Comparative Biochemical and Transcriptomic Analyses Provide New Insights into Phytoplasma Infection Responses in Cucumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Detection of Plant Pathogen in Flat Stems
2.3. Quantification of Plant Hormones and Mineral Nutrients
2.4. RNA Extraction, cDNA Library Construction, and Sequencing
2.5. Bioinformatics Analysis of RNA-seq Data
2.6. qRT-PCR Analysis
3. Results
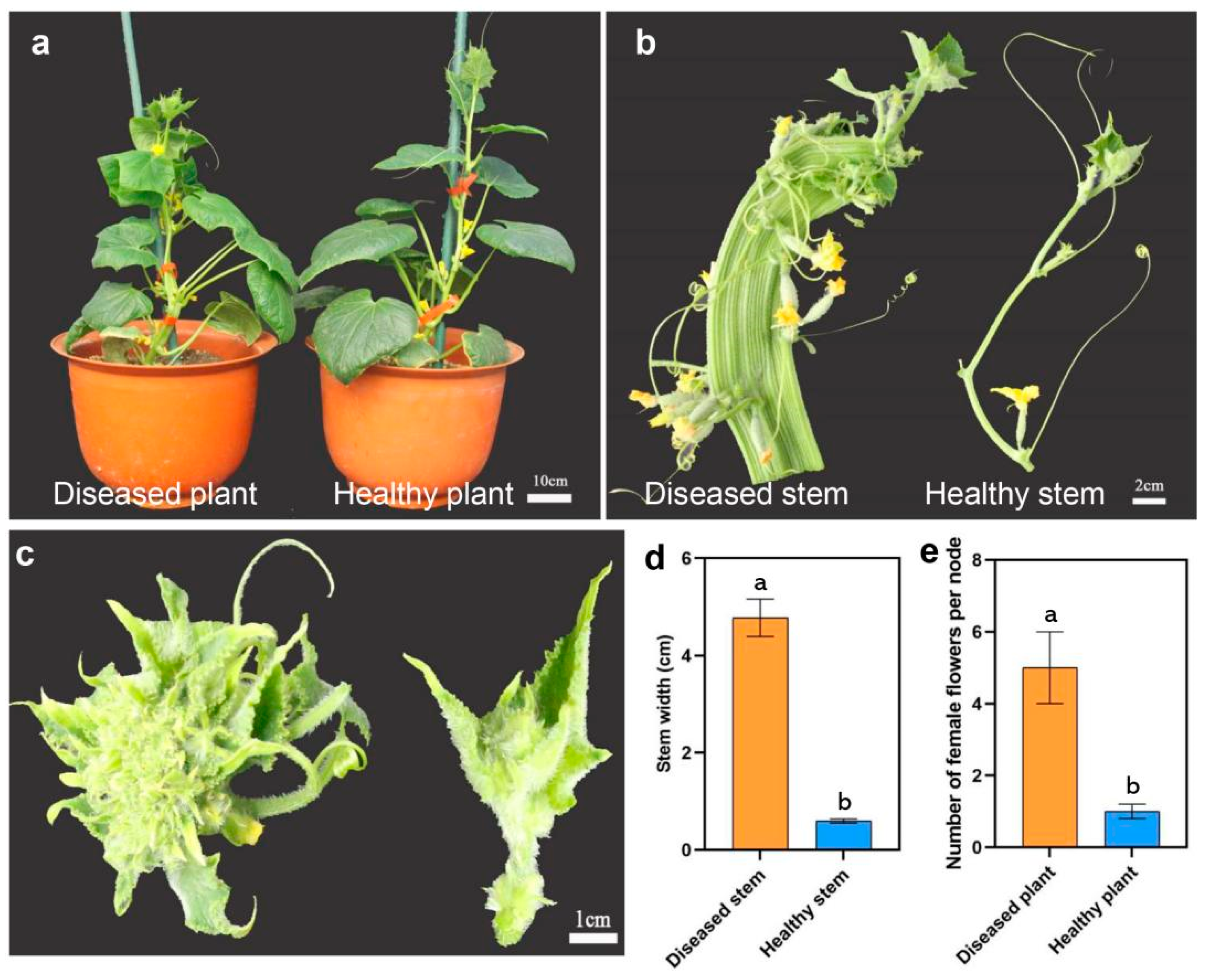
3.1. Phytoplasma Infection Caused Flat Stem Symptoms in Cucumber
3.2. Phytoplasma Infection Caused Cucumber Plant Hormone Disorders
3.3. Phytoplasma Infection Caused Mineral Element Disorders
3.4. Phytoplasma Infection Influences Gene Expression
3.5. DEGs Related to Plant Hormone Signal Transduction
3.6. Identification of DEGs Related to Secondary Metabolite Biosynthesis
3.7. DEGs Involved in Mineral Nutrition Resist Phytoplasma Infection
4. Discussion
4.1. Identification of the Cucumber Flat Stem Pathogen
4.2. Alterations in Plant Hormone Levels in Response to Phytoplasma Infection
4.3. Phenylpropanoid Genes Related to Plant Disease Resistance
4.4. Mineral Nutrient Regulation of Plant-Pathogen Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Nagendran, K.; Rai, A.B.; Singh, B.; Rao, G.P.; Bertaccini, A. Global Status of Phytoplasma Diseases in Vegetable Crops. Front. Microbiol. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ember, I.; Acs, Z.; Munyaneza, J.E.; Crosslin, J.M.; Kolber, M. Survey and molecular detection of phytoplasmas associated with potato in Romania and southern Russia. Eur. J. Plant Pathol. 2011, 130, 367–377. [Google Scholar] [CrossRef]
- Rao, G.P.; Kumar, M. World status of phytoplasma diseases associate with eggplant. Crop Prot. 2017, 96, 22–29. [Google Scholar] [CrossRef]
- Navratil, M.; Valova, P.; Fialova, R.; Lauterer, P.; Safarova, D.; Stary, M. The incidence of “stolbur” disease and associated yield losses in vegetable crops in South Moravia (Czech Republic). Crop Prot. 2009, 28, 898–904. [Google Scholar] [CrossRef]
- Maejima, K.; Oshima, K.; Namba, S. Exploring the phytoplasmas, plant pathogenic bacteria. J. Gen. Plant Pathol. 2014, 80, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Xu, E.; Deng, M.; Zhao, Z.; Niu, S. Phenylpropanoid metabolism, hormone biosynthesis and signal transduction-related genes play crucial roles in the resistance of Paulownia fortunei to paulownia witches’ broom phytoplasma infection. Genes Genom. 2015, 37, 913–929. [Google Scholar] [CrossRef]
- Mou, H.Q.; Lu, J.; Zhu, S.F.; Lin, C.L.; Tian, G.Z.; Xu, X.; Zhao, W.J. Transcriptomic analysis of paulownia infected by paulownia witches’-broom phytoplasma. PLoS ONE 2013, 8, e77217. [Google Scholar] [CrossRef]
- Ye, X.; Wang, H.; Chen, P.; Fu, B.; Zhang, M.; Li, J.; Zheng, X.; Tan, B.; Feng, J. Combination of iTRAQ proteomics and RNA-seq transcriptomics reveals multiple levels of regulation in phytoplasma-infected Ziziphus jujuba Mill. Hortic. Res. 2017, 4, 17080. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhao, J.; Liu, M. Photosynthetic responses to phytoplasma infection in Chinese jujube. Plant Physiol. Biochem. 2016, 105, 12–20. [Google Scholar] [CrossRef]
