Effect of Combined Methamphetamine and Oxycodone Use on the Synaptic Proteome in an In Vitro Model of Polysubstance Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Primary Rat Neuronal Cultures
2.3. Mass Spectrometry Analysis and Protein Identification
2.4. Bioinformatics Analysis
2.5. Western Blot
2.6. Statistical Analysis
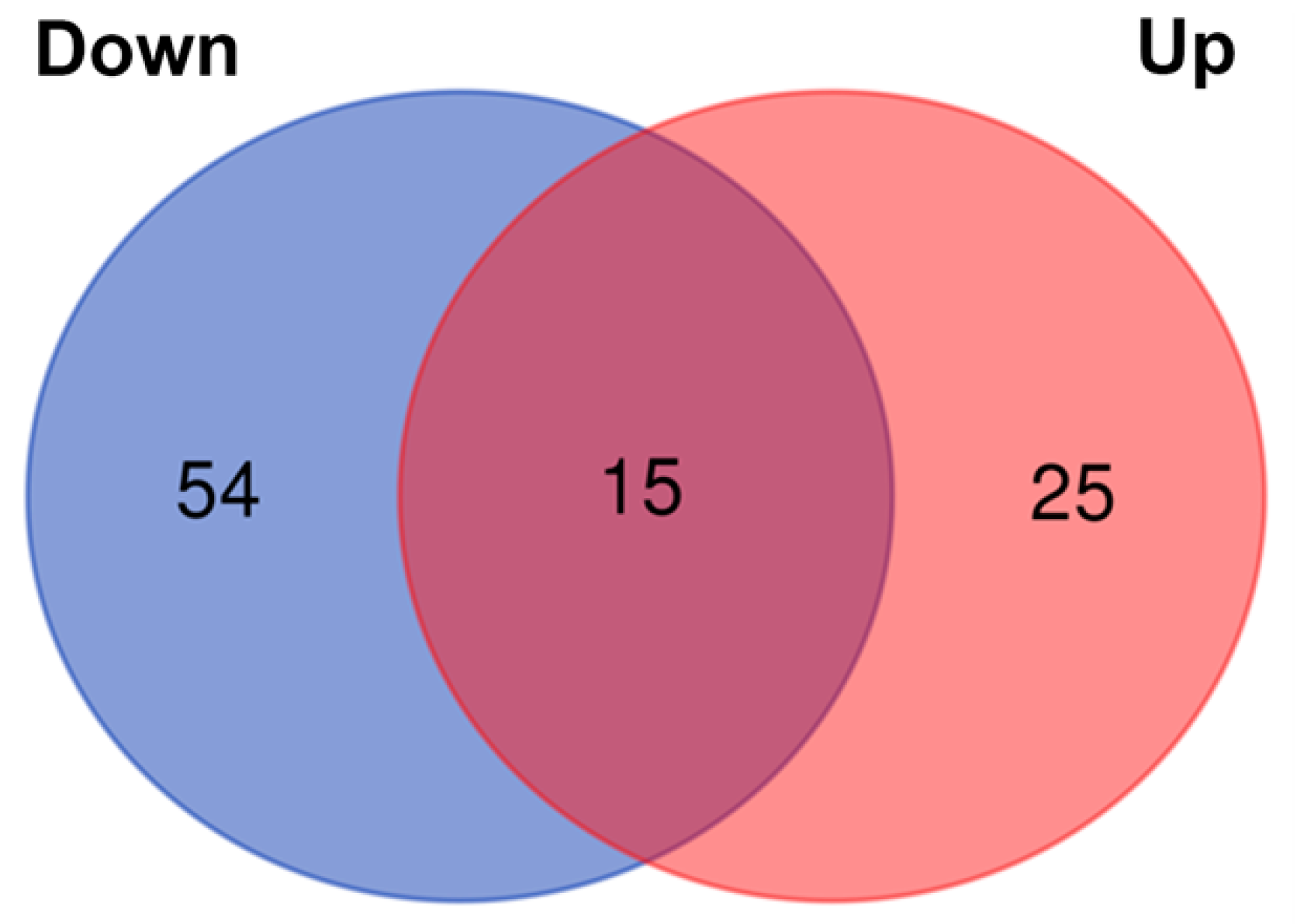
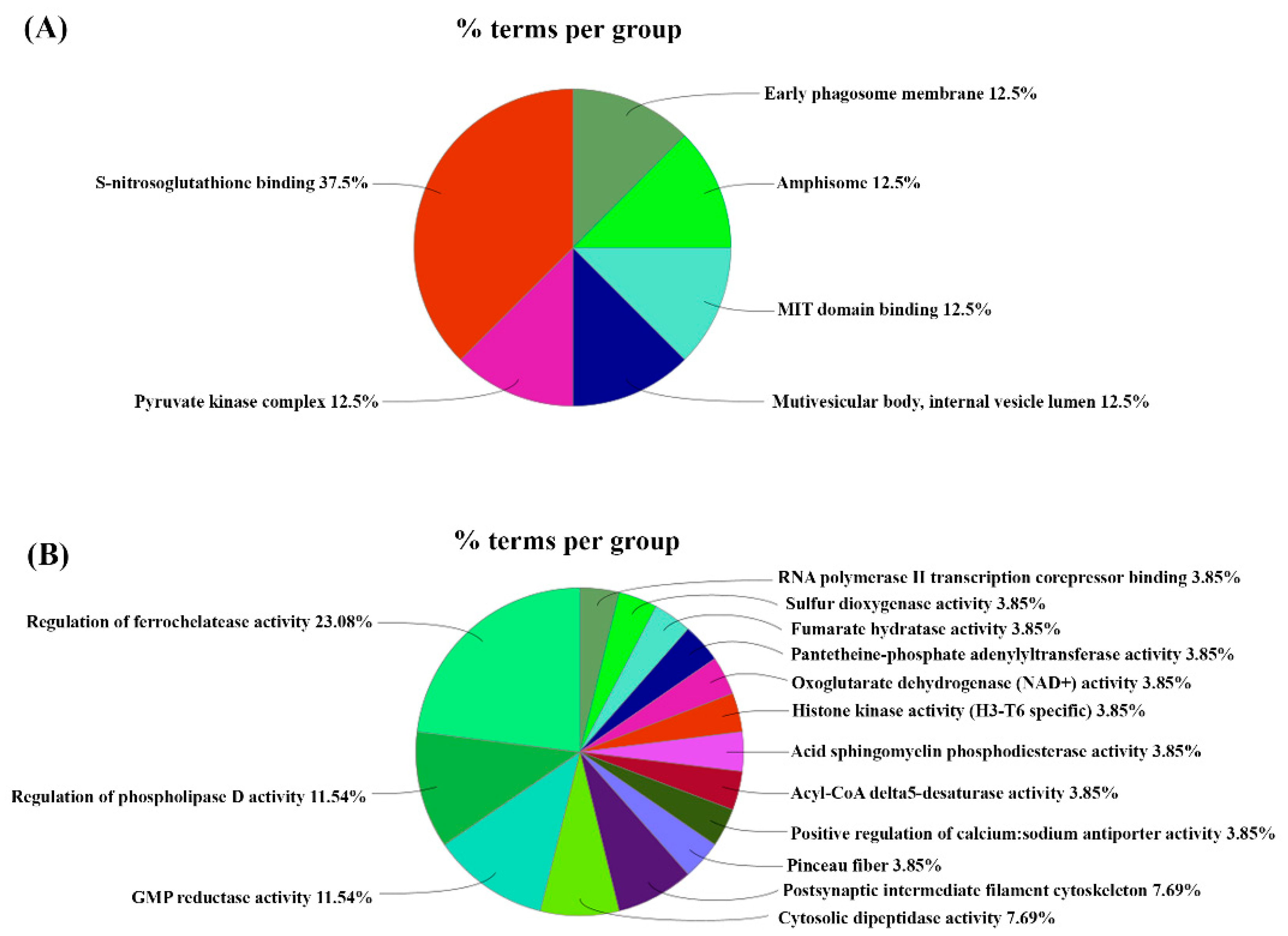
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- NIDA. Overdose Death Rates. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 14 June 2022).
