Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases
Abstract
:1. Introduction
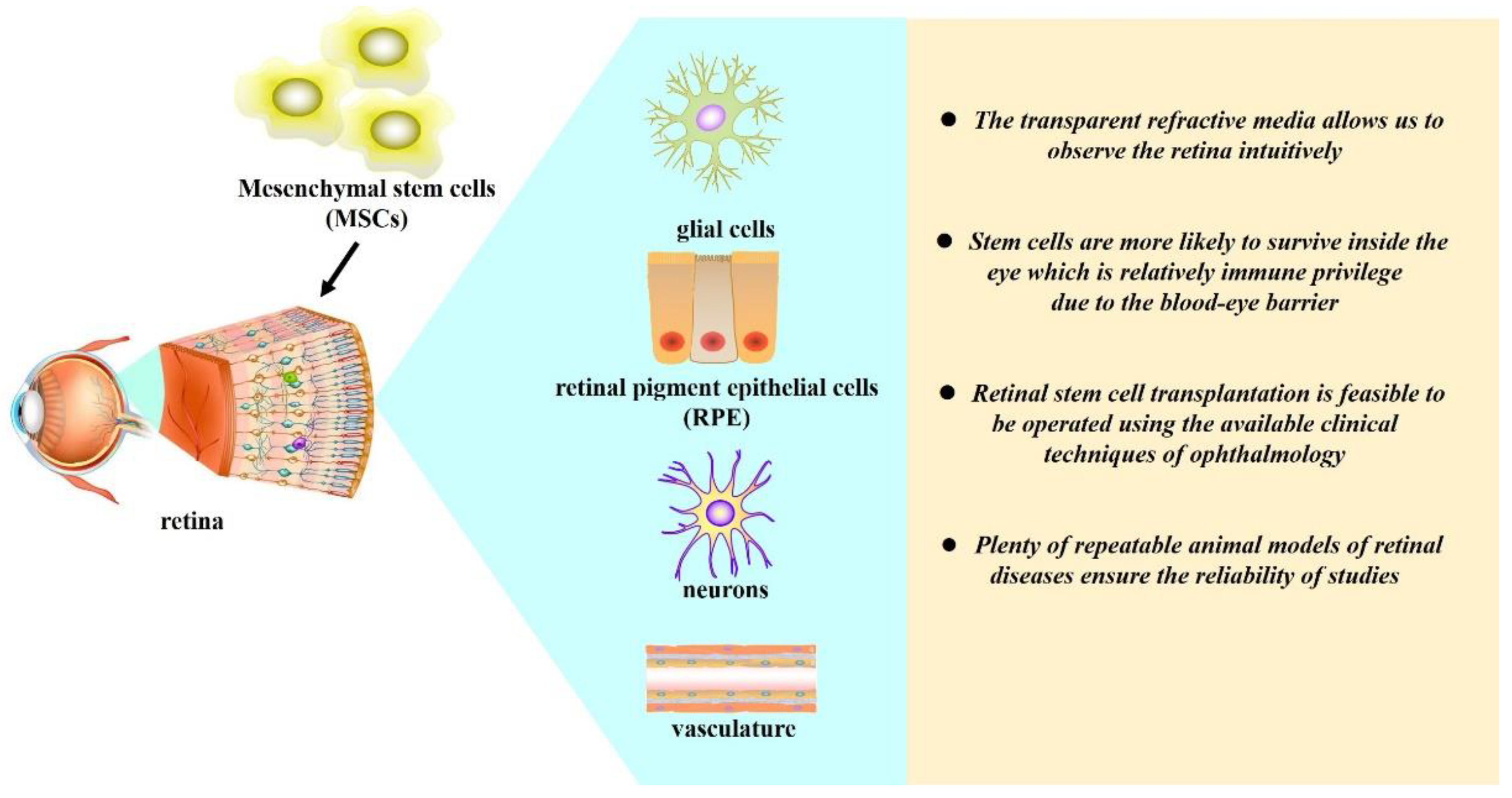
2. Retina Is a Suitable Target for MSC-Based Therapy
3. The Delivery Options
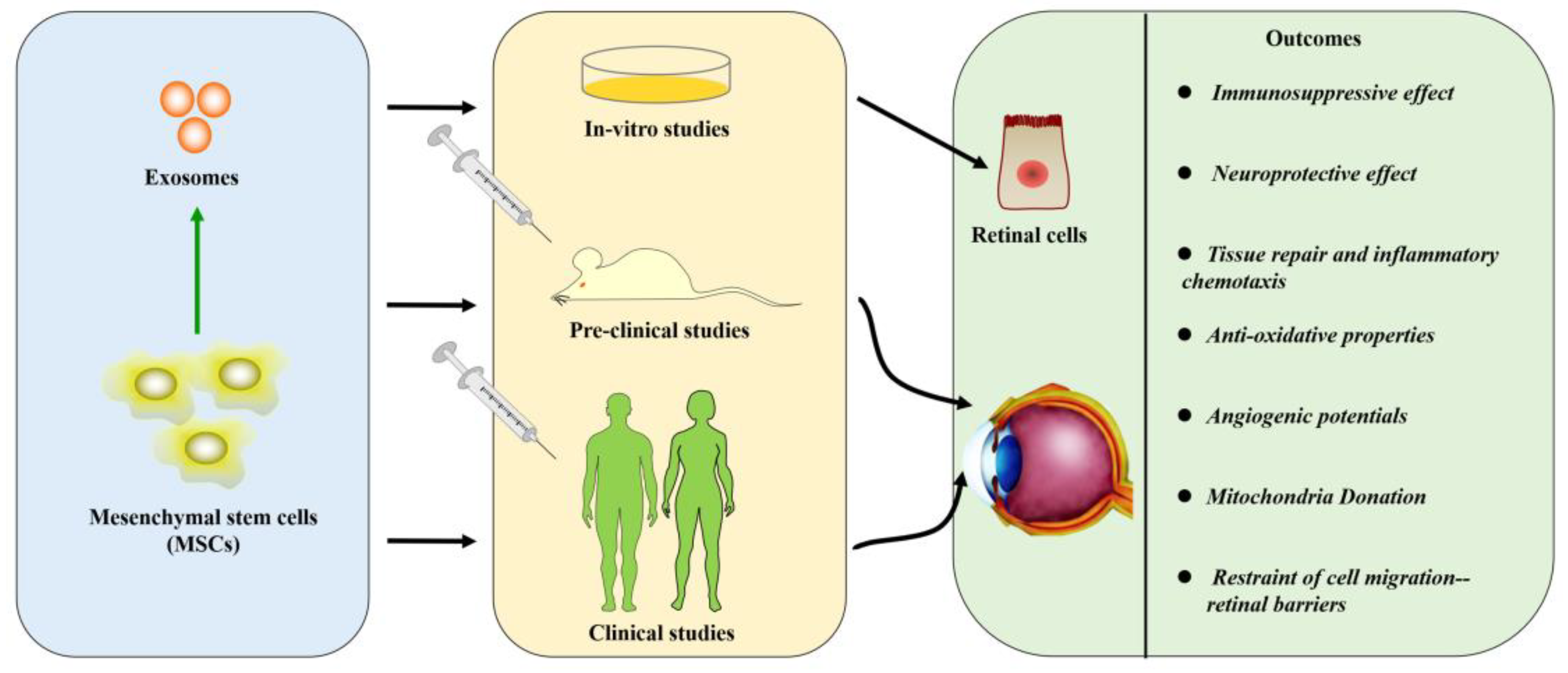
4. Strategies for Treating the Retinal Diseases with Mesenchymal Stem Cells
4.1. Implications of Mesenchymal Stem Cell Therapy in Retinal Degeneration
4.2. Immunosuppressive Effect of Mesenchymal Stem Cells in Uveitis
4.3. Neuroprotective Effect of Mesenchymal Stem Cells in Reducing or Delaying the Retinal Tissue Damage
4.4. Tissue Repair and Inflammatory Chemotaxis of Mesenchymal Stem Cells
4.5. Antioxidative Properties of Mesenchymal Stem Cells
4.6. Angiogenic Potentials of Mesenchymal Stem Cells
4.7. Mitochondria Donation
4.8. Restraint of Cell Migration—Retinal Barriers
4.9. New Insights about Exosomes Derived from Mesenchymal Stem Cells
5. The Pitfalls of Mesenchymal-Stem-Cell-Based Therapies
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet. Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Ashraf, M.; Souka, A.; Adelman, R. Predicting outcomes to anti-vascular endothelial growth factor (VEGF) therapy in diabetic macular oedema: A review of the literature. Br. J. Ophthalmol. 2016, 100, 1596–1604. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, S.N.; Chung, H.; Kim, T.E.; Kim, H.C. Intravitreal Anti-Vascular Endothelial Growth Factor Therapy and Retinal Nerve Fiber Layer Loss in Eyes With Age-Related Macular Degeneration: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1798–1806. [Google Scholar] [CrossRef] [Green Version]
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef]
- Mackie, A.R.; Losordo, D.W. CD34-positive stem cells: In the treatment of heart and vascular disease in human beings. Tex. Heart Inst. J. 2011, 38, 474–485. [Google Scholar]
- Park, S.S. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFj1–ORSFj10. [Google Scholar] [CrossRef] [Green Version]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef]
- Joe, A.W.; Gregory-Evans, K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr. Eye Res. 2010, 35, 941–952. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem. Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, N.S.; Oner, A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: A 6-month follow-up results of a phase 3 trial. Int. J. Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Ozmert, E.; Arslan, U. Management of retinitis pigmentosa by Wharton’s jelly-derived mesenchymal stem cells: Prospective analysis of 1-year results. Stem. Cell Res. Ther. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem. Cell Investig. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. Intravitreal autologous mesenchymal stem cell transplantation: A non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem. Cell. Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Investig. Ophthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: A phase I trial. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef]
