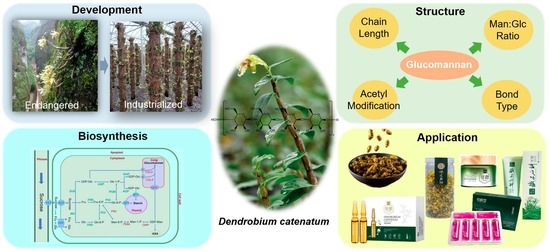
Glucomannan in Dendrobium catenatum: Bioactivities, Biosynthesis and Perspective
Abstract
:1. Introduction
2. Feature and Structure of Glucomannan
3. Applications of Glucomannan
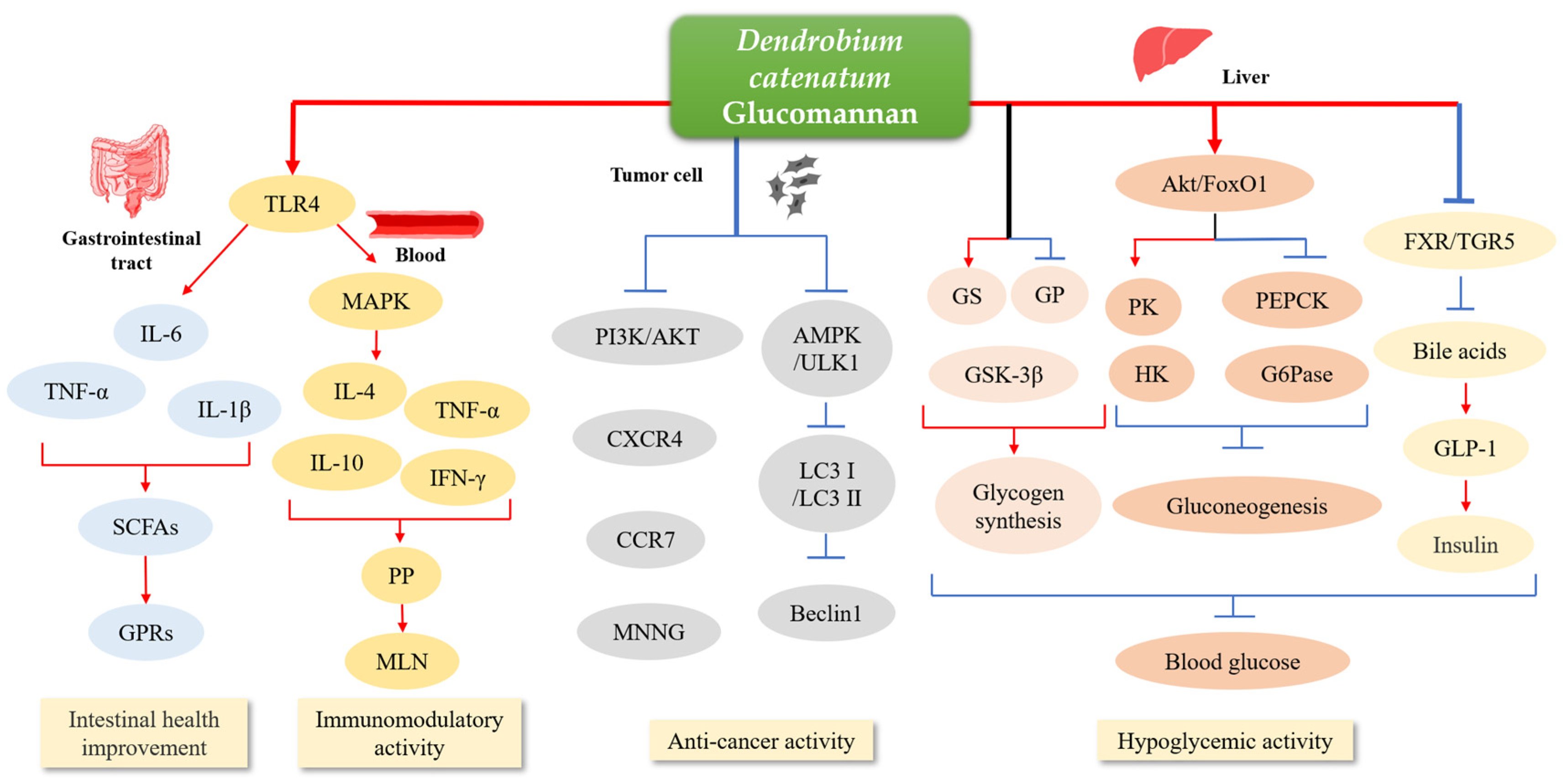
3.1. Medical Applications
3.1.1. Intestinal Health Improvement
3.1.2. Immunomodulatory Activity
3.1.3. Anti-Cancer Activity
3.1.4. Hypoglycemic Activity
3.2. Daily Application
3.2.1. Cosmetics
3.2.2. Food and Functional Food
4. Glucomannan Biosynthesis Pathway in Plant
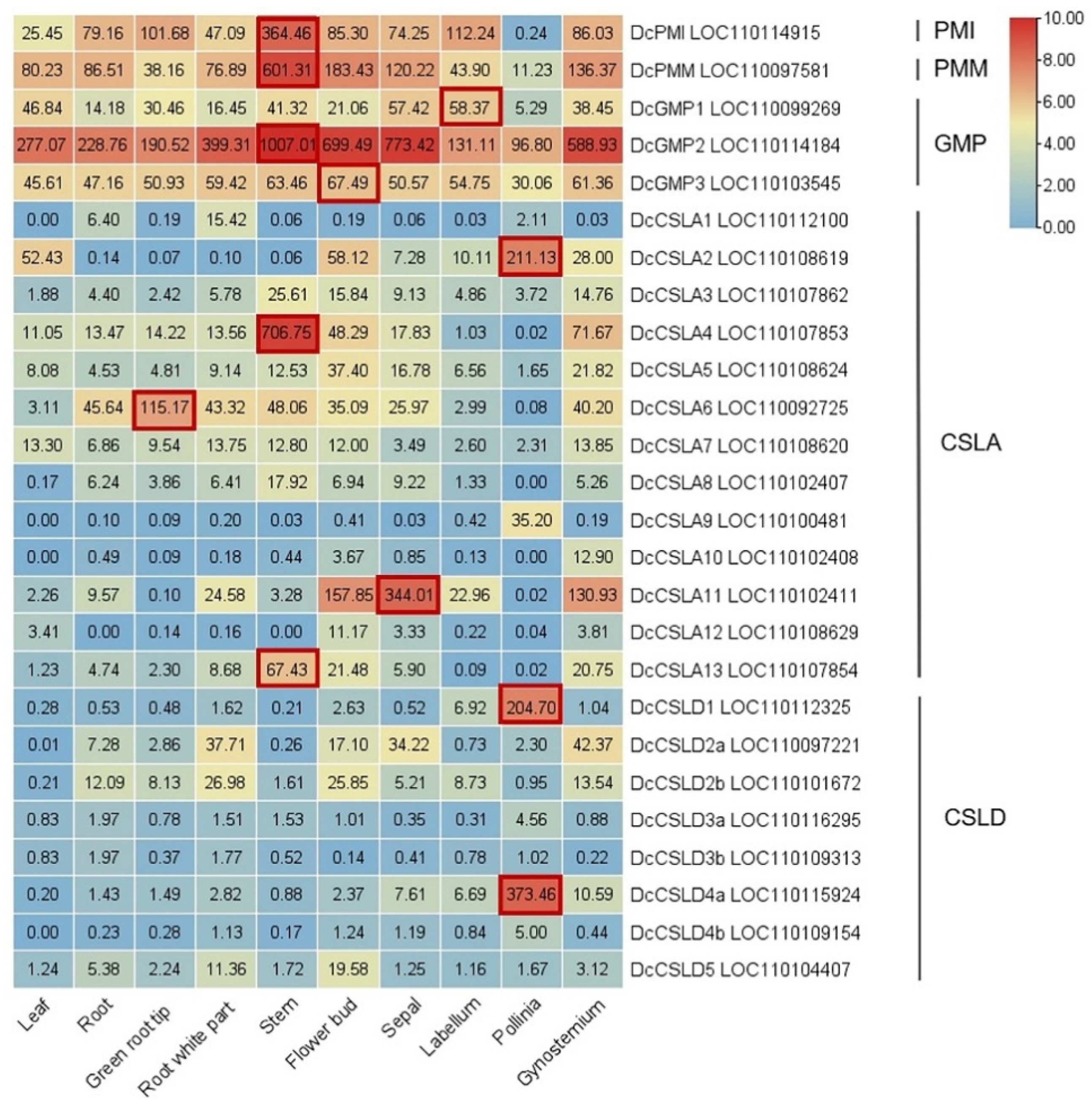
5. Research Progresses of Key Glucomannan Biosynthetic Genes in Plant
5.1. Phosphate Mannose Isomerase (PMI): Fru-6-P ↔ Man-6-P
5.2. Phosphomannomutase (PMM): Man-6-P ↔ Man-1-P
5.3. GDP-Mannose Pyrophosphorylase (GMP): Man-1-P ↔ GDP-Man
5.4. Cellulose-like Synthase A/D (CSLA/D): GDP-Man + UDP/GDP-Glu → Glucomannan
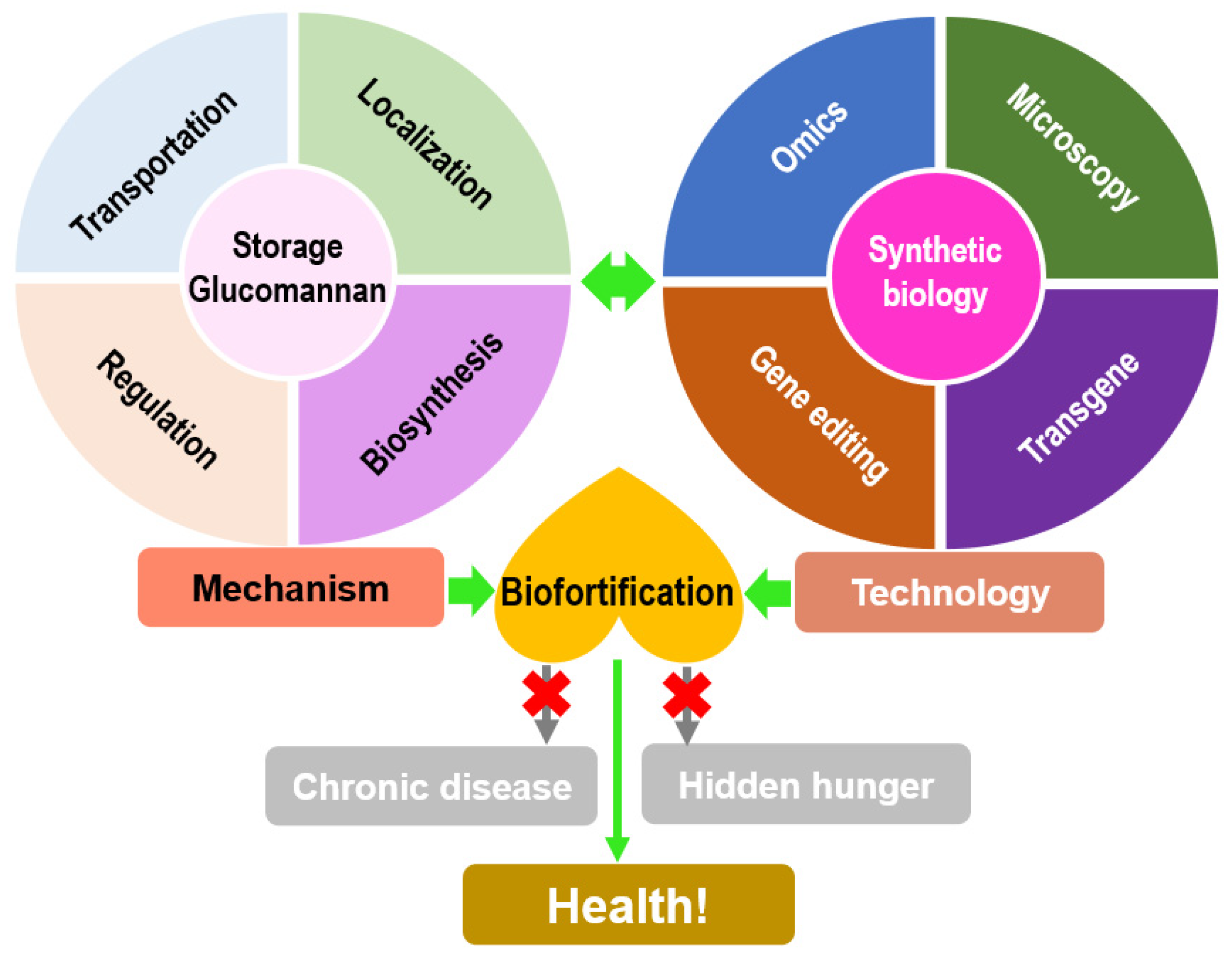
6. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Si, J.; Zhang, Y.; Luo, Y.; Liu, J.; Liu, Z. Herbal textual research on relationship between chinese medicine “shihu” (Dendrobium spp.) and “tiepi shihu” (D. catenatum). China J. Chin. Mater. Med. 2017, 42, 2001–2005. [Google Scholar]
- Cheng, J.; Dang, P.-P.; Zhao, Z.; Yuan, L.-C.; Zhou, Z.-H.; Wolf, D.; Luo, Y.-B. An assessment of the chinese medicinal Dendrobium industry: Supply, demand and sustainability. J. Ethnopharmacol. 2019, 229, 81–88. [Google Scholar] [CrossRef]
- Si, J.; Wang, Q.; Liu, Z.; Liu, J.; Luo, Y. Breakthrough in key science and technologies in Dendrobium catenatum Industry. China J. Chin. Mater. Med. 2017, 42, 2223–2227. [Google Scholar]
- Jiao, C.; Song, C.; Zheng, S.; Zhu, Y.; Jin, Q.; Cai, Y.; Lin, Y. Metabolic profiling of Dendrobium officinale in response to precursors and methyl jasmonate. Int. J. Mol. Sci. 2018, 19, 728. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [Green Version]
- Keithley, J.; Swanson, B. Glucomannan and obesity: A critical review. Altern. Ther. Health Med. 2005, 11, 30–34. [Google Scholar]
- Chen, D.; Han, Z.; Si, J. Huangjing (Polygonati rhizoma) is an emerging crop with great potential to fight chronic and hidden hunger. Sci. China Life Sci. 2021, 64, 1564–1566. [Google Scholar] [CrossRef]
- Gonzalez, P.S.; O’Prey, J.; Cardaci, S.; Barthet, V.J.A.; Sakamaki, J.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Future Smart Food: Rediscovering Hidden Treasures of Neglected and Underutilized Species for Zero Hunger in Asia; Li, X., Siddique, K.H.M., Eds.; UN: Rome, Italy, 2018; ISBN 978-92-1-047392-7. [Google Scholar]
- Siddique, K.H.M.; Li, X.; Gruber, K. Rediscovering Asia’s forgotten crops to fight chronic and hidden hunger. Nat. Plants 2021, 7, 116–122. [Google Scholar] [CrossRef]
- McCarty, M.F. Glucomannan minimizes the postprandial insulin surge: A potential adjuvant for hepatothermic therapy. Med. Hypotheses 2002, 58, 487–490. [Google Scholar] [CrossRef]
- Chua, M.; Hocking, T.J.; Chan, K.; Baldwin, T.C. Temporal and spatial regulation of glucomannan deposition and mobilization in corms of Amorphophallus konjac (Araceae). Am. J. Bot. 2013, 100, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Sagardia, S.; Seguel, O.; Torres, C.; Tapia, C.; Franck, N.; Cardemil, L. Effect of water availability on growth and water use efficiency for biomass and gel production in Aloe Vera (Aloe barbadensis M.). Ind. Crops Prod. 2010, 31, 20–27. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Li, B. Identification of molecular driving forces involved in the gelation of konjac glucomannan: Effect of degree of deacetylation on hydrophobic association. Carbohydr. Polym. 2011, 86, 865–871. [Google Scholar] [CrossRef]
- Cescutti, P.; Campa, C.; Delben, F.; Rizzo, R. Structure of the oligomers obtained by enzymatic hydrolysis of the glucomannan produced by the plant Amorphophallus konjac. Carbohydr. Res. 2002, 337, 2505–2511. [Google Scholar] [CrossRef]
- Shi, X.-D.; Nie, S.-P.; Yin, J.-Y.; Que, Z.-Q.; Zhang, L.-J.; Huang, X.-J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocoll. 2017, 73, 176–183. [Google Scholar] [CrossRef]
- Pereira, J.H.; Chen, Z.; McAndrew, R.P.; Sapra, R.; Chhabra, S.R.; Sale, K.L.; Simmons, B.A.; Adams, P.D. Biochemical characterization and crystal structure of endoglucanase Cel5A from the hyperthermophilic Thermotoga maritima. J. Struct. Biol. 2010, 172, 372–379. [Google Scholar] [CrossRef]
- Tester, R.; Al-Ghazzewi, F. Glucomannans and nutrition. Food Hydrocoll. 2017, 68, 246–254. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Yang, F. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydr. Polym. 2014, 104, 175–181. [Google Scholar] [CrossRef]
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003, 53, 183–189. [Google Scholar] [CrossRef]
