RNA-Seq and Iso-Seq Reveal the Important Role of COMT and CCoAOMT Genes in Accumulation of Scopoletin in Noni (Morinda citrifolia)
Abstract
:1. Introduction
2. Methods and Materials
2.1. Plant Material Preparation
2.2. RNA Extraction and Quality Assessment
2.3. PacBio Iso-Seq Library Preparation and Sequencing
2.4. Illumina RNA-Seq Library Preparation and Sequencing
2.5. Iso-Seq Data Analysis
2.6. Illumina RNA-Seq Data Quality Control
2.7. Transcript Correction and Redundancy Removal
2.8. Gene Functional Annotation
2.9. Differential Expression Analysis
2.10. Phylogenetic Analysis of CCoAOMTs and COMTs
2.11. q-PCR
2.12. Treatment of Noni Fruit and Scopoletin Content Determination
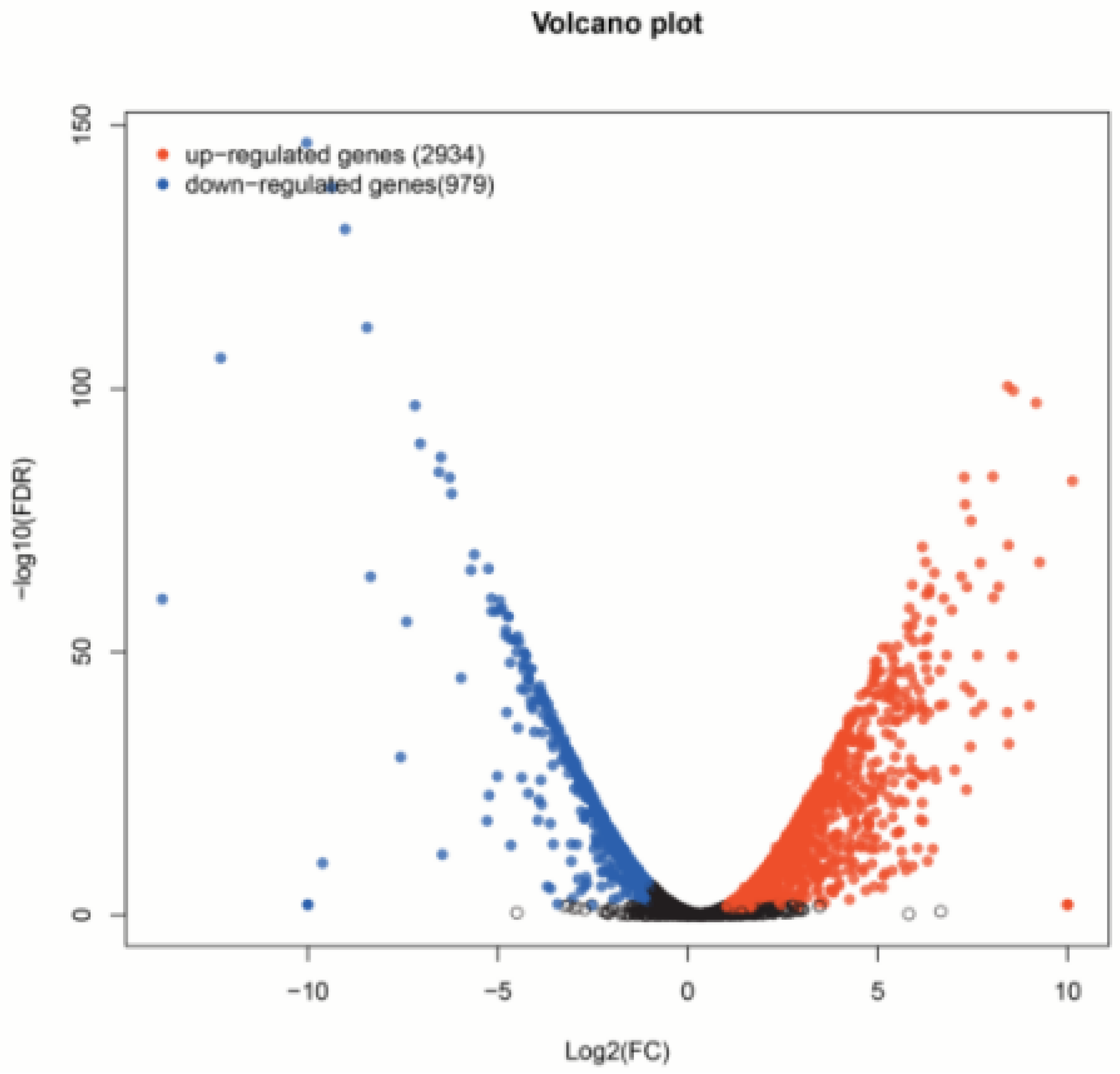
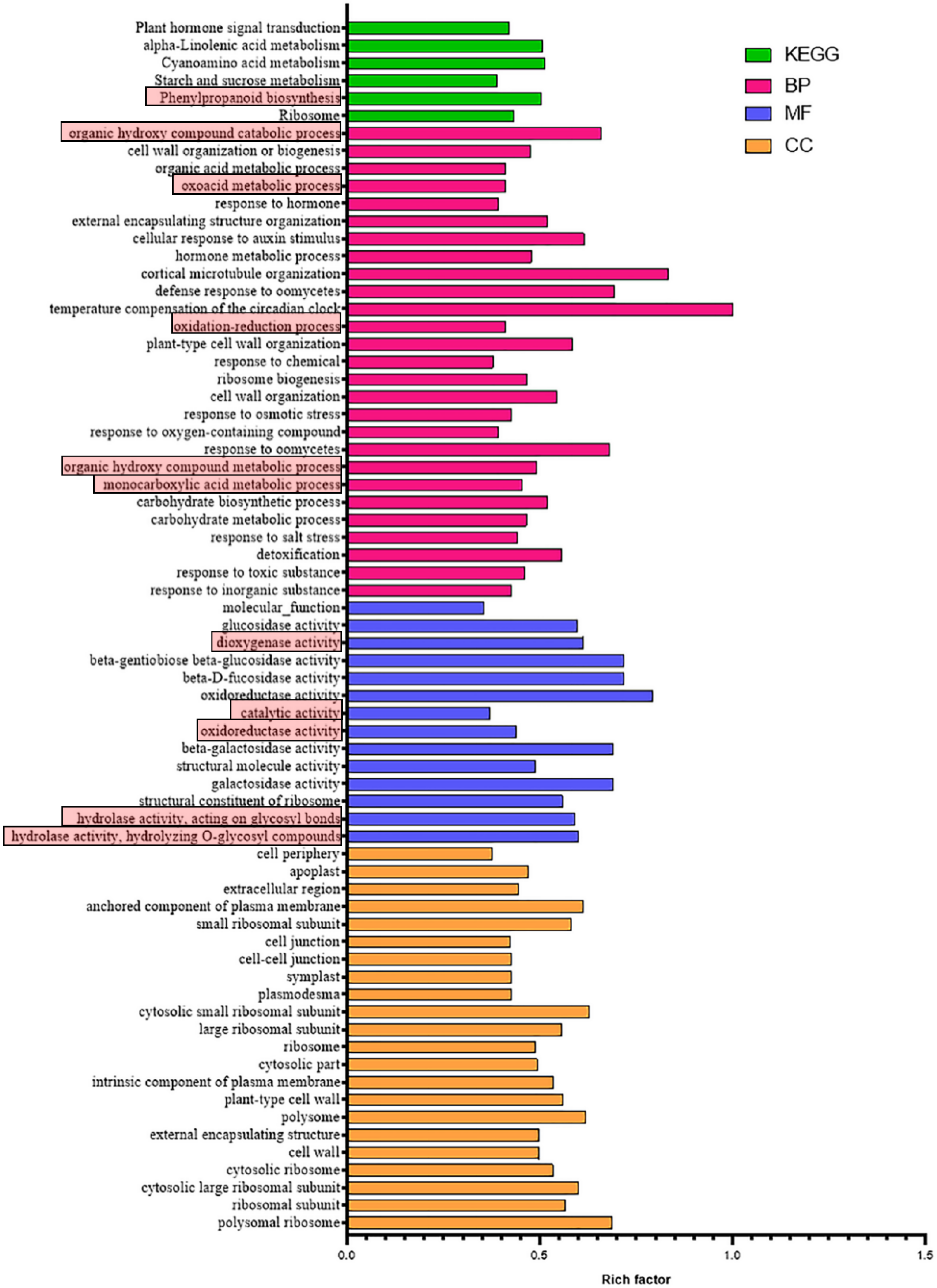
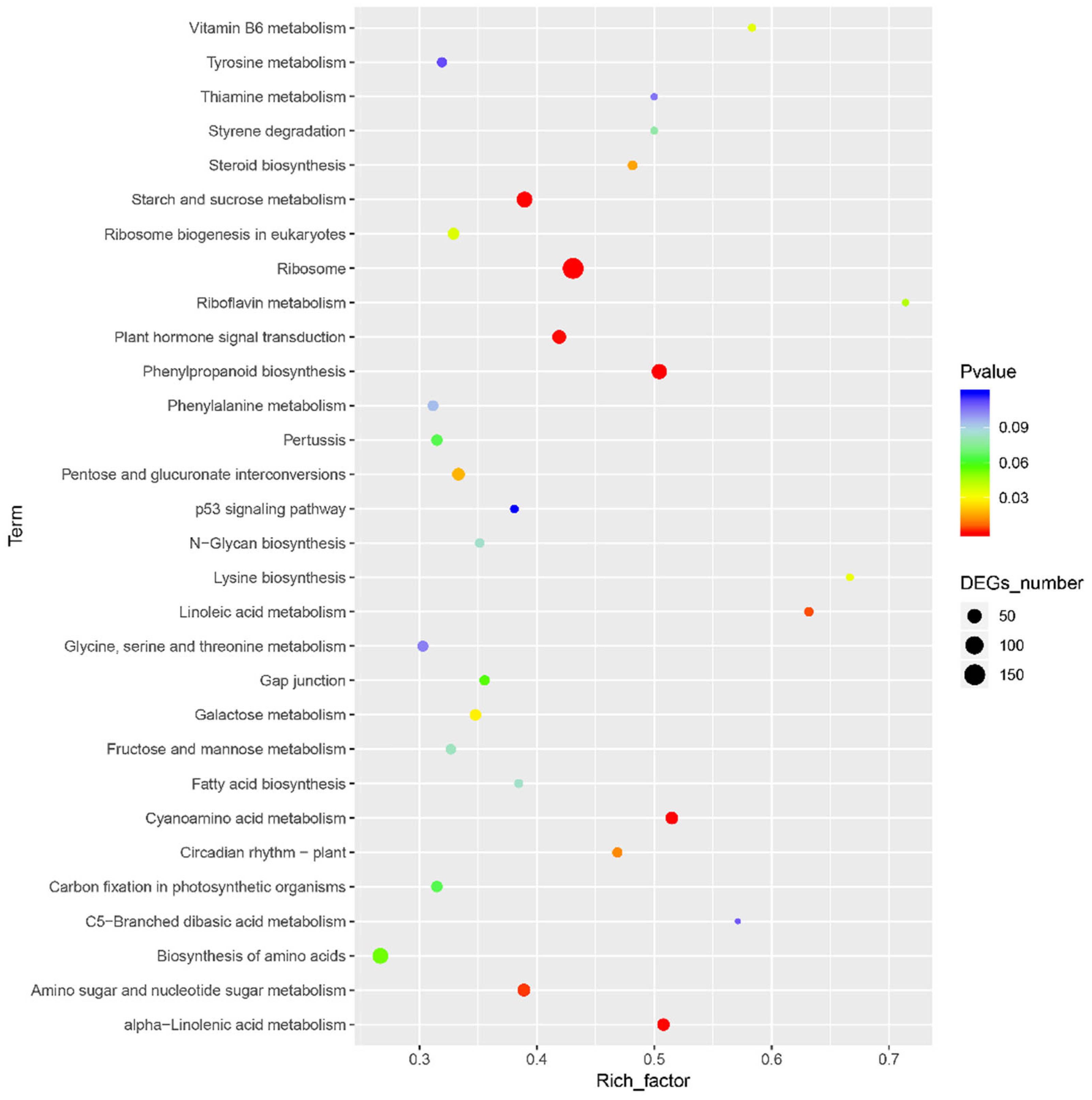
