A Novel Transcriptome Approach to the Investigation of the Molecular Pathology of Vitreous and Retinal Detachment
Abstract
1. Introduction
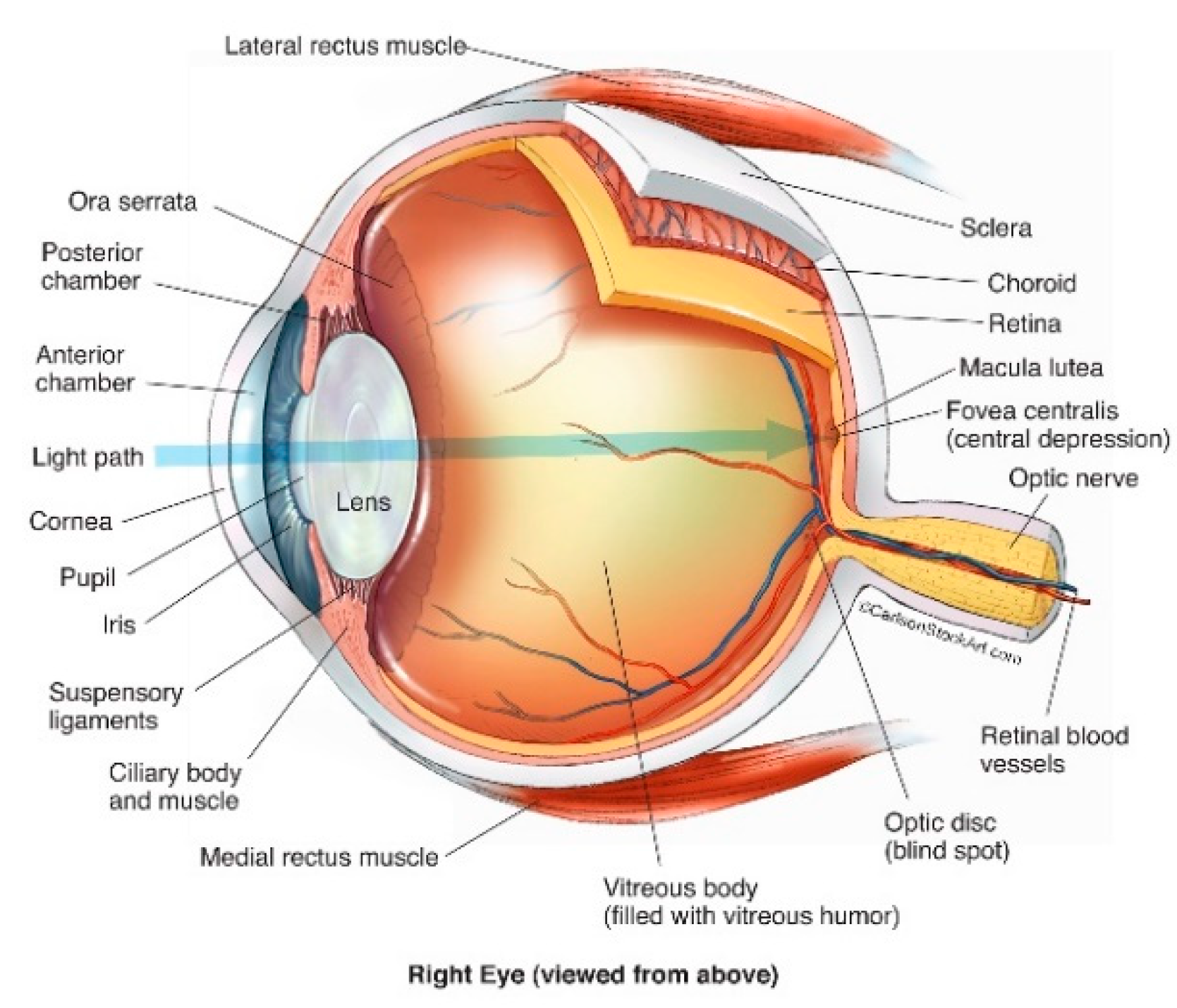
2. Anatomy of the Human Eye
3. Posterior Vitreous Detachment
4. Retinal Detachment
5. Rhegmatogenous Retinal Detachment
5.1. Horseshoe Retinal Tears
5.2. Giant Retinal Tears
5.3. Macular Hole-Associated Retinal Detachment