- Zafari, S.; Niknam, V.; Musetti, R.; Noorbakhsh, S.N. Effect of phytoplasma infection on metabolite content and antioxidant enzyme activity in lime (Citrus aurantifolia). Acta Physiol. Plant. 2012, 34, 561–568. [Google Scholar] [CrossRef]
- Albertazzi, G.; Milc, J.; Caffagni, A.; Francia, E.; Roncaglia, E.; Ferrari, F.; Tagliafico, E.; Stefani, E.; Pecchioni, N. Gene expression in grapevine cultivars in response to Bois Noir phytoplasma infection. Plant Sci. 2009, 176, 792–804. [Google Scholar] [CrossRef]
- Hren, M.; Nikolić, P.; Rotter, A.; Blejec, A.; Terrier, N.; Ravnikar, M.; Dermastia, M.; Gruden, K. ‘Bois noir’ phytoplasma induces significant reprogramming of the leaf transcriptome in the field grown grapevine. BMC Genom. 2009, 10, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fofana, B.; Benhamou, N.; McNally, D.J.; Labbé, C.; Séguin, A.; Bélanger, R.R. Suppression of induced resistance in cucumber through disruption of the flavonoid pathway. Phytopathology 2005, 95, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunkel, B.N.; Harper, C.P. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J. Exp. Bot. 2018, 69, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Nejat, N.; Cahill, D.M.; Vadamalai, G.; Ziemann, M.; Rookes, J.; Naderali, N. Transcriptomics-based analysis using RNA-Seq of the coconut (Cocos nucifera) leaf in response to yellow decline phytoplasma infection. Mol. Genet. Genom. 2015, 290, 1899–1910. [Google Scholar] [CrossRef]
- Dermastia, M. Plant hormones in phytoplasma infected plants. Front. Plant Sci. 2019, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Riemann, M.; Haga, K.; Shimizu, T.; Okada, K.; Ando, S.; Mochizuki, S.; Nishizawa, Y.; Yamanouchi, U.; Nick, P.; Yano, M. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013, 74, 226–238. [Google Scholar] [CrossRef]
- Takatsuji, H.; Jiang, C.J.; Sugano, S. Salicylic acid signaling pathway in rice and the potential applications of its regulators. Jpn. Agric. Res. Q. 2010, 44, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between nutrition, plant diseases and pests. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 283–298. [Google Scholar]
- Bhaskar, C.V.; Rao, G.R.; Reddy, K. Effect of nitrogen and potassium nutrition on sheath rot incidence and phenol content in rice (Oryza sativa L.). Indian J. Plant Physiol. 2001, 6, 254–257. [Google Scholar]
- Shearer, B.; Fairman, R. A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australas. Plant Pathol. 2007, 36, 78–86. [Google Scholar] [CrossRef]
- Conway, W.S.; Sams, C.E.; Kelman, A. Enhancing the natural resistance of plant tissues to postharvest diseases through calcium applications. HortScience 1994, 29, 751–754. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, D.; Verma, S.K. In silico identification of copper-binding proteins of Xanthomonas translucens pv. undulosa for their probable role in plant-pathogen interactions. Physiol. Mol. Plant Pathol. 2019, 106, 187–195. [Google Scholar] [CrossRef]