- Gu, X.; Yu, X.; Zhao, C.; Duan, P.; Zhao, T.; Liu, Y.; Li, S.; Yang, Z.; Li, Y.; Qian, C.; et al. Efficacy and Safety of Autologous Bone Marrow Mesenchymal Stem Cell Transplantation in Patients with Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 49, 40–52. [Google Scholar] [CrossRef]
- Weiss, J.N.; Levy, S.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A preliminary report. Neural Regen. Res. 2015, 10, 982–988. [Google Scholar]
- Weiss, J.N.; Levy, S.; Benes, S.C. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem. Cell. Investig. 2017, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E., 2nd; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W., Jr.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. A 2018, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Meirelles Lda, S.; Nardi, N.B. Methodology, biology and clinical applications of mesenchymal stem cells. Front. Biosci. 2009, 14, 4281–4298. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.; Dembinski, J.; Sasser, A.K.; Studeny, M.; Andreeff, M.; Marini, F. Mesenchymal stem cells in cancer: Tumor-associated fibroblasts and cell-based delivery vehicles. Int. J. Hematol. 2007, 86, 8–16. [Google Scholar] [CrossRef]
- Yang, J.; Cai, B.; Glencer, P.; Li, Z.; Zhang, X.; Li, X. Induced Pluripotent Stem Cells and Outer Retinal Disease. Stem. Cells Int 2016, 2016, 2850873. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Tzameret, A.; Sher, I.; Belkin, M.; Treves, A.J.; Meir, A.; Nagler, A.; Levkovitch-Verbin, H.; Barshack, I.; Rosner, M.; Rotenstreich, Y. Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp. Eye Res. 2014, 118, 135–144. [Google Scholar] [CrossRef]
- Leow, S.N.; Luu, C.D.; Hairul Nizam, M.H.; Mok, P.L.; Ruhaslizan, R.; Wong, H.S.; Wan Abdul Halim, W.H.; Ng, M.H.; Ruszymah, B.H.; Chowdhury, S.R.; et al. Safety and Efficacy of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells Therapy for Retinal Degeneration. PLoS ONE 2015, 10, e0128973. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Q.; Sun, X.; Shen, W.; Liu, B.; Zhong, X.; Leng, Y.; Li, C.; Zhang, W.; Chai, F.; et al. Generation of retinal ganglion-like cells from reprogrammed mouse fibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5970–5978. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Yao, K.; Kim, J.C. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: Engraftment and involvement in wound healing. Eye 2006, 20, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.Y.; Liang, H.L.; Wang, Y.S.; Zhang, Z.X.; Wang, B.R.; Shi, Y.Y.; Dong, X.; Cai, Y. A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol. Ther. 2010, 18, 1837–1845. [Google Scholar] [CrossRef]
- Ruster, B.; Gottig, S.; Ludwig, R.J.; Bistrian, R.; Muller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef]
- Rajashekhar, G. Mesenchymal stem cells: New players in retinopathy therapy. Front. Endocrinol. (Lausanne) 2014, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem. Cell. Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.X.; Xu, H.W.; Zheng, Q.Y.; FitzGibbon, T. Noggin induces human bone marrow-derived mesenchymal stem cells to differentiate into neural and photoreceptor cells. Indian J. Exp. Biol. 2010, 48, 444–452. [Google Scholar]