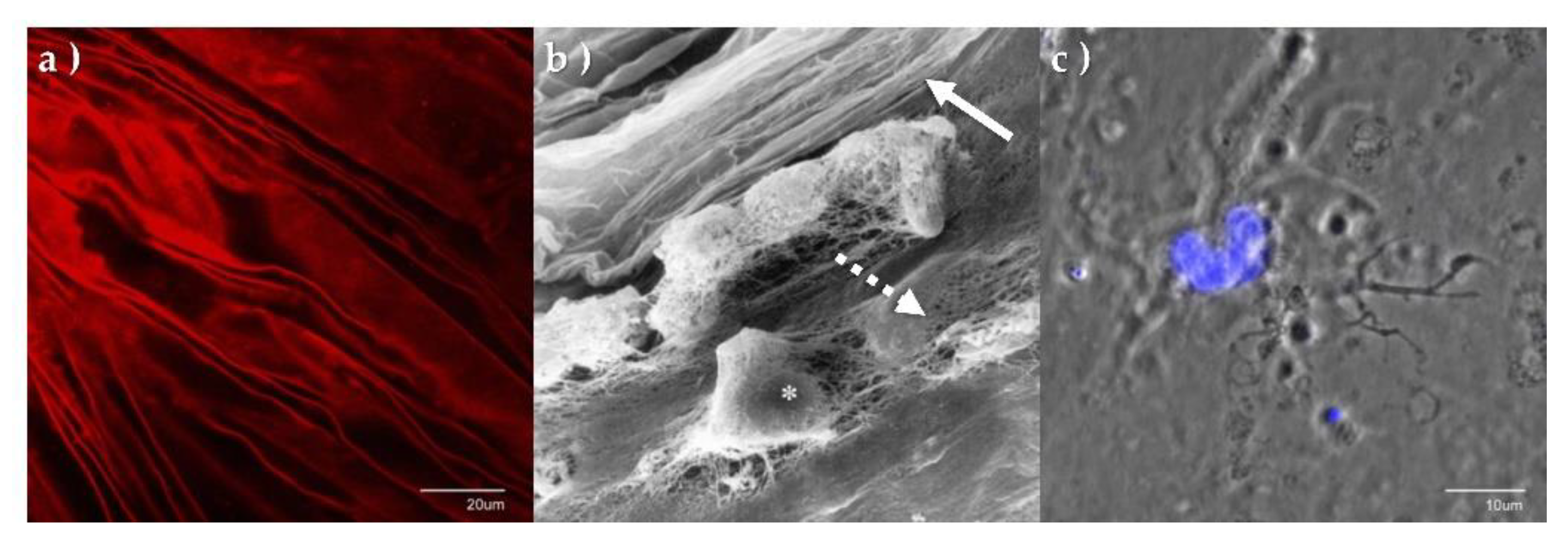
6. The Laminocyte
7. Discussion and Future Projections
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NHS Digital. Latest Figures on Registered Blind and Partially Sighted People in England are Published. 2021. Available online: https://digital.nhs.uk/news/2021/latest-figures-on-registered-blind-and-partially-sighted-people-in-england-are-published (accessed on 17 May 2022).