- Salehi, M.; Siampour, M.; Esmailzadeh Hosseini, S.; Bertaccini, A. Characterization and vector identification of phytoplasmas associated with cucumber and squash phyllody in Iran. Bull. Insectology 2015, 68, 311–319. [Google Scholar]
- Namba, S.; Kato, S.; Iwanami, S.; Oyaizu, H.; Shiozawa, H.; Tsuchizaki, T. Detection and differentiation of plant-pathogenic mycoplasmalike organisms using polymerase chain reaction. Phytopathology 1993, 83, 786–791. [Google Scholar] [CrossRef]
- Deng, S.; Hiruki, C. Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. J. Microbiol. Methods. 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Gundersen, D.; Lee, I.M. Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol. Mediterr. 1996, 35, 144–151. [Google Scholar]
- Schneider, B. Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasma. Mol. Diagn. Proced. Mycoplasmolgy 1995, 1, 369–380. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Chu, J.; Sun, X.; Wang, J.; Yan, C. Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Anal. Sci. 2012, 28, 1081–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanvir, E.M.; Whitfield, K.M.; Ng, J.C.; Shaw, P.N. Development and Validation of an ICP-MS Method and Its Application to Determine Multiple Trace Elements in Small Volumes of Whole Blood and Plasma. J. Anal. Toxicol. 2021, 44, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florea, L.; Song, L.; Salzberg, S.L. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000Research 2013, 2, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J. topGO: Enrichment analysis for Gene Ontology. R package version 2.28. 0. Cranio 2016. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, L.; Che, H.Y.; Cao, X.R.; Luo, D.Q. Phytoplasma in Association With Rubber Tree (Hevea brasiliensis) Stem Fasciation in China. Plant Dis. 2016, 100, 2520. [Google Scholar] [CrossRef]
- Bräutigam, K.; Soolanayakanahally, R.; Champigny, M.; Mansfield, S.; Douglas, C.; Campbell, M.M.; Cronk, Q. Sexual epigenetics: Gender-specific methylation of a gene in the sex determining region of Populus balsamifera. Sci. Rep. 2017, 7, 45388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Nagendran, K.; Kumar, R.; Pandey, K.K.; Singh, J.; Rao, G.P. First report of ‘Candidatus Phytoplasma asteris’ associated with flat stem disease of spinach (Spinacia oleracea L.) in India. J. Plant Pathol. 2021, 103, 699. [Google Scholar] [CrossRef]
- Panda, P.; Nigam, A.; Rao, G. Multilocus gene analysis reveals the presence of two phytoplasma groups in Impatiens balsamina showing flat stem and phyllody. 3 Biotech 2021, 11, 122. [Google Scholar] [CrossRef]
- Yadav, A.; Thorat, V.; Rao, G. Molecular detection of 16SrI-B and 16SrII-D subgroups of phytoplasma associated with flat stem and witches’ broom disease of Celosia argentea L. 3 Biotech 2017, 7, 311. [Google Scholar]
