Molecular Characteristics and Promoter Analysis of Porcine COL1A1
Abstract
:1. Introduction
2. Materials and Method
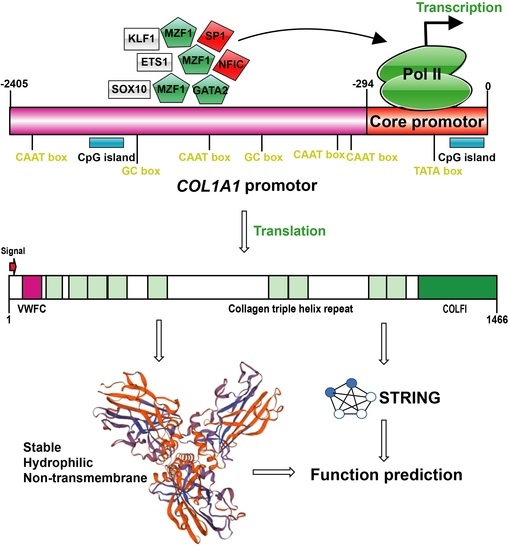
2.1. Bioinformatics Analysis of 5′ Flanking Region of Porcine COL1A1 Gene
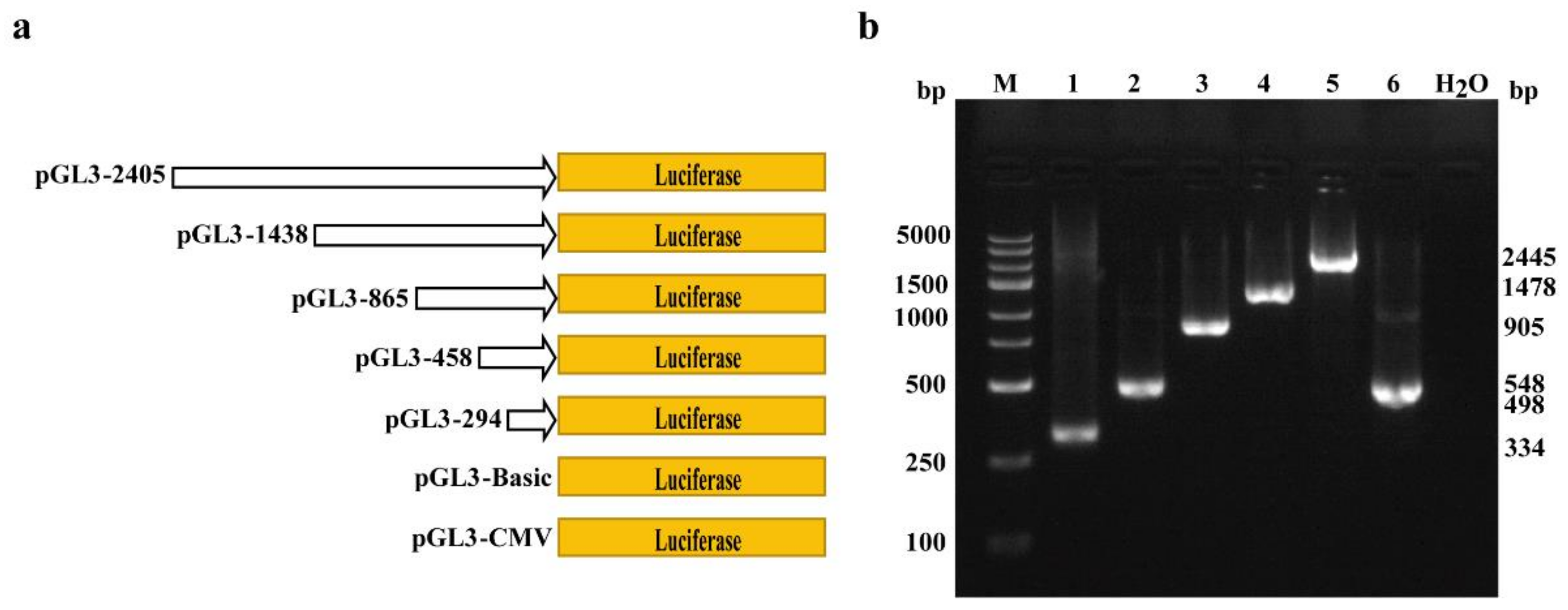
2.2. Amplification of Porcine COL1A1 Promoter with Different Lengths
2.3. Luciferase Vector Construction
2.4. Cell Culture and Transfection
2.5. Luciferase Reporter Gene Assay
2.6. Structural Analysis of Porcine COL1A1 Protein
2.7. Statistical Analysis
3. Results
3.1. Bioinformatics Analysis of 5′ Flanking Region of Porcine COL1A1
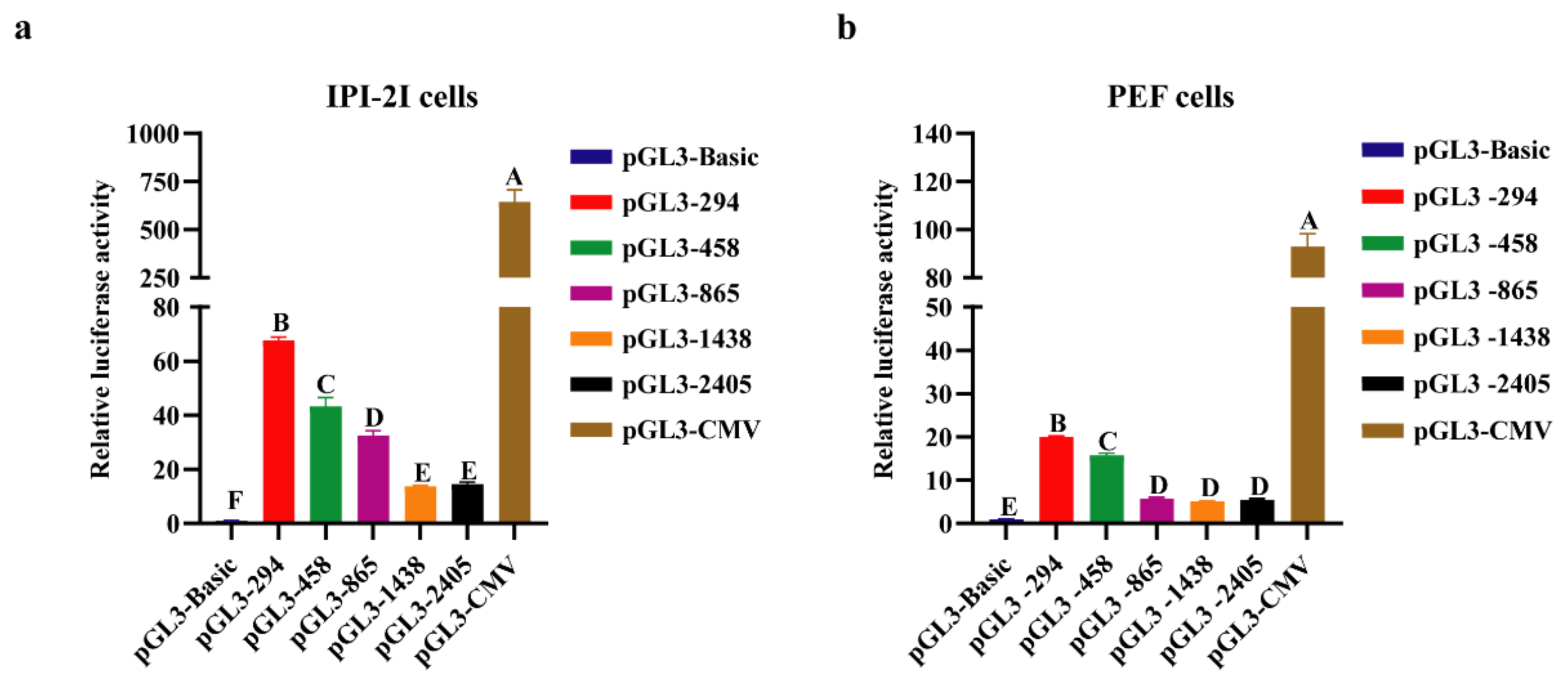
3.2. Analysis of Transcriptional Activity of Porcine COL1A1 Promoter by Luciferase Reporters
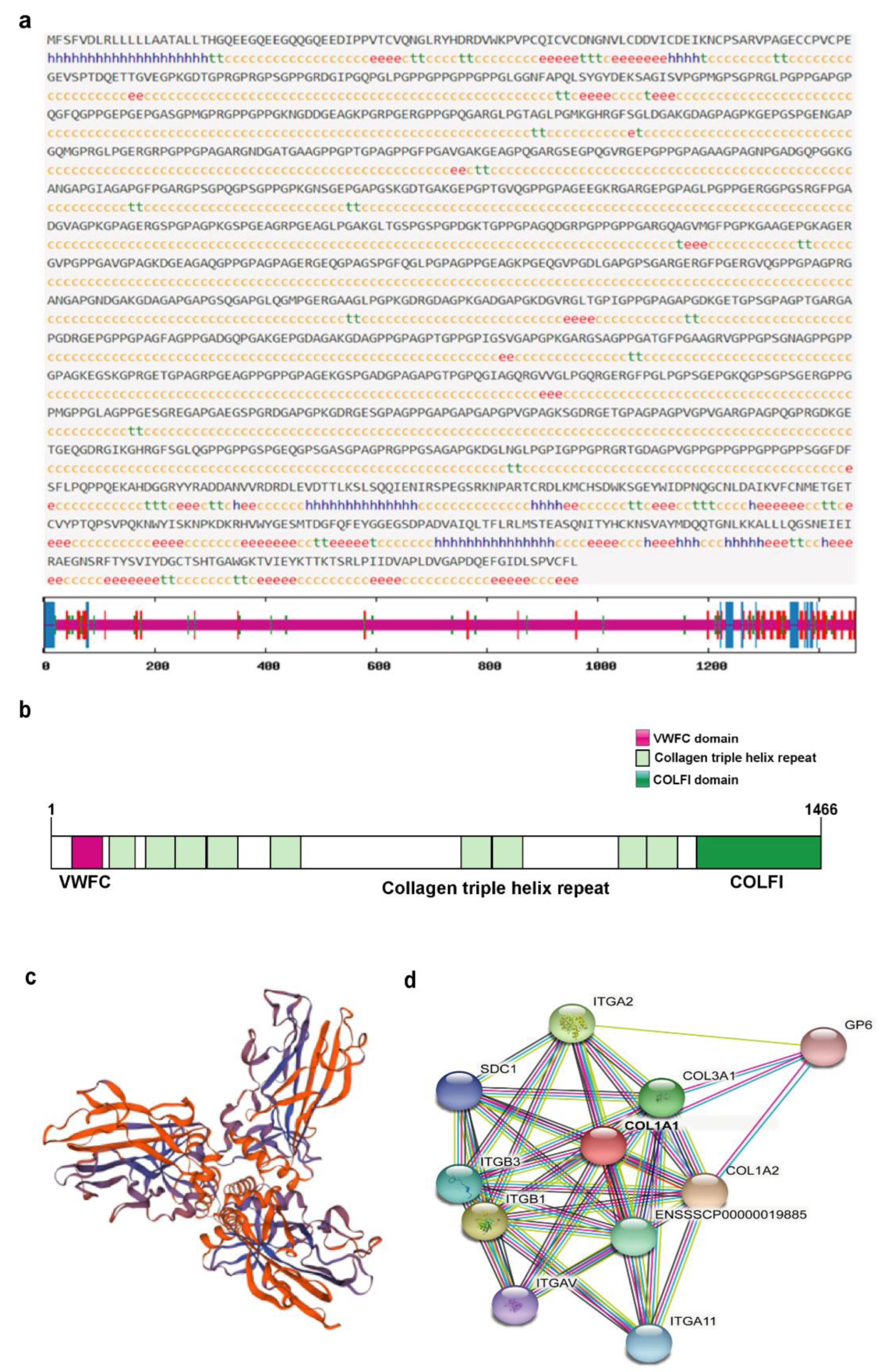
3.3. Amino Acid Sequence Analysis of Porcine COL1A1
3.4. Structural and Functional Prediction of Porcine COL1A1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, U.; Carrique, L.; Vadon-Le Goff, S.; Mariano, N.; Georges, R.N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural basis of homo-and heterotrimerization of collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulmes, D.J. Building collagen molecules, fibrils, and suprafibrillar structures. J. Struct. Biol. 2002, 137, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; McBride, D.J.; Losert, W.; Leikin, S. Segregation of type I collagen homo-and heterotrimers in fibrils. J. Mol. Biol. 2008, 383, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Uitto, J. Collagen polymorphism: Isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch. Biochem. Biophys. 1979, 192, 371–379. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Bashey, R.I.; Benditt, M.; Yankowski, R. Identification of collagen alpha1(I) trimer in embryonic chick tendons and calvaria. Biochem. Biophys. Res. Commun. 1977, 78, 1354–1361. [Google Scholar] [CrossRef]
- McBride, D.J., Jr.; Kadler, K.E.; Hojima, Y.; Prockop, D.J. Self-assembly into fibrils of a homotrimer of type I collagen. Matrix 1992, 12, 256–263. [Google Scholar] [CrossRef]