- Quartilho, A.; Simkiss, P.; Zekite, A.; Xing, W.; Wormald, R.; Bunce, C. Leading causes of certifiable visual loss in England and Wales during the year ending 31 March 2013. Eye 2016, 30, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.; Aylward, B. Rhegmatogenous retinal detachment: A reappraisal of its pathophysiology and treatment. Ophthalmic Res. 2014, 51, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Vitreous and Vision Degrading Myodesopsia. Prog. Retin. Eye Res. 2020, 79, 100847. [Google Scholar] [CrossRef] [PubMed]
- Foos, R.Y.; Wheeler, N.C. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology 1982, 89, 1502–1512. [Google Scholar] [CrossRef]
- Sebag, J. Age-related changes in human vitreous structure. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Akiba, J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology 1993, 100, 1384–1388. [Google Scholar] [CrossRef]
- Weber-Krause, B.; Eckardt, C. Incidence of posterior vitreous detachment in the elderly. Ophthalmologe 1997, 94, 619–623. [Google Scholar] [CrossRef]
- Snead, M.P.; Snead, D.R.J.; Mahmood, A.S.; Scott, J.D. Vitreous detachment and the posterior hyaloid membrane: A clinicopathological study. Eye 1994, 8, 204–209. [Google Scholar] [CrossRef]
- Ang, A.; Poulson, A.V.; Snead, D.R.; Snead, M.P. Posterior vitreous detachment: Current concepts and management. Comp. Ophthalmol. Update 2005, 6, 167. [Google Scholar]
- Kakehashi, A.; Takezawa, M.; Akiba, J. Classification of posterior vitreous detachment. Clin. Ophthalmol. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Snead, M.P.; Snead, D.R.; James, S.; Richards, A.J. Clinicopathological changes at the vitreoretinal junction: Posterior vitreous detachment. Eye 2008, 22, 1257–1262. [Google Scholar] [CrossRef]
- Snead, M.P.; Snead, D.R.; Richards, A.J.; Harrison, J.B.; Poulson, A.V.; Morris, A.H.; Sheard, R.M.; Scott, J.D. Clinical, histological and ultrastructural studies of the posterior hyaloid membrane. Eye 2002, 16, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Fincham, G.; James, S.; Spickett, C.; Hollingshead, M.; Thrasivoulou, C.; Poulson, A.V.; McNinch, A.; Richards, A.J.; Snead, D.; Limb, G.A.; et al. Posterior vitreous detachment and the posterior hyaloid membrane. Ophthalmology 2018, 125, 227–236. [Google Scholar] [CrossRef]
- Snead, D.R.; James, S.; Snead, M.P. Pathological changes in the vitreoretinal junction 1: Epiretinal membrane formation. Eye 2008, 22, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Snead, D.R.J.; Cullen, N.; James, S.; Poulson, A.V.; Morris, A.H.C.; Lukaris, A.; Scott, J.D.; Richards, A.J.; Snead, M.P. Hyperconvolution of the inner limiting membrane in vitreomaculopathies. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 853–862. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, F.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, M.S. Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10885/ (accessed on 1 September 2021).
- Snell, R.S.; Lemp, M.A. Clinical Anatomy of the Eye, 2nd ed.; Wiley: New York, NY, USA, 2013; p. 164. [Google Scholar]
- Snell, R.S.; Lemp, M.A. Clinical Anatomy of the Eye, 2nd ed.; Wiley: New York, NY, USA, 2013; pp. 157, 159. [Google Scholar]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- DaveCarlson/CarlsonStockArt. Available online: https://www.carlsonstockart.com/ (accessed on 25 May 2022).
- Provis, J.M.; Penfold, P.L.; Cornish, E.E.; Sandercoe, T.M.; Madigan, M.C. Anatomy and development of the macula: Specialisation and the vulnerability to macular degeneration. Clin. Exp. Optom. 2005, 88, 269–281. [Google Scholar] [CrossRef]
- Kang, H.K.; Luff, A.J. Management of retinal detachment: A guide for non-ophthalmologists. BMJ 2008, 336, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants 2019, 9, 7. [Google Scholar] [CrossRef]
- Silva, A.F.; Alves, M.A.; Oliveira, M.S.N. Rheological behaviour of vitreous humour. Rheol. Acta 2017, 56, 377–386. [Google Scholar] [CrossRef]