- Xi, H.; Li, Q.; Chen, X.; Liu, C.; Zhao, Y.; Yao, J.; Chen, D.; Liu, J.; Si, J.; Zhang, L. Genome-wide identification of cellulose-like synthase D gene family in Dendrobium catenatum. Biotechnol. Biotechnol. Equip. 2021, 35, 1163–1176. [Google Scholar] [CrossRef]
- Li, L.; Yao, H.; Li, X.; Zhang, Q.; Wu, X.; Wong, T.; Zheng, H.; Fung, H.; Yang, B.; Ma, D.; et al. Destiny of Dendrobium officinale Polysaccharide after Oral Administration: Indigestible and Nonabsorbing, Ends in Modulating Gut Microbiota. J. Agric. Food Chem. 2019, 67, 5968–5977. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.L.; Tang, Z.H.; Zhang, X.F.; Wang, L.S.; Lin, C.W.; Luo, X. Isolation, Purification and Chemical Composition Analysis of Polysaccharides from Dendrobium officinale. J. Guangxi Univ. (Nat. Sci. Ed.) 2016, 41, 2060–2066. [Google Scholar]
- Hsieh, Y.S.-Y.; Chien, C.; Liao, S.K.-S.; Liao, S.-F.; Hung, W.-T.; Yang, W.-B.; Lin, C.-C.; Cheng, T.-J.R.; Chang, C.-C.; Fang, J.-M.; et al. Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 2008, 16, 6054–6068. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, M.; Fu, C.; Chen, Z.; Chan, G.Y.S. Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr. Res. 2004, 339, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, X.; Wang, Y.; Jiang, Z.; Zhang, H.; Zhang, M.; Hu, P. Primary structural analysis of polysaccharides from Dendrobium officinale. Chem. J. Univ. 2018, 39, 934–940. [Google Scholar]
- Kuang, M.-T.; Li, J.-Y.; Yang, X.-B.; Yang, L.; Xu, J.-Y.; Yan, S.; Lv, Y.-F.; Ren, F.-C.; Hu, J.-M.; Zhou, J. Structural characterization and hypoglycemic effect via stimulating glucagon-like peptide-1 secretion of two polysaccharides from Dendrobium officinale. Carbohydr. Polym. 2020, 241, 116326. [Google Scholar] [CrossRef]
- Li, M.; Feng, G.; Wang, H.; Yang, R.; Xu, Z.; Sun, Y.-M. Deacetylated konjac glucomannan is less effective in reducing dietary-induced hyperlipidemia and hepatic steatosis in C57BL/6 mice. J. Agric. Food Chem. 2017, 65, 1556–1565. [Google Scholar] [CrossRef]
- Tao, S.; Lei, Z.; Huang, K.; Li, Y.; Ren, Z.; Zhang, X.; Wei, G.; Chen, H. Structural characterization and immunomodulatory activity of two novel polysaccharides derived from the stem of Dendrobium officinale Kimura et Migo. J. Funct. Foods 2019, 57, 121–134. [Google Scholar] [CrossRef]
- Xing, X.; Cui, S.W.; Nie, S.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale o-acetyl-glucomannan (dendronan®): Part II. fine structures of o-acetylated residues. Carbohydr. Polym. 2015, 117, 422–433. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Zhang, D.-D.; Zha, X.-Q.; Pan, L.-H.; Luo, J.-P. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. 2016, 7, 2789–2799. [Google Scholar] [CrossRef]
- Wei, W.; Feng, L.; Bao, W.-R.; Ma, D.-L.; Leung, C.-H.; Nie, S.-P.; Han, Q.-B. Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 2016, 64, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ren, Z.; Zhang, X.; Xing, S.; Tao, S.; Liu, C.; Wei, G.; Yuan, Y.; Lei, Z. Structural characterization of polysaccharides from Dendrobium officinale and their effects on apoptosis of hela cell line. Molecules 2018, 23, 2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Wang, L.; Wang, D.; Wang, D.; Wen, C.; Han, B.; Ouyang, Z. Characterization and anti-tumor activity of a polysaccharide isolated from Dendrobium officinale grown in the Huoshan County. Chin. Med. 2018, 13, 47. [Google Scholar] [CrossRef]
- Liang, J.; Chen, S.; Hu, Y.; Yang, Y.; Yuan, J.; Wu, Y.; Li, S.; Lin, J.; He, L.; Hou, S.; et al. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018, 107, 2201–2210. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Wang, C.; Zhang, Y.; Xi, H.; Si, J.; Zhang, L.; Yan, J. Preparation, structural features and in vitro immunostimulatory activity of a glucomannan from fresh Dendrobium catenatum stems. Front. Nutr. 2022, 8, 823803. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, J.; Li, X.; Lu, J.; Wu, W.; Sun, Y.; Zhu, B.; Qin, L. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 184, 1000–1013. [Google Scholar] [CrossRef]
- Chen, H.-L.; Fan, Y.-H.; Chen, M.-E.; Chan, Y. Unhydrolyzed and hydrolyzed konjac glucomannans modulated cecal and fecal microflora in Balb/c mice. Nutrition 2005, 21, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Nakamura, Y.K.; Omaye, S.T. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr. Metab. 2012, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Colantonio, A.G.; Werner, S.L.; Brown, M. The Effects of prebiotics and substances with prebiotic properties on metabolic and inflammatory biomarkers in individuals with type 2 diabetes mellitus: A systematic review. J. Acad. Nutr. Diet. 2020, 120, 587–607.