- Bai, Y.; Kissoudis, C.; Yan, Z.; Visser, R.G.; van der Linden, G. Plant behaviour under combined stress: Tomato responses to combined salinity and pathogen stress. Plant J. 2018, 93, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.F.; Lin, C.P.; Sung, Y.C.; Chen, J.C. Auxin influences symptom expression and phytoplasma colonisation in periwinkle infected with periwinkle leaf yellowing phytoplasma. Ann. Appl. Biol. 2013, 163, 420–429. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, X.; Cao, J. Small auxin upregulated RNA (SAUR) gene family in maize: Identification, evolution, and its phylogenetic comparison with Arabidopsis, rice, and sorghum. J. Integr. Plant Biol. 2014, 56, 133–150. [Google Scholar] [CrossRef]
- Mardi, M.; Karimi Farsad, L.; Gharechahi, J.; Salekdeh, G.H. In-depth transcriptome sequencing of Mexican lime trees infected with Candidatus Phytoplasma aurantifolia. PLoS ONE 2015, 10, e0130425. [Google Scholar] [CrossRef] [Green Version]
- Gai, Y.P.; Li, Y.Q.; Guo, F.Y.; Yuan, C.Z.; Mo, Y.Y.; Zhang, H.L.; Wang, H.; Ji, X.L. Analysis of phytoplasma-responsive sRNAs provide insight into the pathogenic mechanisms of mulberry yellow dwarf disease. Sci. Rep. 2014, 4, 5378. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Dong, Y.; Deng, M.; Zhao, Z.; Niu, S.; Xu, E. Plant-pathogen interaction, circadian rhythm, and hormone-related gene expression provide indicators of phytoplasma infection in Paulownia fortunei. Int. J. Mol. Sci. 2014, 15, 23141–23162. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liang, Y.; Deng, Y.; Chen, Q.; Zhang, J.; Yang, X.; Zuo, J. Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res. 2009, 19, 1178–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Xie, Z.; Zhang, Y.; Wang, S. The FvCYP714C2 gene plays an important role in gibberellin synthesis in the woodland strawberry. Genes Genom. 2021, 43, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.; Yan, D.; Dong, W.; Yang, W.; Li, Q.; Zeng, L.; Wang, J.; Wang, L.; Hicks, L.M. Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellin deactivation. Plant J. 2011, 67, 342–353. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Li, L.; Gao, Y.; Zhao, L.; Patil, S.B.; Fang, J.; Zhang, W.; Yang, Y.; Li, M. The Arabidopsis U-box E3 ubiquitin ligase PUB30 negatively regulates salt tolerance by facilitating BRI1 kinase inhibitor 1 (BKI1) degradation. Plant Cell Environ. 2017, 40, 2831–2843. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Argueso, C.T. No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, A.R.; Catarcione, G.; Ederli, L.; Zadra, C.; Pasqualini, S.; Badiani, M.; Musetti, R.; Santi, S.; Ciaffi, M. Jasmonate-mediated defence responses, unlike salicylate-mediated responses, are involved in the recovery of grapevine from bois noir disease. BMC Plant Biol. 2017, 17, 118. [Google Scholar] [CrossRef]
- Guan, C.; Wang, C.; Li, Q.; Ji, J.; Wang, G.; Jin, C.; Tong, Y. LcSABP2, a salicylic acid binding protein 2 gene from Lycium chinense, confers resistance to triclosan stress in Nicotiana tabacum. Ecotoxicol. Environ. Saf. 2019, 183, 109516. [Google Scholar] [CrossRef]