- Palmer, A.; Scott, N.; Dietze, P.; Higgs, P. Motivations for crystal methamphetamine-opioid co-injection/co-use amongst community-recruited people who inject drugs: A qualitative study. Harm Reduct. J. 2020, 17, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusyniak, D.E. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr. Clin. N. Am. 2013, 36, 261–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIDA. How is Methamphetamine Different from Other Stimulants, Such as Cocaine? Available online: https://nida.nih.gov/publications/research-reports/methamphetamine/how-methamphetamine-different-other-stimulants-such-cocaine (accessed on 14 June 2022).
- Radfar, S.R.; Rawson, R.A. Current Research on Methamphetamine: Epidemiology, Medical and Psychiatric Effects, Treatment, and Harm Reduction Efforts. Addict. Health 2015, 6, 146–154. [Google Scholar]
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deodhar, M.; Turgeon, J.; Michaud, V. Contribution of CYP2D6 Functional Activity to Oxycodone Efficacy in Pain Management: Genetic Polymorphisms, Phenoconversion, and Tissue-Selective Metabolism. Pharmaceutics 2021, 13, 1466. [Google Scholar] [CrossRef]
- CDC. U.S. Opioid Dispensing Rate Maps. Available online: https://www.cdc.gov/drugoverdose/rxrate-maps/index.html (accessed on 30 July 2022).
- Sadiq, N.M.D.; Travis, J.; Therese, M. Oxycodone; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Guda, R.S.; Odegaard, K.E.; Tan, C.; Schaal, V.L.; Yelamanchili, S.V. Integrated Systems Analysis of Mixed Neuroglial Cultures Proteome Post Oxycodone Exposure. Int. J. Mol. Sci. 2021, 22, 6421. [Google Scholar] [CrossRef] [PubMed]
- Natividad, L.A.; Buczynski, M.W.; McClatchy, D.B.; Yates, J.R., 3rd. From Synapse to Function: A Perspective on the Role of Neuroproteomics in Elucidating Mechanisms of Drug Addiction. Proteomes 2018, 6, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abul-Husn, N.S.; Devi, L.A. Neuroproteomics of the Synapse and Drug Addiction. J. Pharmacol. Exp. Ther. 2006, 318, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.M.; Vellichirammal, N.N.; Guda, C.; Pendyala, G. Decoding the Synaptic Proteome with Long-Term Exposure to Midazolam during Early Development. Int. J. Mol. Sci. 2022, 23, 4137. [Google Scholar] [CrossRef] [PubMed]
- Koul, S.; Schaal, V.L.; Chand, S.; Pittenger, S.T.; Vellichirammal, N.N.; Kumar, V.; Guda, C.; Bevins, R.A.; Yelamanchili, S.V.; Pendyala, G. Role of Brain Derived Extracellular Vesicles in Decoding Sex Differences Associated with Nicotine Self-Administration. Cells 2020, 9, 1883. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, K.E.; Schaal, V.L.; Clark, A.R.; Koul, S.; Sankarasubramanian, J.; Xia, Z.; Mellon, M.; Uberti, M.; Liu, Y.; Stothert, A.; et al. A Holistic Systems Approach to Characterize the Impact of Pre- and Post-natal Oxycodone Exposure on Neurodevelopment and Behavior. Front. Cell Dev. Biol. 2021, 8, 619199. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Gowen, A.; Savine, M.; Moore, D.; Clark, A.; Huynh, W.; Wu, N.; Odegaard, K.; Weyrich, L.; Bevins, R.A.; et al. A comprehensive study to delineate the role of an extracellular vesicle-associated microRNA-29a in chronic methamphetamine use disorder. J. Extracell. Vesicles 2021, 10, e12177. [Google Scholar] [CrossRef]
- Odegaard, K.E. In utero and Postnatal Oxycodone Exposure: Implications for Intergenerational Effects. Ph.D. Thesis, University of Nebraska Medical Center, Omaha, NE, USA, 2021. [Google Scholar]