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, K.; Nakamura, S.; Moriyama-Hashiguchi, M.; Kitajima, M.; Ejima, H.; Imori, C.; Oku, T. Dietary fructooligosaccharide and glucomannan alter gut microbiota and improve bone metabolism in senescence-accelerated mouse. J. Agric. Food Chem. 2019, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. J. Funct. Foods 2010, 2, 219–224. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Khanna, S.; Tester, R.F.; Piggott, J. The potential use of hydrolysed konjac glucomannan as a prebiotic. J. Sci. Food Agric. 2007, 87, 1758–1766. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Zhang, Z.; Liang, X.; Liu, T.; Yi, H.; Gong, P.; Wang, L.; Yang, W.; Zhang, X.; et al. Konjac glucomannan with probiotics acts as a combination laxative to relieve constipation in mice by increasing short-chain fatty acid metabolism and 5-hydroxytryptamine hormone release. Nutrition 2021, 84, 111112. [Google Scholar] [CrossRef]
- Pongsapipatana, N.; Charoenwattanasatien, R.; Pramanpol, N.; Nguyen, T.-H.; Haltrich, D.; Nitisinprasert, S.; Keawsompong, S. Crystallization, structural characterization and kinetic analysis of a GH26 β-mannanase from Klebsiella oxytoca KUB-CW2-3. Acta Crystallogr. Sect. Struct. Biol. 2021, 77, 1425–1436. [Google Scholar] [CrossRef]
- Li, J.; Jiao, G.; Sun, Y.; Chen, J.; Zhong, Y.; Yan, L.; Jiang, D.; Ma, Y.; Xia, L. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. J. 2021, 19, 937–951. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Liu, J.; Zheng, Z.; Li, Q.; Wang, H.; Chen, Z.; Wang, K. Identification of the core active structure of a Dendrobium officinale polysaccharide and its protective effect against dextran sulfate sodium-induced colitis via alleviating gut microbiota dysbiosis. Food Res. Int. 2020, 137, 109641. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Huang, X.-J.; Shi, X.-D.; Chen, H.-H.; Cui, S.W.; Nie, S.-P. Protective effect of three glucomannans from different plants against DSS induced colitis in female BALB/c mice. Food Funct. 2019, 10, 1928–1939. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Wang, J.; Zhou, Y.; Qi, W.; Chen, H.; Nie, S.; Xie, M. Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohydr. Polym. 2021, 264, 118018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.; Tian, W.; Chen, H.; Yang, Y.; Xu, Y.; Chen, Y.; Chen, P.; Zhu, S.; Li, P.; Du, B. Structural characterization and immunoregulatory activity of polysaccharides from Dendrobium officinale leaves. J. Food Biochem. 2022, 46, e14023. [Google Scholar] [CrossRef] [PubMed]
- Gurusmatika, S.; Nishi, K.; Harmayani, E.; Pranoto, Y.; Sugahara, T. Immunomodulatory activity of octenyl succinic anhydride modified porang (Amorphophallus oncophyllus) glucomannan on mouse macrophage-like J774.1 cells and mouse primary peritoneal macrophages. Molecules 2017, 22, 1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, K.; Kim, S.; Yu, K.; Chung, Y.B.; Kim, W.J.; Suh, H.J.; Kim, H. Changes in the component sugar and immunostimulating activity of polysaccharides isolated from Dendrobium officinale in the pretreatments. J. Sci. Food Agric. 2022, 102, 3021–3028. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Ren, Z.; Yang, Z.; Duan, S.; Wan, Z.; Huang, J.; Liu, C.; Wei, G. Effects of different molecular weight polysaccharides from Dendrobium officinale kimura & migo on human colorectal cancer and transcriptome analysis of differentially expressed genes. Front. Pharmacol. 2021, 12, 704486. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014, 4, 00064. [Google Scholar] [CrossRef] [Green Version]
- Guanen, Q.; Junjie, S.; Baolin, W.; Chaoyang, W.; Yajuan, Y.; Jing, L.; Junpeng, L.; Gaili, N.; Zhongping, W.; Jun, W. MiR-214 promotes cell meastasis and inhibites apoptosis of esophageal squamous cell carcinoma via PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2018, 105, 350–361. [Google Scholar] [CrossRef]
- Wu, C.; Qiu, S.; Liu, P.; Ge, Y.; Gao, X. Rhizoma Amorphophalli inhibits TNBC cell proliferation, migration, invasion and metastasis through the PI3K/Akt/mTOR pathway. J. Ethnopharmacol. 2018, 211, 89–100. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, J.; He, L.; Du, X.; Zhou, L.; Xiong, Q.; Lai, X.; Yang, Y.; Huang, S.; et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol. Res. 2019, 148, 104417. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Wang, G.; Ge, S.; Lan, X.; Xu, G.; Liu, H. Dendrobium officinale polysaccharides inhibit 1-methyl-2-nitro-1-nitrosoguanidine induced precancerous lesions of gastric cancer in rats through regulating wnt/β-catenin pathway and altering serum endogenous metabolites. Molecules 2019, 24, 2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Liu, W.; Chen, F.; Li, N. Effects of Dendrobium officinale polysaccharide on AMPK/ULK1 Pathway Related Autophagy in Astrocytes Induced by Hypoxia/Reoxygenation. China J. Mod. Appl. Pharm. 2021, 38, 2101–2115. [Google Scholar]