- Le Goff, M.; Bishop, P. Adult vitreous structure and postnatal changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ishibashi, T. Hyalocytes: Essential cells of the vitreous cavity in vitreoretinal pathophysiology? Retina 2011, 31, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. The vitreous. In Adlers Physiology of the Eye, 9th ed.; Hart, W., Ed.; Mosby: St. Louis, MO, USA, 1992; pp. 268–347. [Google Scholar]
- Mitry, D.; Charteris, D.G.; Yorston, D.; Siddiqui, M.A.; Campbell, H.; Murphy, A.L.; Fleck, B.W.; Wright, A.F.; Singh, J. Scottish RD Study Group. The epidemiology and socioeconomic associations of retinal detachment in Scotland: A two-year prospective population-based study. Invest Ophthalmol. Vis. Sci. 2010, 51, 4963–4968. [Google Scholar] [CrossRef]
- Lewis, H. Peripheral retinal degenerations and the risk of retinal detachment. Am. J. Ophthalmol. 2003, 136, 155–160. [Google Scholar] [CrossRef]
- Ghazi, N.; Green, W. Pathology and pathogenesis of retinal detachment. Eye 2002, 16, 411–421. [Google Scholar] [CrossRef]
- Mitry, D.; Charteris, D.G.; Fleck, B.W.; Campbell, H.; Singh, J. The epidemiology of rhegmatogenous retinal detachment: Geographical variation and clinical associations. BJO 2010, 94, 678–684. [Google Scholar] [CrossRef]
- Sultan, Z.N.; Agorogiannis, E.I.; Iannetta, D.; Steel, D.; Sandinha, T. Rhegmatogenous retinal detachment: A review of current practice in diagnosis and management. BMJ Open Ophthalmol. 2020, 5, e000474. [Google Scholar] [CrossRef]
- Sodhi, A.; Leung, L.S.; Do, D.V.; Gower, E.W.; Schein, O.D.; Handa, J.T. Recent Trends in the Management of Rhegmatogenous Retinal Detachment. Surv. Ophthalmol. 2008, 53, 50–67. [Google Scholar] [CrossRef]
- Kirin, M.; Chandra, A.; Charteris, D.G.; Hayward, C.; Campbell, S.; Celap, I.; Bencic, G.; Vatavuk, Z.; Kirac, I.; Richards, A.J.; et al. Genome-wide association study identifies genetic risk underlying primary rhegmatogenous retinal detachment. Hum. Mol. Genet. 2013, 22, 3174–3185. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Tielsch, J.M.; Schein, O.D. Racial difference in the incidence of retinal detachment in Singapore. Arch. Ophthalmol. 1999, 117, 379–383. [Google Scholar] [CrossRef]
- Peters, A.L. Retinal detachment in black South Africans. S. Afr. Med. J. 1995, 85, 158–159. [Google Scholar] [PubMed]
- Go, S.L.; Hoyng, C.B.; Klaver, C.C. Genetic risk of rhegmatogenous retinal detachment: A familial aggregation study. Arch. Ophthalmol. 2005, 123, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.; Hysi, P.; Hammond, C.J.; Prescott, A.; Fincham, G.S.; Poulson, A.V.; McNinch, A.M.; Richards, A.J.; Snead, M.P. Deep Intronic Sequence Variants in COL2A1 Affect the Alternative Splicing Efficiency of Exon 2, and May Confer a Risk for Rhegmatogenous Retinal Detachment. Hum. Mutat. 2016, 37, 1085–1096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ung, T.; Comer, M.; Ang, A.; Sheard, R.; Lee, C.; Poulson, A.V.; Newman, D.K.; Scott, J.D.; Richards, A.J.; Snead, M.P. Clinical features and surgical management of retinal detachment secondary to round retinal holes. Eye 2005, 19, 665–669. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Method Prim. 2021, 1, 59. [Google Scholar] [CrossRef]
- Ross, W.H. Retinal dialysis: Lack of evidence for a genetic cause. Can. J. Ophthalmol. 1991, 26, 309–312. [Google Scholar]
- Freeman, H.M. Fellow eyes of giant retinal breaks. Trans. Am. Ophthalmol. Soc. 1978, 76, 343–382. [Google Scholar]
- Kanski, J.J. Giant retinal tears. Am. J. Ophthalmol. 1975, 79, 846–852. [Google Scholar] [CrossRef]
- Thompson, J.A.; Snead, M.P.; Billington, B.M.; Barrie, T.; Thompson, J.R.; Sparrow, J.M. National audit of the outcome of primary surgery for rhegmatogenous retinal detachment. II. Clinical outcomes. Eye 2002, 16, 771–777. [Google Scholar] [CrossRef]
- Margehria, R.R.; Schepens, C.L. Macular breaks 1. Diagnosis, etiology and observations. Am. J. Ophthalmol. 1972, 74, 219–232. [Google Scholar]
