158. [Google Scholar] [CrossRef]
- Tladi, M.; Dalu, T.; Rogers, D.C.; Nyamukondiwa, C.; Emami-Khoyi, A.; Oliver, J.C.; Teske, P.R.; Wasserman, R.J. The complete mitogenome of an undescribed clam shrimp of the genus Gondwanalimnadia (Branchiopoda: Spinicaudata), from a temporary wetland in Central District, Botswana. Mitochondrial DNA Part B 2020, 5, 1238–1240. [Google Scholar] [CrossRef] [Green Version]
- Emami-Khoyi, A.; Tladi, M.; Dalu, T.; Teske, P.R.; van Vuuren, B.J.; Rogers, D.C.; Nyamukondiwa, C.; Wasserman, R.J. The complete mitogenome of Leptestheria brevirostris Barnard, 1924, a rock pool clam shrimp (Branchiopoda: Spinicaudata) from Central District, Botswana. Mitochondrial DNA Part B 2021, 6, 608–610. [Google Scholar] [CrossRef]
- Umetsu, K.; Iwabuchi, N.; Yuasa, I.; Saitou, N.; Clark, P.F.; Boxshall, G.; Osawa, M.; Igarashi, K. Complete mitochondrial DNA sequence of a tadpole shrimp (Triops cancriformis) and analysis of museum samples. Electrophoresis 2002, 23, 4080–4084. [Google Scholar] [CrossRef]
- Gan, H.; Tan, M.; Austin, C.M. The complete mitogenome of the Australian tadpole shrimp Triops australiensis (Spencer and Hall, 1895) (Crustacea: Branchiopoda: Notostraca). Mitochondrial DNA 2016, 27, 2028–2029. [Google Scholar] [CrossRef]
- Horn, R.L.; Cowley, D.E. Evolutionary relationships within Triops (Branchiopoda: Notostraca) using complete mitochondrial genomes. J. Crustac. Biol. 2014, 34, 795–800. [Google Scholar] [CrossRef]
- Ryu, J.; Hwang, U. Complete mitochondrial genome of the longtail tadpole shrimp Triops longicaudatus (Crustacea, Branchiopoda, Notostraca). Mitochondrial DNA 2010, 21, 170–172. [Google Scholar] [CrossRef]




| Gene | Strand a | OP133270 and MW136376 | MN660045 | ||||
|---|---|---|---|---|---|---|---|
| Size (nts) | Initiator/Terminator | IGN b | Size (nts) | Initiator/Terminator | IGN b | ||
| trnI | + | 64 | 3 | 64 | 1 | ||
| trnW | - | 63 | 5 | 65 | 3 | ||
| nad2 | - | 888 | ATA/TAA | 0 | 888 | ATA/TAA | 0 |
| trnM | - | 63 | 18, 16 | 63 | 3 | ||
| trnQ | - | 67 | 4 | 68 | 6 | ||
| trnC | - | 61 | 24, 10 | 60 | 20 | ||
| trnY | - | 62 | 23, 28 | 62 | 15 | ||
| cox1 | + | 1534 | TTG/T | 0, −4 | 1534 | TTG/T | 0 |
| trnL2-UUR | + | 63, 64 | 0, 3 | 64 | 0 | ||
| cox2 | + | 687 | ATG/TAA | −5, 0 | 682 | ATG/T | 0 |
| trnK | + | 65 | 5, 2 | 65 | 0 | ||
| trnD | + | 64 | 0, 4 | 62 | 0 | ||
| atp8 | + | 159 | ATT/TAA | −7 | 159 | ATT/TAA | −7 |
| atp6 | + | 660 | ATG/TAA | −1 | 660 | GTG/TAA | −1 |
| cox3 | + | 784 | ATG/T | 0, −1 | 784 | ATG/T | −2 |
| trnG | + | 61 | 0, 1 | 61 | 0 | ||
| nad3 | + | 345 | ATT/TAG | −2 | 345 | ATT/TAA | 5 |
| trnA | + | 63 | 0 | 62 | 0 | ||
| trnR | + | 61 | 7, 8 | 61 | 2 | ||
| trnN | + | 65, 63 | 0, 1 | 65 | 0 | ||
| trnS1-AGN | + | 67, 65 | 0, 1 | 67 | −1 | ||
| trnE | + | 63 | −2 | 64 | −3 | ||
| trnF | - | 63 | −1, 0 | 63 | 0 | ||
| nad5 | - | 1614 | ATT/TAA | 12 | 1624 | TTG/T | 0 |
| trnH | - | 61 | 0 | 63 | 0 | ||
| nad4 | - | 1195 | ATG/T | −8 | 1195 | ATG/T | −8 |
| nad4L | - | 264 | ATT/TAA | 1 | 258 | ATT/TAA | 4 |
| trnT | + | 64 | 0 | 63 | 0 | ||
| trnP | - | 61 | 2 | 61 | 2 | ||
| nad6 | + | 450 | ATT/TAA | −1 | 450 | ATT/TAA | −1 |
| cytb | + | 1137 | ATG/TAA | −2 | 1137 | ATG/TAA | −2 |
| trnS2-UCN | + | 66 | −2 | 66 | −2 | ||
| nad1 | - | 897 | TTG/TAA | 11 | 897 | TTG/TAA | 11 |
| trnL1-CUN | - | 62 | 0 | 62 | 0 | ||
| rrnL | - | 1181, 1179 | 0 | 1180 | 0 | ||
| trnV | - | 65 | 0 | 65 | 0 | ||
| rrnS | - | 707, 706 | 0 | 709 | 0 | ||
| CR | + | 147 | 0 | 1182 | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Cheng, J. Comparative Mitogenomic Analyses and New Insights into the Phylogeny of Thamnocephalidae (Branchiopoda: Anostraca). Genes 2022, 13, 1765. https://doi.org/10.3390/genes13101765
Sun X, Cheng J. Comparative Mitogenomic Analyses and New Insights into the Phylogeny of Thamnocephalidae (Branchiopoda: Anostraca). Genes. 2022; 13(10):1765. https://doi.org/10.3390/genes13101765
Chicago/Turabian StyleSun, Xiaoyan, and Jinhui Cheng. 2022. "Comparative Mitogenomic Analyses and New Insights into the Phylogeny of Thamnocephalidae (Branchiopoda: Anostraca)" Genes 13, no. 10: 1765. https://doi.org/10.3390/genes13101765