- Liu, Y.; Yang, L.; Zhang, Y.; Liu, X.; Wu, Z.; Gilbert, R.G.; Deng, B.; Wang, K. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Huang, W.; Nie, S. Metabolism amelioration of Dendrobium officinale polysaccharide on type II diabetic rats. Food Hydrocoll. 2020, 102, 105582. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Liu, Y.; Shui, W.; Wang, J.; Cao, P.; Wang, H.; You, R.; Zhang, Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Chen, J.; Wan, L.; Zheng, Q.; Lan, M.; Zhang, X.; Li, Y.; Li, B.; Li, L. Structural characterization and in vitro hypoglycaemic activity of glucomannan from Anemarrhena asphodeloides bunge. Food Funct. 2022, 13, 1797–1807. [Google Scholar] [CrossRef]
- Walsh, D.E.; Yaghoubian, V.; Behforooz, A. Effect of glucomannan on obese patients: A clinical study. Int. J. Obes. 1984, 8, 289–293. [Google Scholar]
- Li, Q.; Xie, C.; Li, X.; Wang, X. Chemical constituents of Dendrobium officinale and their development and utilization in cosmetics. China Surfactant Deterg. Cosmet. 2017, 47, 109–113. [Google Scholar]
- Chen, M.; Sun, Y.; Zhao, Y. Study on moisturizing properties of Dendrobium officinale extract. J Shanghai Uni Tradit. Chin Med. 2015, 29, 70–73. [Google Scholar] [CrossRef]
- Bao, S.; Zha, X.; Hao, J.; Luo, J. Study on antioxidant activity of polysaccharide from Dendrobium officinale with different molecular weight in vitro. Food Sci 2009, 30, 123–127. [Google Scholar]
- Luo, Q.; Tang, Z.; Zhang, X.; Zhong, Y.; Yao, S.; Wang, L.; Lin, C.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, Z.; Zhang, J. Effects of fermentation on active constituents of Dendrobium officinale and its application in cosmetics. China Surfactant Deterg. 2021, 44, 46–50. [Google Scholar]
- Gu, F.; Jiang, X.; Chen, Y.; Han, B.; Chen, N.; Wei, C. Study on hygroscopic and moisturizing properties and skin irritation of polysaccharides from Dendrobium huoshanense. Nat. Prod. Res. Dev. 2018, 30, 1701–1705. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, M.; Li, C.; Zhang, Z.; He, S. Development of functional yoghurt of Dendrobium officinale. Fujian Agric. Sci. Technol. 2021, 51, 19–23. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, H.; Yang, S.; Zhang, Z.; Chen, L.; Liu, B.; Wang, L. Preparation technology and function of Dendrobium officinale mixed flower tea. Food Ferment. Ind. 2021, 47, 170–179. [Google Scholar] [CrossRef]
- Luo, M.; Xie, W. Development of Dendrobium officinale leaf health tea bag. Food Ind. 2021, 42, 38–43. [Google Scholar]
- Tang, W.; Xia, J.; Chen, Y. Effects of different cutting methods on active components and antioxidant activity of Dendrobium officinale leaf tea. Food Sci. Technol. 2021, 46, 74–82. [Google Scholar] [CrossRef]
- Chen, S.; Yan, M.; Lv, G.; Liu, X. Development status and progress of Dendrobium officinale health food. Chin. J. Pharm. 2013, 48, 1625–1628. [Google Scholar]
- Tan, Y.; Liu, X.; Yuan, F. Structure, properties and application of Konjac Glucomannan in Food. China Condiment 2019, 44, 168–174+178. [Google Scholar]
- Baianu, I.C.; Ozu, E.M. Gelling mechanisms of glucomannan polysaccharides and their interactions with proteins. ACS 2002, 8, 298–305. [Google Scholar]
- Xue, H.; Wu, D.; Xu, Q.; Zhu, Y.; Cheng, C. Application and research progress of Konjac Glucomannan in yogurt. Packag. Food Mach. 2021, 39, 58–62. [Google Scholar]
- Chen, J.; Zhang, K.; Du, J.; Hu, Y.; Wang, L.; Wang, C.; Ni, X.; Jiang, F. Effects of konjac glucomannan and its derivatives on the physical properties of poultry reconstituted ham. Food Sci. 2010, 31, 36–39. [Google Scholar]
- Zhao, D.; Zhou, Y.; Liu, H.; Liang, J.; Cheng, Y.; Nirasawa, S. Effects of dough mixing time before adding konjac glucomannan on the quality of noodles. J. Food Sci. Technol. 2017, 54, 3837–3846. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Y.; Xu, X.; Zhong, G. Optimization of konjac glucomannan edible film formulation. Sci. Technol. Food Ind. 2016, 37, 330–336. [Google Scholar] [CrossRef]
- Gilbert, L.; Alhagdow, M.; Nunes-Nesi, A.; Quemener, B.; Guillon, F.; Bouchet, B.; Faurobert, M.; Gouble, B.; Page, D.; Garcia, V.; et al. GDP-d-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 2009, 60, 499–508. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Salmona, J.; Doulbeau, S.; Descroix, F.; Bertrand, B.; Lashermes, P.; Dussert, S. Regulation of galactomannan biosynthesis in coffee seeds. J. Exp. Bot. 2014, 65, 323–337. [Google Scholar] [CrossRef]