- Williamson, A.; Darke, S.; Ross, J.; Teesson, M. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend. 2006, 81, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Staiger, P.K.; Long, C.M.; Carr, V.; Marlatt, G.A.; Richardson, B. Overlooked and underestimated? Problematic alcohol use in clients recovering from drug dependence. Addiction 2012, 108, 1188–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, L.; Molist, G.; Espelt, A.; Barrio, G.; Guitart, A.; Bravo, M.J.; Brugal, M.T. Mortality risk factors and excess mortality in a cohort of cocaine users admitted to drug treatment in Spain. J. Subst. Abus. Treat. 2014, 46, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kedia, S.; Sell, M.A.; Relyea, G. Mono- versus polydrug abuse patterns among publicly funded clients. Subst. Abus. Treat Prev. Policy 2007, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Roy, É.; Richer, I.; Arruda, N.; Vandermeerschen, J.; Bruneau, J. Patterns of cocaine and opioid co-use and polyroutes of administration among street-based cocaine users in Montréal, Canada. Int. J. Drug Policy 2013, 24, 142–149. [Google Scholar] [CrossRef] [Green Version]
- John, W.S.; Wu, L.-T. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend. 2017, 180, 376–384. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular Neurobiology of Addiction. Am. J. Addict. 2001, 10, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular mechanisms of drug addiction. Neuropharmacology 2004, 47, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Badiani, A.; Belin, D.; Epstein, D.; Calu, D.; Shaham, Y. Opiate versus psychostimulant addiction: The differences do matter. Nat. Rev. Neurosci. 2011, 12, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Crummy, E.A.; O’Neal, T.J.; Baskin, B.M.; Ferguson, S.M. One Is Not Enough: Understanding and Modeling Polysubstance Use. Front. Neurosci. 2020, 14, 569. [Google Scholar] [CrossRef]
- Bonci, A.; Williams, J.T. A Common Mechanism Mediates Long-Term Changes in Synaptic Transmission after Chronic Cocaine and Morphine. Neuron 1996, 16, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Saal, D.; Dong, Y.; Bonci, A.; Malenka, R.C. Drugs of Abuse and Stress Trigger a Common Synaptic Adaptation in Dopamine Neurons. Neuron 2003, 37, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Badiani, A.; Oates, M.M.; Day, H.E.; Watson, S.J.; Akil, H.; Robinson, T.E. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav. Brain Res. 1999, 103, 203–209. [Google Scholar] [CrossRef]
- Van den Oever, M.C.; Goriounova, N.A.; Li, K.W.; Van der Schors, R.C.; Binnekade, R.; Schoffelmeer, A.N.; Mansvelder, H.D.; Smit, A.B.; Spijker, S.; De Vries, T.J. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat. Neurosci. 2008, 11, 1053–1058. [Google Scholar] [CrossRef]
- Siegel, A. Neurobiology of Aggression and Rage; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Siegel, A.; Bhatt, S.; Bhatt, R.; Zalcman, S.S. The Neurobiological Bases for Development of Pharmacological Treatments of Aggressive Disorders. Curr. Neuropharmacol. 2007, 5, 135–147. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahadevia, D.; Saha, R.; Manganaro, A.; Chuhma, N.; Ziolkowski-Blake, A.; Morgan, A.A.; Dumitriu, D.; Rayport, S.; Ansorge, M.S. Dopamine promotes aggression in mice via ventral tegmental area to lateral septum projections. Nat. Commun. 2021, 12, 6796. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Dietz, D.M.; Dumitriu, D.; Morrison, J.H.; Malenka, R.C.; Nestler, E.J. The addicted synapse: Mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010, 33, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiga, S.; Mulas, G.; Piras, F.; Diana, M. The “addicted” spine. Front. Neuroanat. 2014, 8, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killinger, B.A.; Moszczynska, A. Epothilone D prevents binge methamphetamine-mediated loss of striatal dopaminergic markers. J. Neurochem. 2015, 136, 510–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ujike, H.; Takaki, M.; Kodama, M.; Kuroda, S. Gene Expression Related to Synaptogenesis, Neuritogenesis, and MAP Kinase in Behavioral Sensitization to Psychostimulants. Ann. N. Y. Acad. Sci. 2006, 965, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Fujii, Y.; Kasai, N.; Okajima, T.; Nakashima, H. Regulation of neuritogenesis in hippocampal neurons using stiffness of extracellular microenvironment. PLoS ONE 2018, 13, e0191928. [Google Scholar] [CrossRef] [Green Version]