- Aizat, W.M.; Able, J.A.; Stangoulis, J.C.; Able, A.J. Characterisation of ethylene pathway components in non-climacteric capsicum. BMC Plant Biol. 2013, 13, 191. [Google Scholar] [CrossRef] [Green Version]
- Broekaert, W.F.; Delauré, S.L.; De Bolle, M.F.; Cammue, B.P. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006, 44, 393–416. [Google Scholar] [CrossRef]
- Koo, A.J.; Cooke, T.F.; Howe, G.A. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proc. Natl. Acad. Sci. USA 2011, 108, 9298–9303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, D.M.; Haneklaus, S. Managing nutrition to control plant disease. Landbauforsch. Volkenrode 2007, 57, 313. [Google Scholar]
- Bakeer, A.R.T.; Abdel-latef, M.A.; Afifi, M.A.; Barakat, M.E. Application of microelements and sodium and potassium salts on tomato plants and their role in suppressing powdery mildew disease. World 2012, 1, 01–10. [Google Scholar]
- Masaoka, Y.; Pustika, A.; Subandiyah, S.; Okada, A.; Hanundin, E.; Purwanto, B.; Okuda, M.; Okada, Y.; Saito, A.; Holford, P. Lower concentrations of microelements in leaves of citrus infected with ‘Candidatus Liberibacter asiaticus’. Jpn. Agric. Res. Q. 2011, 45, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, H. Relation between resistance to bacterial wilt and calcium nutrition in tomato seedlings. Jpn. Agric. Res. Q. 2001, 35, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Buoso, S.; Musetti, R.; Marroni, F.; Calderan, A.; Schmidt, W.; Santi, S. Infection by phloem-limited phytoplasma affects mineral nutrient homeostasis in tomato leaf tissues. J. Plant Physiol. 2022, 271, 153659. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Ji, P.; Longo, L.M.; Olson, S.; Momol, T.M. Bacterial wilt induced changes in nutrient distribution and biomass and the effect of acibenzolar-S-methyl on bacterial wilt in tomato. Crop Prot. 2007, 26, 978–982. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghaithi, A.G.; Hanif, M.A.; Al-Busaidi, W.M.; Al-Sadi, A.M. Increased sodium and fluctuations in minerals in acid limes expressing witches’ broom symptoms. SpringerPlus 2016, 5, 418. [Google Scholar] [CrossRef]
- Raiesi, T.; Golmohammadi, M. Changes in nutrient concentrations and biochemical characteristics of Mexican lime (Citrus aurantifolia) infected by phytoplasma. J. Gen. Plant Pathol. 2020, 86, 486–493. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, Q.; Wang, J.; Lou, L.; Xu, X.; Chen, X. Comparative Biochemical and Transcriptomic Analyses Provide New Insights into Phytoplasma Infection Responses in Cucumber. Genes 2022, 13, 1903. https://doi.org/10.3390/genes13101903
Wang X, Hu Q, Wang J, Lou L, Xu X, Chen X. Comparative Biochemical and Transcriptomic Analyses Provide New Insights into Phytoplasma Infection Responses in Cucumber. Genes. 2022; 13(10):1903. https://doi.org/10.3390/genes13101903
Chicago/Turabian StyleWang, Xueting, Qiming Hu, Jiaxi Wang, Lina Lou, Xuewen Xu, and Xuehao Chen. 2022. "Comparative Biochemical and Transcriptomic Analyses Provide New Insights into Phytoplasma Infection Responses in Cucumber" Genes 13, no. 10: 1903. https://doi.org/10.3390/genes13101903