- Manzoor, S.; Wani, S.M.; Ahmad Mir, S.; Rizwan, D. Role of probiotics and prebiotics in mitigation of different diseases. Nutrition 2022, 96, 111602. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Roux, C.; Gresh, N.; Perera, L.E.; Piquemal, J.-P.; Salmon, L. Binding of 5-phospho-D-arabinonohydroxamate and 5-phospho-D-arabinonate inhibitors to zinc phosphomannose isomerase fromCandida albicans studied by polarizable molecular mechanics and quantum mechanics. J. Comput. Chem. 2007, 28, 938–957. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Natre 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Maruta, T.; Yonemitsu, M.; Yabuta, Y.; Tamoi, M.; Ishikawa, T.; Shigeoka, S. Arabidopsis Phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J. Biol. Chem. 2008, 283, 28842–28851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Hu, D.; Zhao, X.; Li, Y.; Liu, T.; Wang, J.; Hou, X.; Li, Y. BcPMI2, isolated from non-heading Chinese cabbage encoding phosphomannose isomerase, improves stress tolerance in transgenic tobacco. Mol. Biol. Rep. 2014, 41, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Agbayani, R.; McCafferty, H.; Albert, H.H.; Moore, P.H. Effective selection of transgenic papaya plants with the PMI/Man selection system. Plant Cell Rep. 2005, 24, 426–432. [Google Scholar] [CrossRef]
- He, Z.; Fu, Y.; Si, H.; Hu, G.; Zhang, S.; Yu, Y.; Sun, Z. Phosphomannose-isomerase (PMI) gene as a selectable marker for rice transformation via Agrobacterium. Plant Sci. 2004, 166, 17–22. [Google Scholar] [CrossRef]
- Fujiki, Y.; Yoshikawa, Y.; Sato, T.; Inada, N.; Ito, M.; Nishida, I.; Watanabe, A. Dark-inducible genes from Arabidopsis thaliana are associated with leaf senescence and repressed by sugars. Physiol. Plant. 2001, 111, 345–352. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Tang, Y.; Zhang, X.; Zhang, M.; Zheng, Y.; Zhang, F. Using the phosphomannose isomerase (PMI) gene from saccharomyces cerevisiae for selection in rice transformation. J. Integr. Agric. 2012, 11, 1391–1398. [Google Scholar] [CrossRef]
- Duan, Y.; Zhai, C.; Li, H.; Li, J.; Mei, W.; Gui, H.; Ni, D.; Song, F.; Li, L.; Zhang, W.; et al. An efficient and high-throughput protocol for Agrobacterium-mediated transformation based on phosphomannose isomerase positive selection in Japonica rice (Oryza sativa L.). Plant Cell Rep. 2012, 31, 1611–1624. [Google Scholar] [CrossRef]
- Hu, L.; Li, H.; Qin, R.; Xu, R.; Li, J.; Li, L.; Wei, P.; Yang, J. Plant phosphomannose isomerase as a selectable marker for rice transformation. Sci. Rep. 2016, 6, 25921. [Google Scholar] [CrossRef] [Green Version]
- Joersbo, M.; Donaldson, I.; Kreiberg, J.; Petersen, S.G.; Brunstedt, J.; Okkels, F.T. Analysis of mannose selection used for transformation of sugar beet. Mol. Breed. 1998, 4, 111–117. [Google Scholar] [CrossRef]
- Miki, B.; McHugh, S. Selectable marker genes in transgenic plants: Applications, alternatives and biosafety. J. Biotechnol. 2004, 107, 193–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, J. Characterization of an algal phosphomannose isomerase gene and its application as a selectable marker for genetic manipulation of tomato. Plant Divers. 2021, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Matheson, N.K. Phosphomannomutase and phosphoglucomutase in developing Cassia corymbosa seeds. Phytochemistry 1979, 18, 1147–1150. [Google Scholar] [CrossRef]
- Qian, W.; Yu, C.; Qin, H.; Liu, X.; Zhang, A.; Johansen, I.E.; Wang, D. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana: Functional analysis of plant phosphomannomutase. Plant J. 2007, 49, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Badejo, A.A.; Fujikawa, Y.; Esaka, M. Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J. Plant Physiol. 2009, 166, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xia, Z.; Zhang, J.; Wang, D.; Zhai, W. Transgenosis of the phosphomannomutase transgene increases vitamin C content in rice. Chin. J. Rice Sci. 2016, 30, 441–446. [Google Scholar]
- He, C.; Zeng, S.; Teixeira da Silva, J.A.; Yu, Z.; Tan, J.; Duan, J. Molecular cloning and functional analysis of the phosphomannomutase (PMM) gene from Dendrobium officinale and evidence for the involvement of an abiotic stress response during germination. Protoplasma 2017, 254, 1693–1704. [Google Scholar] [CrossRef]
- Veljovic-Jovanovic, S.D.; Pignocchi, C.; Noctor, G.; Foyer, C.H. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001, 127, 426–435. [Google Scholar] [CrossRef]
- Sawake, S.; Tajima, N.; Mortimer, J.C.; Lao, J.; Ishikawa, T.; Yu, X.; Yamanashi, Y.; Yoshimi, Y.; Kawai-Yamada, M.; Dupree, P.; et al. Konjac1 and 2 are key factors for gdp-mannose generation and affect l-ascorbic acid and glucomannan biosynthesis in Arabidopsis. Plant Cell 2015, 27, 3397–3409. [Google Scholar] [CrossRef]