- Vossmerbaeumer, U.; Ohnesorge, S.; Kuehl, S.; Haapalahti, M.; Kluter, H.; Jonas, J.B.; Thierse, H.J.; Bieback, K. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2009, 11, 177–188. [Google Scholar] [CrossRef]
- Kicic, A.; Shen, W.Y.; Wilson, A.S.; Constable, I.J.; Robertson, T.; Rakoczy, P.E. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J. Neurosci. 2003, 23, 7742–7749. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lu, B.; Girman, S.; Duan, J.; McFarland, T.; Zhang, Q.S.; Grompe, M.; Adamus, G.; Appukuttan, B.; Lund, R. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS ONE 2010, 5, e9200. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, P.; Torquetti, L.; Nehemy, M.B.; Goes, A.M. Retinal incorporation and differentiation of mesenchymal stem cells intravitreally injected in the injured retina of rats. Arq. Bras. Oftalmol. 2008, 71, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Ripolles-Garcia, A.; Dolgova, N.; Phillips, M.J.; Savina, S.; Ludwig, A.L.; Stuedemann, S.A.; Nlebedum, U.; Wolfe, J.H.; Garden, O.A.; Maminishkis, A.; et al. Systemic immunosuppression promotes survival and integration of subretinally implanted human ESC-derived photoreceptor precursors in dogs. Stem. Cell. Rep. 2022, 17, 1824–1841. [Google Scholar] [CrossRef]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem. Cell. Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Emre, E.; Yuksel, N.; Duruksu, G.; Pirhan, D.; Subasi, C.; Erman, G.; Karaoz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Karaoz, E.; Demircan, P.C.; Saglam, O.; Aksoy, A.; Kaymaz, F.; Duruksu, G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell. Biol. 2011, 136, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Nakahara, T.; Ishikawa, H.; Sato, S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 2013, 101, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Arner, K.; Ehinger, B.; Perez, M.T. Limitation of anatomical integration between subretinal transplants and the host retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Yellowlees Douglas, J.; Bhatwadekar, A.D.; Li Calzi, S.; Shaw, L.C.; Carnegie, D.; Caballero, S.; Li, Q.; Stitt, A.W.; Raizada, M.K.; Grant, M.B. Bone marrow-CNS connections: Implications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2012, 31, 481–494. [Google Scholar] [CrossRef] [Green Version]
- Radtke, N.D.; Seiler, M.J.; Aramant, R.B.; Petry, H.M.; Pidwell, D.J. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am. J. Ophthalmol. 2002, 133, 544–550. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sevim, D.G.; Smim Kahraman, N.; Unlu, M. Suprachoroidal Adipose Tissue-Derived Mesenchymal Stem Cell Implantation in Patients with Dry-Type Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: 6-Month Follow-Up Results of a Phase 2 Study. Cell. Reprogram. 2018, 20, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem. Cell. Res. Ther. 2011, 2, 34. [Google Scholar] [CrossRef] [Green Version]