- McBride, D.J., Jr.; Choe, V.; Shapiro, J.R.; Brodsky, B. Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J. Mol. Biol. 1997, 270, 275–284. [Google Scholar] [CrossRef]
- Jia, R.; Wang, C. MiR-29b-3p Reverses Cisplatin Resistance by Targeting COL1A1 in Non-Small-Cell Lung Cancer A549/DDP Cells. Cancer Manag. Res. 2020, 12, 2559–2566. [Google Scholar] [CrossRef]
- Kim, K.; Kim, Y.J. RhoBTB3 Regulates proliferation and invasion of breast cancer cells via Col1a1. Mol. Cells 2022, 45, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Fan, X.X.; Yu, H.J.; Ai, G.; Zhang, H.Y.; Kong, H.Y.; Song, Q.Q.; Huang, Y.; Huang, J.Q.; Ning, Q. MicroRNA-29b-3p prevents Schistosoma japonicum-induced liver fibrosis by targeting COL1A1 and COL3A1. J. Cell Biochem. 2018, 119, 3199–3209. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Siedlinski, M.; Denby, L.; McGinnigle, E.; Nowak, M.; Cat, A.N.D.; Medina-Ruiz, L.; Cantini, M.; Skiba, D.; Wilk, G.; et al. T-cell-derived miRNA-214 mediates perivascular fibrosis in hypertension. Circ. Res. 2020, 126, 988–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrogeorgis, E.; Mischak, H.; Latosinska, A.; Vlahou, A.; Schanstra, J.P.; Siwy, J.; Jankowski, V.; Beige, J.; Jankowski, J. Collagen-derived peptides in CKD: A link to fibrosis. Toxins 2021, 14, 10. [Google Scholar] [CrossRef]
- Bou-Gharios, G.; Ponticos, M.; Rajkumar, V.; Abraham, D. Extra-cellular matrix in vascular networks. Cell Prolif. 2004, 37, 207–220. [Google Scholar] [CrossRef]
- Robinson, M.E.; Rauch, D.; Glorieux, F.H.; Rauch, F. Pubertal growth in osteogenesis imperfecta caused by pathogenic variants in COL1A1/COL1A2. Genet. Med. 2022, 24, 1920–1926. [Google Scholar] [CrossRef]
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Primers 2017, 3, 17052. [Google Scholar] [CrossRef]
- Moradifard, S.; Hoseinbeyki, M.; Emam, M.M.; Parchiniparchin, F.; Ebrahimi-Rad, M. Association of the Sp1 binding site and -1997 promoter variations in COL1A1 with osteoporosis risk: The application of meta-analysis and bioinformatics approaches offers a new perspective for future research. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108339. [Google Scholar] [CrossRef]