- Badejo, A.A.; Tanaka, N.; Esaka, M. Analysis of GDP-D-mannose pyrophosphorylase gene promoter from Acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the Acerola gene. Plant Cell Physiol. 2008, 49, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Cronje, C.; George, G.M.; Fernie, A.R.; Bekker, J.; Kossmann, J.; Bauer, R. Manipulation of l-ascorbic acid biosynthesis pathways in Solanum lycopersicum: Elevated GDP-mannose pyrophosphorylase activity enhances l-ascorbate levels in red fruit. Planta 2012, 235, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, C.; Zhu, Z.; Yu, X. Overexpression in tobacco of a tomato gmpase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Rep. 2011, 31, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ouyang, B.; Yang, C.; Zhang, X.; Liu, H.; Zhang, Y.; Zhang, J.; Li, H.; Ye, Z. Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-mannose pyrophosphorylase gene in tomato plant. PLoS ONE 2013, 8, e61987. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, L.; Shi, Q.; Li, Q.; Guo, X.; Li, X.; Yu, X. Effect of Tomato GMPase overexpression on tolerance of potato plants to temperature stress. Scientia. Agric. Sin. 2011, 38, 692–700. [Google Scholar]
- Ai, T.; Liao, X.; Li, R.; Fan, L.; Luo, F.; Xu, Y.; Wang, S. GDP-D-mannose pyrophosphorylase from Pogonatherum paniceum enhances salinity and drought tolerance of transgenic tobacco. Z. Für Nat. C 2016, 71, 243–252. [Google Scholar] [CrossRef]
- He, C.; Yu, Z.; Teixeira da Silva, J.A.; Zhang, J.; Liu, X.; Wang, X.; Zhang, X.; Zeng, S.; Wu, K.; Tan, J.; et al. DoGMP1 from Dendrobium officinale contributes to mannose content of water-soluble polysaccharides and plays a role in salt stress response. Sci. Rep. 2017, 7, 41010. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Qian, W.; Wang, W.; Wu, Y.; Yu, C.; Jiang, X.; Wang, D.; Wu, P. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 18308–18313. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.-H.; Kronzucker, H.J.; Shi, W.-M. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity: Root growth inhibition by ammonium. Plant Cell Environ. 2010, 33, 1529–1542. [Google Scholar] [CrossRef]
- Barth, C.; Gouzd, Z.A.; Steele, H.P.; Imperio, R.M. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J. Exp. Bot. 2010, 61, 379–394. [Google Scholar] [CrossRef] [Green Version]
- Kempinski, C.F.; Haffar, R.; Barth, C. Toward the mechanism of NH4+ sensitivity mediated by Arabidopsis GDP-mannose pyrophosphorylase: NH4+ sensitivity mediated by GMPase. Plant Cell Environ. 2011, 34, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Huang, J.; Xu, Y. The cellulose synthase superfamily in fully sequenced plants and algae. BMC Plant Biol. 2009, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, A.; Schwerdt, J.G.; Shirley, N.J.; Khor, S.F.; Neumann, K.; O’Donovan, L.A.; Lahnstein, J.; Collins, H.M.; Henderson, M.; Fincher, G.B.; et al. Revised Phylogeny of the Cellulose Synthase Gene Superfamily: Insights into Cell Wall Evolution. Plant Physiol. 2018, 177, 1124–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richmond, T.A.; Somerville, C.R. The cellulose synthase superfamily. Plant Physiol. 2000, 124, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Keegstra, K.; Walton, J. β-glucans--brewer’s bane, dietician’s delight. Science 2006, 311, 1872–1873. [Google Scholar] [CrossRef]
- Davis, J.; Brandizzi, F.; Liepman, A.H.; Keegstra, K. Arabidopsis mannan synthase CSLA9 and glucan synthase CSLC4 have opposite orientations in the Golgi membrane: Hemicellulosic glycan synthase topology. Plant J. 2010, 64, 1028–1037. [Google Scholar] [CrossRef]
- Liepman, A.H.; Wilkerson, C.G.; Keegstra, K. Expression of cellulose synthase-like (Csl) genes in insect cells reveals that CslA family members encode mannan synthases. Proc. Natl. Acad. Sci. USA 2005, 102, 2221–2226. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Verhertbruggen, Y.; Oikawa, A.; Manisseri, C.; Knierim, B.; Prak, L.; Jensen, J.K.; Knox, J.P.; Auer, M.; Willats, W.G.T.; et al. The Cooperative activities of CSLD2, CSLD3, and CSLD5 Are required for normal arabidopsis development. Mol. Plant 2011, 4, 1024–1037. [Google Scholar] [CrossRef]
- Wang, X.; Cnops, G.; Vanderhaeghen, R.; Block, S.D.; Montagu, M.V.; Lijsebettens, M.V. AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol. 2001, 126, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Hazen, S.P.; Scott-Craig, J.S.; Walton, J.D. Cellulose synthase-like genes of rice. Plant Physiol. 2002, 128, 336–340. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Chen, D.; Liu, J.; Si, J. Genome-wide identification and expression analysis of CSLA gene family of Dendrobium catenatum. China J. Chin Mater. Med. 2020, 45, 3120–3127. [Google Scholar]
- Dhugga, K.S.; Barreiro, R.; Whitten, B.; Stecca, K.; Hazebroek, J.; Randhawa, G.S.; Dolan, M.; Kinney, A.J.; Tomes, D.; Nichols, S.; et al. Guar Seed ß-Mannan Synthase Is a Member of the Cellulose Synthase Super Gene Family. Science 2004, 303, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A.H.; Nairn, C.J.; Willats, W.G.T.; Sørensen, I.; Roberts, A.W.; Keegstra, K. Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggests diverse roles of mannans in plants. Plant Physiol. 2007, 143, 1881–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Li, L.; Sun, Y.-H.; Chiang, V.L. The Cellulose Synthase Gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol. 2006, 142, 1233–1245. [Google Scholar] [CrossRef] [Green Version]