- Maltman, D.J.; Hardy, S.A.; Przyborski, S.A. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem. Int. 2011, 59, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici, O.; Bulle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jager, M.; Sorg, R.; et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrahin, A.; Ahmed, R.K.; Ferrara, G.; Rane, L.; Poiret, T.; Isaikina, Y.; Skrahina, A.; Zumla, A.; Maeurer, M.J. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: An open-label phase 1 safety trial. Lancet Res. Med. 2014, 2, 108–122. [Google Scholar] [CrossRef]
- Gebler, A.; Zabel, O.; Seliger, B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012, 18, 128–134. [Google Scholar] [CrossRef]
- Yang, J.; Ren, X.J.; Chen, X.T.; Jiang, Y.F.; Han, Z.B.; Han, Z.C.; Li, X.R.; Zhang, X.M. Human umbilical cord-derived mesenchymal stem cells treatment for refractory uveitis: A case series. Int. J. Ophthalmol. 2021, 14, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, H.; Shao, H.; Nian, H.; Zhang, Y.; Bai, L.; Su, C.; Liu, X.; Dong, L.; Li, X.; et al. Long-term therapeutic effects of mesenchymal stem cells compared to dexamethasone on recurrent experimental autoimmune uveitis of rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5561–5571. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.T.; Zhang, L.J.; Shao, H.; Bai, L.L.; Yu, B.; Su, C.; Dong, L.J.; Liu, X.; Li, X.R.; Zhang, X.M. Therapeutic effects of mesenchymal stem cells administered at later phase of recurrent experimental autoimmune uveitis. Int. J. Ophthalmol. 2016, 9, 1381–1389. [Google Scholar]
- Chen, X.; Shao, H.; Zhi, Y.; Xiao, Q.; Su, C.; Dong, L.; Liu, X.; Li, X.; Zhang, X. CD73 Pathway Contributes to the Immunosuppressive Ability of Mesenchymal Stem Cells in Intraocular Autoimmune Responses. Stem. Cells Dev. 2016, 25, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Chen, X.; Shao, H.; Bai, L.; Li, X.; Zhang, X. Mesenchymal Stem Cells Inhibited Dendritic Cells Via the Regulation of STAT1 and STAT6 Phosphorylation in Experimental Autoimmune Uveitis. Curr. Mol. Med. 2018, 17, 478–487. [Google Scholar] [CrossRef]
- Saldinger, L.K.; Nelson, S.G.; Bellone, R.R.; Lassaline, M.; Mack, M.; Walker, N.J.; Borjesson, D.L. Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro. Vet. Ophthalmol. 2020, 23, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Xiao, J.; Liu, J.; Tang, S. Human Umbilical Cord Mesenchymal Stem Cells Attenuate Ocular Hypertension-Induced Retinal Neuroinflammation via Toll-Like Receptor 4 Pathway. Stem. Cells Int. 2019, 2019, 9274585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.K.; Fortino, V.R.; Pelaez, D.; Cheung, H.S. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World. J. Stem. Cells 2014, 6, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mandai, M.; Kamao, H.; Hashiguchi, T.; Shikamura, M.; Kawamata, S.; Sugita, S.; Takahashi, M. Protective Effects of Human iPS-Derived Retinal Pigmented Epithelial Cells in Comparison with Human Mesenchymal Stromal Cells and Human Neural Stem Cells on the Degenerating Retina in rd1 mice. Stem. Cells 2015, 33, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shan, Q.; Ma, P.; Jiang, Y.; Chen, P.; Wen, J.; Zhou, Y.; Qian, H.; Pei, X. Differentiation potential of bone marrow mesenchymal stem cells into retina in normal and laser-injured rat eye. Sci. China C Life Sci. 2004, 47, 241–250. [Google Scholar] [CrossRef]