- Giunta, C.; Chambaz, C.; Pedemonte, M.; Scapolan, S.; Steinmann, B. The arthrochalasia type of Ehlers-Danlos syndrome (EDS VIIA and VIIB): The diagnostic value of collagen fibril ultrastructure. Am. J. Med. Genet. Part A 2008, 146A, 1341–1346. [Google Scholar] [CrossRef]
- Ma, H.P.; Chang, H.L.; Bamodu, O.A.; Yadav, V.K.; Huang, T.Y.; Wu, A.T.H.; Yeh, C.T.; Tsai, S.H.; Lee, W.H. Collagen1A1 (COL1A1) is a reliable biomarker and putative therapeutic target for hepatocellular carcinogenesis and metastasis. Cancers 2019, 11, 786. [Google Scholar] [CrossRef]
- Zang, S.; Guo, R.; Xing, R.; Zhang, L.; Li, W.; Zhao, M.; Fang, J.; Hu, F.; Kang, B.; Ren, Y.; et al. Identification of differentially-expressed genes in intestinal gastric cancer by microarray analysis. Genom. Proteom. Bioinform. 2014, 12, 276–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintani, Y.; Hollingsworth, M.A.; Wheelock, M.J.; Johnson, K.R. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006, 66, 11745–11753. [Google Scholar] [CrossRef] [Green Version]
- Erceylan, Ö.F.; Savaş, A.; Göv, E. Targeting the tumor stroma: Integrative analysis reveal GATA2 and TORYAIP1 as novel prognostic targets in breast and ovarian cancer. Turk. J. Biol. 2021, 45, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Papapetrou, E.P.; Bushman, F.D. Safe harbours for the integration of new DNA in the human genome. Nat. Rev. Cancer 2011, 12, 51–58. [Google Scholar] [CrossRef]
- Inukai, S.; Kock, K.H.; Bulyk, M.L. Transcription factor-DNA binding: Beyond binding site motifs. Curr. Opin. Genet. Dev. 2017, 43, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q. Seamless cloning and gene fusion. Trends Biotechnol. 2005, 23, 199–207. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, J.; Li, Y.; Xu, Y. MZF1 Transcriptionally activated microrna-328-3p suppresses the malignancy of stomach adenocarcinoma via inhibiting CD44. J. Immunol. Res. 2022, 2022, 5819295. [Google Scholar] [CrossRef]
- Liu, T.; Ye, P.; Ye, Y.; Lu, S.; Han, B. Circular RNA hsa_circRNA_002178 silencing retards breast cancer progression via microRNA-328-3p-mediated inhibition of COL1A1. J. Cell Mol. Med. 2020, 24, 2189–2201. [Google Scholar] [CrossRef]