- Gille, S.; Cheng, K.; Skinner, M.E.; Liepman, A.H.; Wilkerson, C.G.; Pauly, M. Deep sequencing of voodoo lily (Amorphophallus konjac): An approach to identify relevant genes involved in the synthesis of the hemicellulose glucomannan. Planta 2011, 234, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Alonso, A.P.; Wilkerson, C.G.; Keegstra, K. Deep EST profiling of developing fenugreek endosperm to investigate galactomannan biosynthesis and its regulation. Plant Mol. Biol. 2012, 79, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Goubet, F.; Barton, C.J.; Mortimer, J.C.; Yu, X.; Zhang, Z.; Miles, G.P.; Richens, J.; Liepman, A.H.; Seffen, K.; Dupree, P. Cell wall glucomannan in Arabidopsis is synthesised by CSLA glycosyltransferases, and influences the progression of embryogenesis. Plant J. 2009, 60, 527–538. [Google Scholar] [CrossRef]
- Goubet, F.; Misrahi, A.; Park, S.K.; Zhang, Z.; Twell, D.; Dupree, P. AtCSLA7, a cellulose synthase-like putative glycosyltransferase, is important for pollen tube growth and embryogenesis in Arabidopsis. Plant Physiol. 2003, 131, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Voiniciuc, C.; Dama, M.; Gawenda, N.; Stritt, F.; Pauly, M. Mechanistic insights from plant heteromannan synthesis in yeast. Proc. Natl. Acad. Sci. USA 2019, 116, 522–527. [Google Scholar] [CrossRef]
- Robert, M.; Waldhauer, J.; Stritt, F.; Yang, B.; Pauly, M.; Voiniciuc, C. Modular biosynthesis of plant hemicellulose and its impact on yeast cells. Biotechnol. Biofuels 2021, 14, 140. [Google Scholar] [CrossRef]
- Wang, Y.; Mortimer, J.C.; Davis, J.; Dupree, P.; Keegstra, K. Identification of an additional protein involved in mannan biosynthesis. Plant J. 2013, 73, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.-C.; Reca, I.-B.; Kim, Y.; Park, S.; Thomashow, M.F.; Keegstra, K.; Han, K.-H. Transcription factors that directly regulate the expression of CSLA9 encoding mannan synthase in Arabidopsis thaliana. Plant Mol. Biol. 2014, 84, 577–587. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wu, K.; Zhang, J.; Liu, X.; Zeng, S.; Yu, Z.; Zhang, X.; Teixeira da Silva, J.A.; Deng, R.; Tan, J.; et al. Cytochemical localization of polysaccharides in Dendrobium officinale and the involvement of DoCSLA6 in the synthesis of mannan polysaccharides. Front. Plant Sci. 2017, 8, 00173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, A.J.; Yoo, C.-M.; Mutwil, M.; Jensen, J.K.; Hou, G.; Blaukopf, C.; Sørensen, I.; Blancaflor, E.B.; Scheller, H.V.; Willats, W.G.T. Functional analysis of the cellulose synthase-like genes CSLD1, CSLD2, and CSLD4 in tip-growing Arabidopsis cells. Plant Physiol. 2008, 148, 1238–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnall, J.A.; Quatrano, R.S. Abscisic acid elicits the water-stress response in root hairs of Arabidopsis thaliana. Plant Physiol. 1992, 100, 216–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favery, B.; Ryan, E.; Foreman, J.; Linstead, P.; Boudonck, K.; Steer, M.; Shaw, P.; Dolan, L. KOJAK encodes a cellulose synthase-like protein required for root hair cell morphogenesis in Arabidopsis. Genes Dev. 2001, 15, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Galway, M.E.; Eng, R.C.; Schiefelbein, J.W.; Wasteneys, G.O. Root hair-specific disruption of cellulose and xyloglucan in AtCSLD3 mutants, and factors affecting the post-rupture resumption of mutant root hair growth. Planta 2011, 233, 985–999. [Google Scholar] [CrossRef]
- Bernal, A.J.; Jensen, J.K.; Harholt, J.; Sørensen, S.; Moller, I.; Blaukopf, C.; Johansen, B.; de Lotto, R.; Pauly, M.; Scheller, H.V.; et al. Disruption of ATCSLD5 results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. Plant J. 2007, 52, 791–802. [Google Scholar] [CrossRef]
- Yoo, C.-M.; Quan, L.; Blancaflor, E.B. Divergence and redundancy in CSLD2 and CSLD3 function during Arabidopsis Thaliana root hair and female gametophyte development. Front. Plant Sci. 2012, 3, 00111. [Google Scholar] [CrossRef]