- Arnhold, S.; Heiduschka, P.; Klein, H.; Absenger, Y.; Basnaoglu, S.; Kreppel, F.; Henke-Fahle, S.; Kochanek, S.; Bartz-Schmidt, K.U.; Addicks, K.; et al. Adenovirally transduced bone marrow stromal cells differentiate into pigment epithelial cells and induce rescue effects in RCS rats. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4121–4129. [Google Scholar] [CrossRef]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef]
- Li, N.; Li, X.R.; Yuan, J.Q. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 503–514. [Google Scholar]
- Johnson, T.V.; Bull, N.D.; Hunt, D.P.; Marina, N.; Tomarev, S.I.; Martin, K.R. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2051–2059. [Google Scholar] [CrossRef]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014, 137, 503–519. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Zhang, L.; Wang, M.; Zhang, X.; Li, X. Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. Int. J. Mol. Sci. 2014, 15, 9372–9385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motegi, S.I.; Ishikawa, O. Mesenchymal stem cells: The roles and functions in cutaneous wound healing and tumor growth. J. Dermatol. Sci. 2017, 86, 83–89. [Google Scholar] [CrossRef]
- Eseonu, O.I.; De Bari, C. Homing of mesenchymal stem cells: Mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology 2015, 54, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Neuss, S.; Becher, E.; Woltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem. Cells 2004, 22, 405–414. [Google Scholar] [CrossRef]
- Shi, M.; Li, J.; Liao, L.; Chen, B.; Li, B.; Chen, L.; Jia, H.; Zhao, R.C. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: Role in homing efficiency in NOD/SCID mice. Haematologica 2007, 92, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Son, B.R.; Marquez-Curtis, L.A.; Kucia, M.; Wysoczynski, M.; Turner, A.R.; Ratajczak, J.; Ratajczak, M.Z.; Janowska-Wieczorek, A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem. Cells 2006, 24, 1254–1264. [Google Scholar] [CrossRef]
- De Becker, A.; Van Hummelen, P.; Bakkus, M.; Vande Broek, I.; De Wever, J.; De Waele, M.; Van Riet, I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007, 92, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Huang, K.; Zhou, J.; Yan, D.; Tang, Y.L.; Zhao, T.C.; Miller, R.J.; Kishore, R.; Losordo, D.W.; Qin, G. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J. Mol. Cell. Cardiol. 2015, 81, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkouchi, S.; Block, G.J.; Katsha, A.M.; Kanehira, M.; Ebina, M.; Kikuchi, T.; Saijo, Y.; Nukiwa, T.; Prockop, D.J. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol. Ther. 2012, 20, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzelay, A.; Weisthal Algor, S.; Niztan, A.; Katz, S.; Benhamou, M.; Nakdimon, I.; Azmon, N.; Gozlan, S.; Mezad-Koursh, D.; Neudorfer, M.; et al. Adipose-Derived Mesenchymal Stem Cells Migrate and Rescue RPE in the Setting of Oxidative Stress. Stem. Cells Int. 2018, 2018, 9682856. [Google Scholar] [CrossRef] [Green Version]