- Kanki, Y.; Kohro, T.; Jiang, S.; Tsutsumi, S.; Mimura, I.; Suehiro, J.; Wada, Y.; Ohta, Y.; Ihara, S.; Iwanari, H.; et al. Epigenetically coordinated GATA2 binding is necessary for endothelium-specific endomucin expression. EMBO J. 2011, 30, 2582–2595. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Artlett, C.M.; Jimenez, S.A.; Hall, D.J.; Varga, J. Positive regulation of human alpha 1 (I) collagen promoter activity by transcription factor Sp1. Gene 1995, 164, 229–234. [Google Scholar] [CrossRef]
- Nehls, M.C.; Rippe, R.A.; Veloz, L.; Brenner, D.A. Transcription factors nuclear factor I and Sp1 interact with the murine collagen alpha 1 (I) promoter. Mol. Cell Biol. 1991, 11, 4065–4073. [Google Scholar] [PubMed] [Green Version]
- Chen, L.; Shioda, T.; Coser, K.R.; Lynch, M.C.; Yang, C.; Schmidt, E.V. Genome-wide analysis of YY2 versus YY1 target genes. Nucleic Acids. Res. 2010, 38, 4011–4026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Zamudio, R.I.; Roux, P.F.; de Freitas, J.; Robinson, L.; Doré, G.; Sun, B.; Belenki, D.; Milanovic, M.; Herbig, U.; Schmitt, C.A.; et al. AP-1 imprints a reversible transcriptional programme of senescent cells. Nat. Cell Biol. 2020, 22, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Care, M.A.; Cocco, M.; Laye, J.P.; Barnes, N.; Huang, Y.; Wang, M.; Barrans, S.; Du, M.; Jack, A.; Westhead, D.R.; et al. SPIB and BATF provide alternate determinants of IRF4 occupancy in diffuse large B-cell lymphoma linked to disease heterogeneity. Nucleic Acids. Res. 2014, 42, 7591–7610. [Google Scholar] [CrossRef] [PubMed]
- Palomero, J.; Vegliante, M.C.; Rodríguez, M.L.; Eguileor, A.; Castellano, G.; Planas-Rigol, E.; Jares, P.; Ribera-Cortada, I.; Cid, M.C.; Campo, E.; et al. SOX11 promotes tumor angiogenesis through transcriptional regulation of PDGFA in mantle cell lymphoma. Blood 2014, 124, 2235–2247. [Google Scholar] [CrossRef]