- Li, M.; Xiong, G.; Li, R.; Cui, J.; Tang, D.; Zhang, B.; Pauly, M.; Cheng, Z.; Zhou, Y. Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth: Rice cellulose synthase-like D4 is essential for normal cell-wall. Plant J. 2009, 60, 1055–1069. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Yin, L.; Oikawa, A.; Scheller, H.V. Mannan synthase activity in the CSLD family. Plant Signal. Behav. 2011, 6, 1620–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Szumlanski, A.L.; Gu, F.; Guo, F.; Nielsen, E. A role for CSLD3 during cell-wall synthesis in apical plasma membranes of tip-growing root-hair cells. Nat. Cell Biol. 2011, 13, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bak, G.; Burgin, T.; Barnes, W.J.; Mayes, H.B.; Peña, M.J.; Urbanowicz, B.R.; Nielsen, E. Biochemical and genetic analysis identify CSLD3 as a beta-1,4-glucan synthase that functions during plant cell wall synthesis. Plant Cell 2020, 32, 1749–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Zhang, R.; Tang, Y.; Peng, C.; Wu, L.; Feng, S.; Chen, P.; Wang, Y.; Du, X.; Peng, L. Cotton CSLD3 restores cell elongation and cell wall integrity mainly by enhancing primary cellulose production in the Arabidopsis cesa6 mutant. Plant Mol. Biol. 2019, 101, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Johns, M.A.; Cao, H.; Rupani, M. A survey of plant and algal genomes and transcriptomes reveals new insights into the evolution and function of the cellulose synthase superfamily. BMC Genom. 2014, 15, 260. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Zhu, Y. Polygonati Rhizome—A new high-quality crop with great potential and not occupying farmland. Sci. Sin. Vitae. 2021, 51, 1477–1484. [Google Scholar] [CrossRef]
- Lowe, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef]
- Akhtar, S. Malnutrition in South Asia—A Critical Reappraisal. Crit. Rev. Food Sci. Nutr. 2016, 56, 2320–2330. [Google Scholar] [CrossRef]
- Li, X.; Yadav, R.; Siddique, K.H.M. Neglected and underutilized crop species: The key to improving dietary diversity and fighting hunger and malnutrition in Asia and the Pacific. Front. Nutr. 2020, 7, 593711. [Google Scholar] [CrossRef]
- Jin, P.; Liang, Z.; Li, H.; Chen, C.; Xue, Y.; Du, Q. Biosynthesis of low-molecular-weight mannan using metabolically engineered Bacillus subtilis 168. Carbohydr. Polym. 2021, 251, 117115. [Google Scholar] [CrossRef]
- Zhu, Q.; Zeng, D.; Yu, S.; Cui, C.; Li, J.; Li, H.; Chen, J.; Zhang, R.; Zhao, X.; Chen, L.; et al. From golden rice to aSTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol. Plant 2018, 11, 1440–1448. [Google Scholar] [CrossRef] [PubMed]


| Name | Molecular Weights (Mw, kDa) | Monosaccharide Compositions | Bioactivities | References |
|---|---|---|---|---|
| DOP2 | 4699 | Man:Glc = 7.64:1.00 | Unknown | [23] |
| DOP3 | 5480 | Man:Glc = 4.50:1.00 | Unknown | [23] |
| DOP4 | 5408 | Man:Glc = 3.57:1.00 | Unknown | [23] |
| DOP-1-A1 | 130 | Man:Glc = 40.2:8.4 | Unknown | [25] |
| SDOP | 1660 | Man:Glc = 4.9:1.0 | Unknown | [26] |
| DOPW-1 | 389.98 | Man:Glc = 10.75:1.00 | Unknown | [29] |
| DOPW-2 | 374.11 | Man:Glc = 8.82:1.00 | Unknown | [29] |
| DOP-1 | 389.98 | Man:Glc = 5.18:1.00 | Immunomodulatory activity | [27] |
| DOP-2 | 374.11 | Man:Glc = 4.78:1.00 | Immunomodulatory activity | [27] |
| DOP-W3-b | 15.43 | Man:Glc = 4.5:1.0 | Immunomodulatory activity | [31] |
| DOP-I-1 | 730 | Man:Glc = 5.8:1.0 | Immunomodulatory activity | [32] |
| DOPa | 810 | Man:Glc = 5.6:1.0 | Immunomodulatory activity | [32] |
| DOPb | 670 | Man:Glc = 5.9:1.0 | Immunomodulatory activity | [32] |
| DOPA-1 | 394 | Man:Glc = 5.8:1.0 | Immunomodulatory activity | [5] |
| DOPA-2 | 362 | Man:Glc = 4.5:1.0 | Immunomodulatory activity | [5] |
| DWDOP1 | 1341 | Man: Glc = 6.79:1.00 | Unknown | [33] |
| FWDOP1 | 1415 | Man: Glc = 7.46:1.00 | Anti-tumor activity | [33] |
| DOPA-1 | 229 | Man:Glc:Gal = 1.00:0.42:0.27 | Anti-tumor activity | [34] |
| DOP1-DES | 298 | Man:Glc = 2.2:1.0 | Unknown | [35] |
| DOP2-DES | 30 | Man:Glc = 3.7:1.0 | Unknown | [35] |
| LDOP-1 | 91.8 | Man:Gal:Glc:Gal:Ara = 2.0:1.7:1.3:1.6:0.7 | Anti-inflammatory activity | [30] |
| DLP-1 | 1380 | Man:Glc = 71.69:22.89 | Immunomodulatory activity | |
| DCP | 221 | Man:Glc:Gal = 69.5:30.2:0.3 | Immunomodulatory activity | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, L.; Shi, Y.; Li, C.; Liu, J.; Chong, S.-L.; Lim, K.-J.; Si, J.; Han, Z.; Chen, D. Glucomannan in Dendrobium catenatum: Bioactivities, Biosynthesis and Perspective. Genes 2022, 13, 1957. https://doi.org/10.3390/genes13111957
Qi L, Shi Y, Li C, Liu J, Chong S-L, Lim K-J, Si J, Han Z, Chen D. Glucomannan in Dendrobium catenatum: Bioactivities, Biosynthesis and Perspective. Genes. 2022; 13(11):1957. https://doi.org/10.3390/genes13111957
Chicago/Turabian StyleQi, Luyan, Yan Shi, Cong Li, Jingjing Liu, Sun-Li Chong, Kean-Jin Lim, Jinping Si, Zhigang Han, and Donghong Chen. 2022. "Glucomannan in Dendrobium catenatum: Bioactivities, Biosynthesis and Perspective" Genes 13, no. 11: 1957. https://doi.org/10.3390/genes13111957
APA StyleQi, L., Shi, Y., Li, C., Liu, J., Chong, S. -L., Lim, K. -J., Si, J., Han, Z., & Chen, D. (2022). Glucomannan in Dendrobium catenatum: Bioactivities, Biosynthesis and Perspective. Genes, 13(11), 1957. https://doi.org/10.3390/genes13111957