- Psaltis, P.J.; Zannettino, A.C.; Worthley, S.G.; Gronthos, S. Concise review: Mesenchymal stromal cells: Potential for cardiovascular repair. Stem. Cells 2008, 26, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, J.M.; Kong, T.; Kim, C.; Bae, S.H.; Kim, H.W.; Moon, J. Retinal Angiogenesis Effects of TGF-beta1 and Paracrine Factors Secreted From Human Placental Stem Cells in Response to a Pathological Environment. Cell. Transplant. 2016, 25, 1145–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribot, J.; Caliaperoumal, G.; Paquet, J.; Boisson-Vidal, C.; Petite, H.; Anagnostou, F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J. Cell. Mol. Med. 2017, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.; Periasamy, R.; Gangaraju, R. Adult Stem Cell Therapeutics in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4876. [Google Scholar] [CrossRef] [Green Version]
- Elshaer, S.L.; Evans, W.; Pentecost, M.; Lenin, R.; Periasamy, R.; Jha, K.A.; Alli, S.; Gentry, J.; Thomas, S.M.; Sohl, N.; et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2(Akita) mouse. Stem. Cell. Res. Ther. 2018, 9, 322. [Google Scholar] [CrossRef]
- Herrmann, M.; Bara, J.J.; Sprecher, C.M.; Menzel, U.; Jalowiec, J.M.; Osinga, R.; Scherberich, A.; Alini, M.; Verrier, S. Pericyte plasticity-comparative investigation of the angiogenic and multilineage potential of pericytes from different human tissues. Eur. Cell. Mater. 2016, 31, 236–249. [Google Scholar] [CrossRef]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Leniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef] [Green Version]
- Fiori, A.; Terlizzi, V.; Kremer, H.; Gebauer, J.; Hammes, H.P.; Harmsen, M.C.; Bieback, K. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology 2018, 223, 729–743. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, Z.; Wang, S. Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration. Front. Aging 2022, 3, 926627. [Google Scholar] [CrossRef]
- Jassim, A.H.; Inman, D.M.; Mitchell, C.H. Crosstalk Between Dysfunctional Mitochondria and Inflammation in Glaucomatous Neurodegeneration. Front. Pharmacol. 2021, 12, 699623. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front. Cell. Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Dawson, R.H.; Berridge, M.V. Intercellular Communication in Tumor Biology: A Role for Mitochondrial Transfer. Front. Oncol. 2018, 8, 344. [Google Scholar] [CrossRef]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem. Cell. Rev. Rep. 2021, 17, 1154–1173. [Google Scholar] [CrossRef]
- Jiang, D.; Xiong, G.; Feng, H.; Zhang, Z.; Chen, P.; Yan, B.; Chen, L.; Gandhervin, K.; Ma, C.; Li, C.; et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics 2019, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.; Park, S.H.; Lee, D.; Kim, G.H.; Noh, J.E.; Lee, K.J.; Kim, G.J. Overexpression of pigment epithelium-derived factor in placenta-derived mesenchymal stem cells promotes mitochondrial biogenesis in retinal cells. Lab. Investig. 2021, 101, 51–69. [Google Scholar] [CrossRef]
- Vinores, S.A. Assessment of blood-retinal barrier integrity. Histol. Histopathol. 1995, 10, 141–154. [Google Scholar]