- Fang, X.; Yoon, J.G.; Li, L.; Yu, W.; Shao, J.; Hua, D.; Zheng, S.; Hood, L.; Goodlett, D.R.; Foltz, G.; et al. The SOX2 response program in glioblastoma multiforme: An integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genom. 2011, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Sen, G.L.; Boxer, L.D.; Webster, D.E.; Bussat, R.T.; Qu, K.; Zarnegar, B.J.; Johnston, D.; Siprashvili, Z.; Khavari, P.A. ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Dev. Cell 2012, 22, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Liska, D.J.; Reed, M.J.; Sage, E.H.; Bornstein, P. Cell-specific expression of alpha 1(I) collagen-hGH minigenes in transgenic mice. J. Cell Biol. 1994, 125, 695–704. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Varga, J.; Olsen, A.; Li, L.; Diaz, A.; Herhal, J.; Koch, J. Functional analysis of human alpha 1(I) procollagen gene promoter. Differential activity in collagen-producing and -nonproducing cells and response to transforming growth factor beta 1. J. Biol. Chem. 1994, 269, 12684–12691. [Google Scholar] [CrossRef]
- Haberle, V.; Lenhard, B. Promoter architectures and developmental gene regulation. Semin. Cell Dev. Biol. 2016, 57, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annicotte, J.S.; Schoonjans, K.; Haby, C.; Auwerx, J. An E-box in pGL3 reporter vectors precludes their use for the study of sterol regulatory element-binding proteins. Biotechniques 2001, 31, 993–996. [Google Scholar] [PubMed]
- Wang, J.M.; Lang, B.; Zhu, H.Y.; Du, H.T.; Tian, Y.M.; Su, Y.H. Cloning and transcriptional activity analysis of the porcine cofilin 2 gene promoter. Gene 2014, 547, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Rippe, R.A.; Lorenzen, S.I.; Brenner, D.A.; Breindl, M. Regulatory elements in the 5’-flanking region and the first intron contribute to transcriptional control of the mouse alpha 1 type I collagen gene. Mol. Cell Biol. 1989, 9, 2224–2227. [Google Scholar]
- Perrotti, D.; Melotti, P.; Skorski, T.; Casella, I.; Peschle, C.; Calabretta, B. Overexpression of the zinc finger protein MZF1 inhibits hematopoietic development from embryonic stem cells: Correlation with negative regulation of CD34 and c-myb promoter activity. Mol. Cell Biol. 1995, 15, 6075–6087. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liao, T.; Liu, H.; Yuan, H.; Ouyang, T.; Wang, J.; Chai, S.; Li, J.; Chen, J.; Li, X.; et al. Hypoxic glioma stem cell-derived exosomes containing linc01060 promote progression of glioma by regulating the MZF1/c-Myc/HIF1α axis. Cancer Res. 2021, 81, 114–128. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D.; Azizkhan-Clifford, J. Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell Physiol. 2001, 188, 143–160. [Google Scholar] [CrossRef]
- Gidoni, D.; Dynan, W.S.; Tjian, R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. Nature 1984, 312, 409–413. [Google Scholar] [CrossRef]
- Jones, K.A.; Kadonaga, J.T.; Rosenfeld, P.J.; Kelly, T.J.; Tjian, R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell 1987, 48, 79–89. [Google Scholar] [CrossRef]
- O’Carroll, A.M.; Lolait, S.J.; Howell, G.M. Transcriptional regulation of the rat apelin receptor gene: Promoter cloning and identification of an Sp1 site necessary for promoter activity. J. Mol. Endocrinol. 2006, 36, 221–235. [Google Scholar] [CrossRef] [Green Version]
- Söding, J.; Lupas, A.N. More than the sum of their parts: On the evolution of proteins from peptides. Bioessays 2003, 25, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Bork, P. Shuffled domains in extracellular proteins. FEBS Lett. 1991, 286, 47–54. [Google Scholar] [CrossRef]
- Hunt, L.T.; Barker, W.C. von Willebrand factor shares a distinctive cysteine-rich domain with thrombospondin and procollagen. Biochem. Biophys. Res. Commun. 1987, 144, 876–882. [Google Scholar] [CrossRef]
- Reid, K.B. Structure/function relationships in the collectins (mammalian lectins containing collagen-like regions). Biochem. Soc. Trans. 1993, 21, 464–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterkofsky, B. Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy. Am. J. Clin. Nutr. 1991, 54, 1135s–1140s. [Google Scholar] [CrossRef] [PubMed]
- McElroy, K.; Mouton, L.; Du Pasquier, L.; Qi, W.; Ebert, D. Characterisation of a large family of polymorphic collagen-like proteins in the endospore-forming bacterium Pasteuria ramosa. Res. Microbiol. 2011, 162, 701–714. [Google Scholar] [CrossRef]



| Primer | Primer Sequence (5′→3′) |
|---|---|