- Morita, H.; Ideta, H.; Yonemoto, J.; Sasaki, K.; Tanaka, S. Causative factors of retinal detachment in macular holes. Retina 1991, 11, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.; Schepens, C.L.; Von Pirquet, S.R. Retinoschisis. I. Seniletype: A clinical report of one hundred seven cases. Arch. Ophthalmol. 1960, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hagler, W.S.; Woldroff, H.S. Retinal detachment in relation to senile retinoschisis. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1973, 77, 99–113. [Google Scholar]
- Verdaguer, J. Juvenile retinal detachment. Pan American Association of Ophthalmology and American Journal of Ophthalmology Lecture. Am. J. Ophthalmol. 1982, 93, 145–156. [Google Scholar] [PubMed]
- Westfall, A.C.; Maa, A.; Mieler, W.; Holz, E. Incidence of retinal tears and late-onset retinal breaks in eyes with symptomatic posterior vitreous detachment. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2066. [Google Scholar]
- Mitry, D.; Singh, J.; Yorston, D.; Siddiqui, M.A.; Wright, A.; Fleck, B.W.; Campbell, H.; Charteris, D.G. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology 2011, 118, 1429–1434. [Google Scholar] [CrossRef]
- Boutin, T.S.; Charteris, D.G.; Chandra, A.; Campbell, S.; Hayward, C.; Campbell, A.; Nandakumar, P.; Hinds, D.; UK Biobank Eye & Vision Consortium; 23andMe Research Team; et al. Insights into the genetic basis of retinal detachment. Hum. Mol. Genet. 2020, 29, 689–702. [Google Scholar] [CrossRef]
- Schepens, C.L.; Dobble, J.G.; Mc, M.J. Retinal detachments with giant breaks: Preliminary report. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1962, 66, 471–479. [Google Scholar]
- Snead, M.P. Giant retinal tear. In Essentials in Ophthalmology—Vitreoretinal Surgery; Kirchhof, B., Wong, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 89–100. [Google Scholar]
- Snead, M.P. Retinal detachment in childhood. In Paediatric Ophthalmology and Strabismus, 6th ed.; Lyons, C., Hoyt, C., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2022. [Google Scholar]
- Ang, G.S.; Townend, J.; Lois, N. Epidemiology of giant retinal tears in the United Kingdom: The British giant retinal tear epidemiology eye study (BGEES). Retina 2010, 51, 4781–4787. [Google Scholar] [CrossRef]
- Shunmugam, M.; Ang, G.S.; Lois, N. Giant retinal tears. Surv. Ophthalmol. 2014, 59, 192–216. [Google Scholar] [CrossRef]
- Chang, S.; Lopez, J.M. Giant retinal tears and proliferative vitreoretinopathy. In Retina, 4th ed.; Ryan, S.J., Ed.; Mosby: St. Louis, MO, USA, 2006; pp. 2345–2351. [Google Scholar] [CrossRef]
- Ang, G.S.; Townend, J.; Lois, N. Interventions for prevention of giant retinal tear in the fellow eye. Cochrane Database Syst. Rev. 2009, 2, CD006909. [Google Scholar] [CrossRef]
- Aylward, G.W.; Cooling, R.J.; Leaver, P.K. Trauma-induced retinal detachment associated with giant retinal tears. Retina 1993, 13, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Li, K.H.; Hwang, J.F. Myopic macular hole-associated retinal detachment with proliferative vitreoretinopathy. Taiwan J. Ophthalmol. 2013, 3, 141–145. [Google Scholar] [CrossRef]
- Spaide, R.F. Staphyloma: Part 1. Pathologic Myopia; Springer: New York, NY, USA, 2014; pp. 167–176. [Google Scholar]
- Ohno-Matsui, K.; Jonas, J.B. Posterior staphyloma in pathologic myopia. Prog. Retin. Eye Res. 2019, 70, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Sugden, C.J.; Iorio, V.; Troughton, L.D.; Liu, K.; Morais, M.; Lennon, R.; Bou-Gharios, G.; Hamill, K.J. Laminin N-terminus α31 expression during development is lethal and causes widespread tissue-specific defects in a transgenic mouse model. FASEB J. 2022, 36, e22318. [Google Scholar] [CrossRef] [PubMed]
- Maumenee, A.E. Further Advances in the Study of the Macula. Arch Ophthalmol. 1967, 78, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, H.L.; Schepens, C.L.; Elzeneiny, I.; Freeman, H.M. Macular Pucker Following Retinal Detachment Surgery. Arch. Ophthalmol. 1970, 83, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Fincham, G.S. The Prevention and Pathogenesis of Retinal Detachment. Ph.D. Thesis, UCL (University College London), London, UK, 2015. Available online: https://discovery.ucl.ac.uk/id/eprint/1459426/h (accessed on 17 April 2022).