- Schevzov, G.; Curthoys, N.M.; Gunning, P.W.; Fath, T. Chapter Two—Functional Diversity of Actin Cytoskeleton in Neurons and its Regulation by Tropomyosin. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 298, pp. 33–94. [Google Scholar]
- Chen, S.-Y.; Lin, M.-C.; Tsai, J.-S.; He, P.-L.; Luo, W.-T.; Chiu, I.-M.; Herschman, H.R.; Li, H.-J. Exosomal 2′,3′-CNP from mesenchymal stem cells promotes hippocampus CA1 neurogenesis/neuritogenesis and contributes to rescue of cognition/learning deficiencies of damaged brain. Stem Cells Transl. Med. 2020, 9, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.E.; Green, M.T.; Kinkade, J.A.; Schmidt, R.R.; Willemse, T.E.; Schenk, A.K.; Mao, J.; Rosenfeld, C.S. Maternal Oxycodone Treatment Results in Neurobehavioral Disruptions in Mice Offspring. Eneuro 2021, 8, ENEURO.0150-21.2021. [Google Scholar] [CrossRef] [PubMed]
- Seip-Cammack, K.M.; Shapiro, M.L. Behavioral flexibility and response selection are impaired after limited exposure to oxycodone. Learn. Mem. 2014, 21, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Hui, R.; Wang, J.; Shen, X.; Xie, B.; Gong, M.; Yu, F.; Cong, B.; Ma, C. Effects of molecular hydrogen on methamphetamine-induced neurotoxicity and spatial memory impairment. Front. Pharmacol. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takashima, Y.; Mandyam, C.D. The role of hippocampal adult neurogenesis in methamphetamine addiction. Brain Plast. 2018, 3, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoist, M.; Gaillard, S.; Castets, F. The striatin family: A new signaling platform in dendritic spines. J. Physiol. 2006, 99, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Bartoli, M.; Castets, F.; Monneron, A. Striatin, a calmodulin-dependent scaffolding protein, directly binds caveolin-1. FEBS Lett. 2001, 508, 49–52. [Google Scholar] [CrossRef]
- Kachidian, P.; Vuillet, J.; Bartoli, M.; Castets, F.; Nieoullon, A.; Goff, L.K.-L. Relationships between striatin-containing neurons and cortical or thalamic afferent fibres in the rat striatum. An ultrastructural study by dual labelling. Neuroscience 1998, 85, 111–122. [Google Scholar] [CrossRef]
- Haeberlé, A.-M.; Castets, F.; Bombarde, G.; Baillat, G.; Bailly, Y. Immunogold localization of phocein in dendritic spines. J. Comp. Neurol. 2006, 495, 336–350. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, D.; Athota, P.; Gowen, A.; Nguyen, N.M.; Schaal, V.L.; Yelamanchili, S.V.; Pendyala, G. Effect of Combined Methamphetamine and Oxycodone Use on the Synaptic Proteome in an In Vitro Model of Polysubstance Use. Genes 2022, 13, 1816. https://doi.org/10.3390/genes13101816
Meyer D, Athota P, Gowen A, Nguyen NM, Schaal VL, Yelamanchili SV, Pendyala G. Effect of Combined Methamphetamine and Oxycodone Use on the Synaptic Proteome in an In Vitro Model of Polysubstance Use. Genes. 2022; 13(10):1816. https://doi.org/10.3390/genes13101816
Chicago/Turabian StyleMeyer, Daniel, Pranavi Athota, Austin Gowen, Nghi M. Nguyen, Victoria L. Schaal, Sowmya V. Yelamanchili, and Gurudutt Pendyala. 2022. "Effect of Combined Methamphetamine and Oxycodone Use on the Synaptic Proteome in an In Vitro Model of Polysubstance Use" Genes 13, no. 10: 1816. https://doi.org/10.3390/genes13101816
APA StyleMeyer, D., Athota, P., Gowen, A., Nguyen, N. M., Schaal, V. L., Yelamanchili, S. V., & Pendyala, G. (2022). Effect of Combined Methamphetamine and Oxycodone Use on the Synaptic Proteome in an In Vitro Model of Polysubstance Use. Genes, 13(10), 1816. https://doi.org/10.3390/genes13101816