| P-2405-1F | TCGAGATCTGCGATCTAAGTATGCTTGGAAACCTTCTGCC |
| P-1438-1F | TCGAGATCTGCGATCTAAGTAGACGGAGGCTAGGGTAGAG |
| P-865-1F | TCGAGATCTGCGATCTAAGTCCCACCCCATCTCTCTCAAT |
| P-458-1F | TCGAGATCTGCGATCTAAGTATTGCGCATCCCCGGAAGAA |
| P-294-1F | TCGAGATCTGCGATCTAAGTCTTTCCCTTCCCTTCCCTCC |
| P-1R | ACCAACAGTACCGGAATGCCGTCTAGACCCTAGACATGTAGACT |
| CMV-F | TCGAGATCTGCGATCTAAGTCGTTACATAACTTACGGTAAATG |
| CMV-R | ACCAACAGTACCGGAATGCCAGCTCTGCTTATATAGACCT |
| Number | Start (bp) | End (bp) | Score | Promoter Sequence |
|---|---|---|---|---|
| 1 | −2069 | −2019 | 0.86 | AGACTGCCACTCTAAAGGGGTGCCCACCACCCTGAGGGTCAGGTCCCTGG |
| 2 | −1247 | −1197 | 0.87 | GGAAACATCCTTTAAAAGAAGGACACTCACCTGCAATTCCATTTTGAACT |
| 3 | −783 | −733 | 0.86 | ACAGCCTCAAAATAAAAATCCTCTCATCCGCACCCACCCCCAAATATCTG |
| 4 | −409 | −359 | 0.92 | AGCCCACAGTATATTGAGGACCGAGCCGCACCCCAGCGGCAGCATCTCTG |
| 5 | −157 | −107 | 1 | CTCGGGATGGTATAAAAGGGGCCCGGGCCAGTGGTCGGAGCAGACGGGAG |
| Category | Amount | Property |
|---|---|---|
| Molecular weight | 139 kDa | |
| Theoretical isoelectric point | 5.6 | Partial acid |
| Instability index (II) | 32.31 | Stable protein |
| Aliphatic index | 38.27 | |
| Grand average of hydropathicity | −0.794 | Hydrophilic protein |
| Formula | C5998H9314N1838O1946S31 | |
| Total number of negatively charged residues (Asp + Glu) | 140 | |
| Total number of positively charged residues (Arg + Lys) | 127 | |
| The highest or minor amino acid contents | Gly, 26.5%; Trp, 0.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, G.; Huang, L.; Zhang, X.; Wang, N.; Wang, H.; Mu, Y.; Li, K.; Liu, Z. Molecular Characteristics and Promoter Analysis of Porcine COL1A1. Genes 2022, 13, 1971. https://doi.org/10.3390/genes13111971
Xiang G, Huang L, Zhang X, Wang N, Wang H, Mu Y, Li K, Liu Z. Molecular Characteristics and Promoter Analysis of Porcine COL1A1. Genes. 2022; 13(11):1971. https://doi.org/10.3390/genes13111971
Chicago/Turabian StyleXiang, Guangming, Lei Huang, Xiuling Zhang, Nan Wang, Hui Wang, Yulian Mu, Kui Li, and Zhiguo Liu. 2022. "Molecular Characteristics and Promoter Analysis of Porcine COL1A1" Genes 13, no. 11: 1971. https://doi.org/10.3390/genes13111971