- Sakamoto, T. Cell biology of hyalocytes. Nippon Ganka Gakkai Zasshi 2003, 107, 866–883. [Google Scholar]
- Qiao, H.; Hisatomi, T.; Sonoda, K.H.; Kura, S.; Sassa, Y.; Kinoshita, S.; Nakamura, T.; Sakamoto, T.; Ishibashi, T. The characterisation of hyalocytes: The origin, phenotype, and turnover. Br. J. Ophthalmol. 2005, 89, 513–517. [Google Scholar] [CrossRef]
- Ferenbach, D.; Hughes, J. Macrophages and dendritic cells: What is the difference? Kidney Int. 2008, 74, 5–7. [Google Scholar] [CrossRef]
- Reeves, S.A.; Helman, L.J.; Allison, A.; Israel, M.A. Molecular cloning and primary structure of human glial fibrillary acidic protein. Proc. Natl. Acad. Sci. USA 1989, 86, 5178–5182. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.S.; Hageman, G.S. In situ characterization of the human hyalocyte. Arch. Ophthalmol. 1994, 112, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Hata, Y.; Hisatomi, T.; Nakamura, Y.; Hirayama, K.; Miura, M.; Nakao, S.; Fujisawa, K.; Sakamoto, T.; Ishibashi, T. Functional properties of hyalocytes under PDGF-rich conditions. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed]
- Bumgarner, R. Overview of DNA microarrays: Types, applications, and their future. Curr. Protoc. Mol. Biol. 2013, 101, 22.1.1–22.1.11. [Google Scholar] [CrossRef]
- Zhao, S.; Fung-Leung, W.P.; Bittner, A.; Ngo, K.; Liu, X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS ONE 2014, 9, e78644. [Google Scholar] [CrossRef]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636. [Google Scholar] [CrossRef]
- Byron, S.; Van Keuren-Jensen, K.; Engelthaler, D.; Carpten, J.D.; Craig, D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 11, 951–969. [Google Scholar] [CrossRef]
- Papaspyropoulos, A.; Lagopati, N.; Mourkioti, I.; Angelopoulou, A.; Kyriazis, S.; Liontos, M.; Gorgoulis, V.; Kotsinas, A. Regulatory and Functional Involvement of Long Non-Coding RNAs in DNA Double-Strand Break Repair Mechanisms. Cells 2021, 10, 1506. [Google Scholar] [CrossRef]
- Roberts, K.; Aivazidis, A.; Kleshchevnikov, V.; Li, T.; Fropf, R.; Rhodes, M.; Beechem, J.M.; Hemberg, M.; Bayraktar, O.A. Transcriptome-wide spatial RNA profiling maps the cellular architecture of the developing human neocortex. bioRxiv 2021, in press. [Google Scholar] [CrossRef]


| Break Type | PHM * Status | Vitreous Architecture | Sex | Typical Age Group (Years) | Refractive Error | Fellow-Eye Involvement Pathology | Reference |
|---|---|---|---|---|---|---|---|
| Atraumatic dialysis | On | Normal | M > F | 8–20 | Emmetropia/hypermetropia | 5–15% | [42] |
| Giant retinal tear | Off | Check for anomaly | M = F | 5–50 | Moderate/ high myopia | Variable up to 80% | [43,44] |
| Horseshoe tear | Off | Usually syneretic | M = F | 45–65 | Moderate/ high myopia | 10% | [45] |
| Round retinal hole | On | Usually normal | F > M | 20–40 | Moderate myopia | 45% | [40] |
| Macular hole | Off | Syneretic | M = F | 45–65 | High myopia | Rare | [46,47] |
| Degenerative retinoschisis | On | Normal | M > F | 70+ | Hypermetropia | 80% | [48,49] |
| X-linked retinoschisis | On | Normal, may have haemorrhage | M | 10–20 | Emmetropia | 100% | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranian, M.; Snead, M. A Novel Transcriptome Approach to the Investigation of the Molecular Pathology of Vitreous and Retinal Detachment. Genes 2022, 13, 1885. https://doi.org/10.3390/genes13101885
Maranian M, Snead M. A Novel Transcriptome Approach to the Investigation of the Molecular Pathology of Vitreous and Retinal Detachment. Genes. 2022; 13(10):1885. https://doi.org/10.3390/genes13101885
Chicago/Turabian StyleMaranian, Mel, and Martin Snead. 2022. "A Novel Transcriptome Approach to the Investigation of the Molecular Pathology of Vitreous and Retinal Detachment" Genes 13, no. 10: 1885. https://doi.org/10.3390/genes13101885
APA StyleMaranian, M., & Snead, M. (2022). A Novel Transcriptome Approach to the Investigation of the Molecular Pathology of Vitreous and Retinal Detachment. Genes, 13(10), 1885. https://doi.org/10.3390/genes13101885