- Singhal, S.; Lawrence, J.M.; Bhatia, B.; Ellis, J.S.; Kwan, A.S.; Macneil, A.; Luthert, P.J.; Fawcett, J.W.; Perez, M.T.; Khaw, P.T.; et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Muller stem cells into degenerating retina. Stem. Cells 2008, 26, 1074–1082. [Google Scholar] [CrossRef]
- Johnson, T.V.; Bull, N.D.; Martin, K.R. Identification of barriers to retinal engraftment of transplanted stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Takahashi, M.; Tanihara, H.; Nakano, I.; Takahashi, J.B.; Mizoguchi, A.; Ide, C.; Honda, Y. Incorporation and differentiation of hippocampus-derived neural stem cells transplanted in injured adult rat retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4268–4274. [Google Scholar]
- Yao, J.; Tucker, B.A.; Zhang, X.; Checa-Casalengua, P.; Herrero-Vanrell, R.; Young, M.J. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheRes with retinal progenitor cells. Biomaterials 2011, 32, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, K.; Yang, H.; Li, B.; Zhou, T.; Wang, H.; Zhou, H.; Chen, S.; Zhou, X.; Wei, X.; et al. Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury. J. Nanobiotechnol. 2021, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhong, X.; Yang, S.; Luo, Z.; Li, K.; Liu, Y.; Cai, S.; Gu, H.; Lu, S.; Zhang, H.; et al. HiPSC-derived retinal ganglion cells grow dendritic arbors and functional axons on a tissue-engineered scaffold. Acta Biomater. 2017, 54, 117–127. [Google Scholar] [CrossRef]
- Roozafzoon, R.; Lashay, A.; Vasei, M.; Ai, J.; Khoshzaban, A.; Keshel, S.H.; Barabadi, Z.; Bahrami, H. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem. Biophys. Res. Commun. 2015, 457, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Sokic, S.; Schardt, J.S.; Raiker, R.S.; Lin, J.W.; Jay, S.M. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2015, 21, 45–54. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem. Cell. Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Desrochers, L.M.; Antonyak, M.A.; Cerione, R.A. Extracellular Vesicles: Satellites of Information Transfer in Cancer and Stem Cell Biology. Dev Cell. 2016, 37, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, E.; Fujita, D.; Takahashi, M.; Oba, S.; Nishimatsu, H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J. Stem. Cells 2016, 8, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem. Cells Int. 2016, 2016, 1240301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conforti, A.; Scarsella, M.; Starc, N.; Giorda, E.; Biagini, S.; Proia, A.; Carsetti, R.; Locatelli, F.; Bernardo, M.E. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem. Cells Dev. 2014, 23, 2591–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front Cell. Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Favaro, E.; Carpanetto, A.; Lamorte, S.; Fusco, A.; Caorsi, C.; Deregibus, M.C.; Bruno, S.; Amoroso, A.; Giovarelli, M.; Porta, M.; et al. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia 2014, 57, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jin, S.; Zhang, Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int. J. Clin. Exp. Med. 2015, 8, 3825–3832. [Google Scholar]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, C.H.; Wallace, C.G.; Yuen, C.M.; Kao, G.S.; Chen, Y.L.; Shao, P.L.; Chen, Y.L.; Chai, H.T.; Lin, K.C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562. [Google Scholar] [CrossRef] [Green Version]
- Blennow, O.; Remberger, M.; Torlen, J.; Szakos, A.; Ljungman, P.; Mattsson, J. Risk Factors for Invasive Mold Infections and Implications for Choice of Prophylaxis after Allogeneic Stem Cell Transplantation. Biol. Blood. Marrow Transplant. 2016, 22, 1684–1689. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.A.; Stevenson, K.; Kim, H.T.; Cutler, C.; Ballen, K.; McDonough, S.; Reynolds, C.; Herrera, M.; Liney, D.; Ho, V.; et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood 2010, 115, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Retinal Disease | Number of Patients | Cell Type | Route of Administration | Dosage | Phase of Study | Outcomes |
|---|---|---|---|---|---|---|---|
| Oner [10] (2016) | RP | 11 | AD-MSC | Subretinal | 2.47 × 106 ± 0.11 cells/150 μl | I | Minor ocular complications |
| Kahraman [11] (2020) | RP | 82 | UC-MSC | Suprachoroidal | 5 × 106 cells/2 mL | III | Beneficial effect on BCVA, VF, and mfERG |
| Özmert [12] (2020) | RP | 32 | WJ-MSC | Subtenon | 2–6 × 106 cells/1.5 mL | III | No ocular or systemic adverse events |
| Weiss [13] (2018) | RP | 17 | BM-MSC | Retrobulbar, subtenon, intravitreal and intravenous | 1.2 × 109 cells/14–15 cm3 | NA | Beneficial effect on visual acuity |
| Tuekprakhon [14] (2021) | RP | 14 | BM-MSC | Intravitreal | 1 × 106 cells, 5 × 106 cells, 1 × 107 cells | I | Beneficial effect on visual acuity |
| Park [15] (2014) | IDRD | 6 | BM-MSC | Intravitreal | - | I | No ocular or systemic adverse events associated with treatment |
| Siqueira [16] (2011) | HRD | 6 | BM-MSC | Intravitreal | 10 × 106 cells/0.1 mL | I | No ocular or systemic adverse events associated with treatment |
| Gu [17] (2018) | DR | 17 | BM-MSC | Intravenous | 3 × 106 cells/kg | NA | Beneficial effect on macular thickness and visual acuity |
| Levy [18] (2015) | Optic nerve diseases | 1 | BM-MSC | Retrobulbar, subtenon, intravitreal, and intravenous | 1.2 × 109 cells/14–15 cm3 | NA | Beneficial effect on visual acuity |
| Weiss [19] (2017) | NAION | 10 | BM-MSC | Retrobulbar, subtenon, intravitreal, and intravenous | 1.2 × 109 cells/14–15 cm3 | NA | Beneficial effect on visual acuity |
| Kuriyan [20] (2017) | AMD | 3 | AD-MSC | Intravitreal | - | NA | Severe ocular complications |
| Local Administration | Systemic Administration |
|---|---|
| Intravitreal or subretinal injection | Intravenous injection |
| Advantages | |
|
|
| Disadvantages | |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Jiang, Y.; Duan, Y.; Zhang, X.; Li, X. Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases. Genes 2022, 13, 1901. https://doi.org/10.3390/genes13101901
Chen X, Jiang Y, Duan Y, Zhang X, Li X. Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases. Genes. 2022; 13(10):1901. https://doi.org/10.3390/genes13101901
Chicago/Turabian StyleChen, Xiteng, Yuanfeng Jiang, Yanan Duan, Xiaomin Zhang, and Xiaorong Li. 2022. "Mesenchymal-Stem-Cell-Based Strategies for Retinal Diseases" Genes 13, no. 10: 1901. https://doi.org/10.3390/genes13101901