3. Results
3.1. Transcriptome Sequencing and Annotation
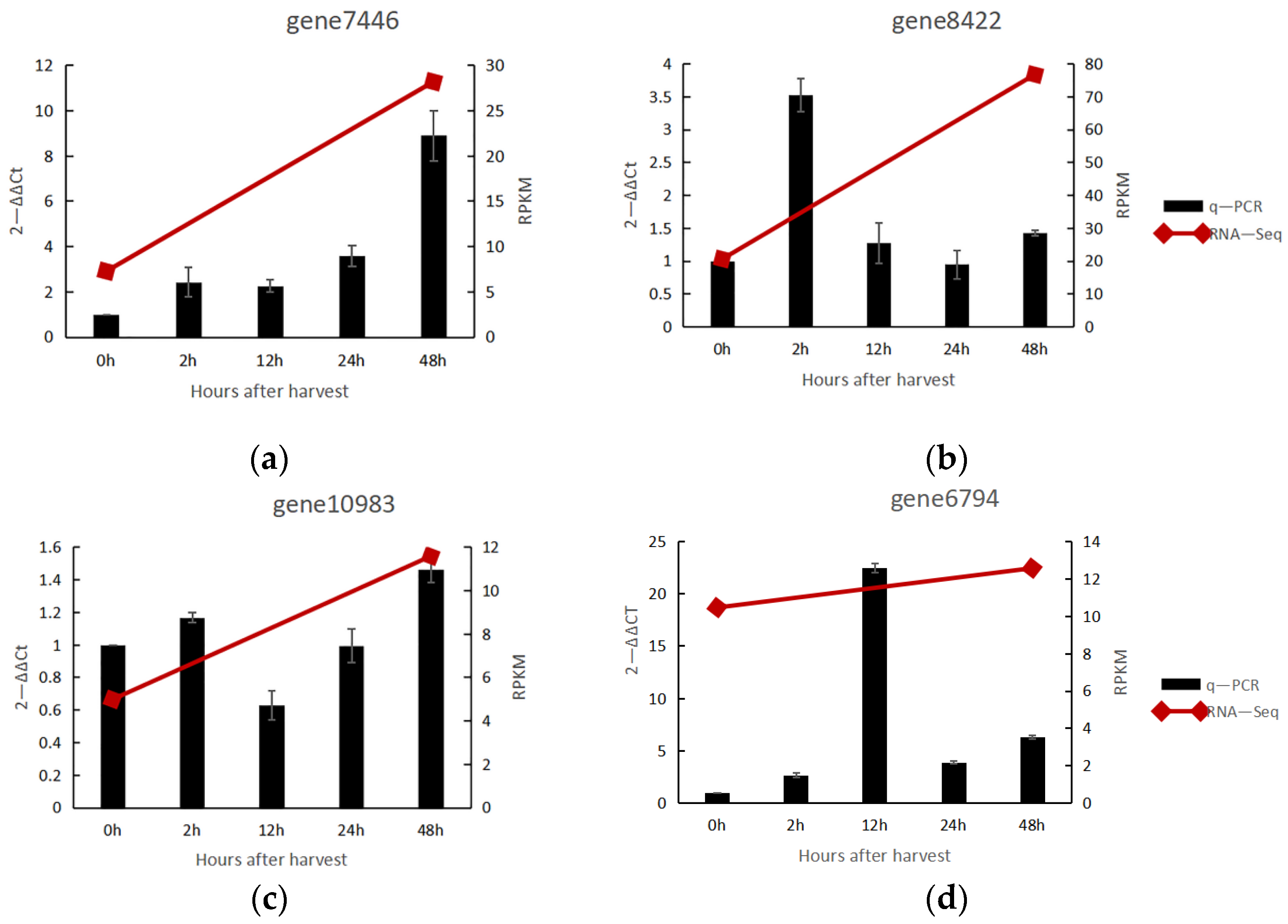
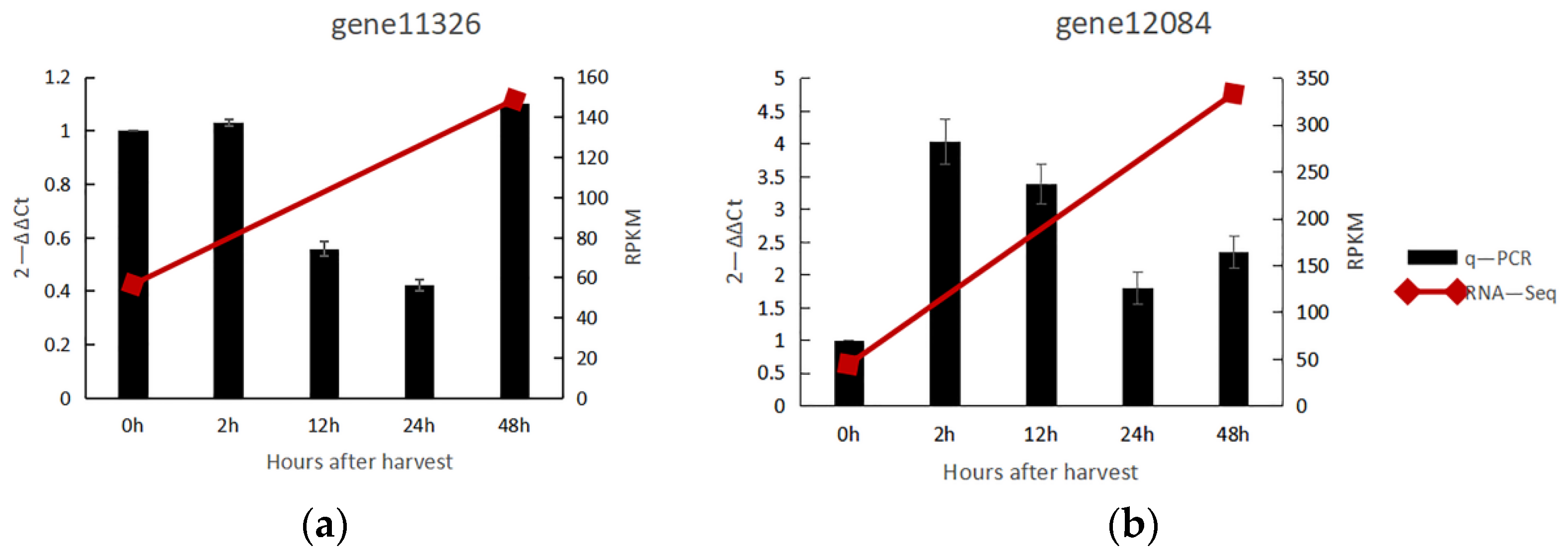
3.2. Transcriptome Data Were Verified by q-PCR
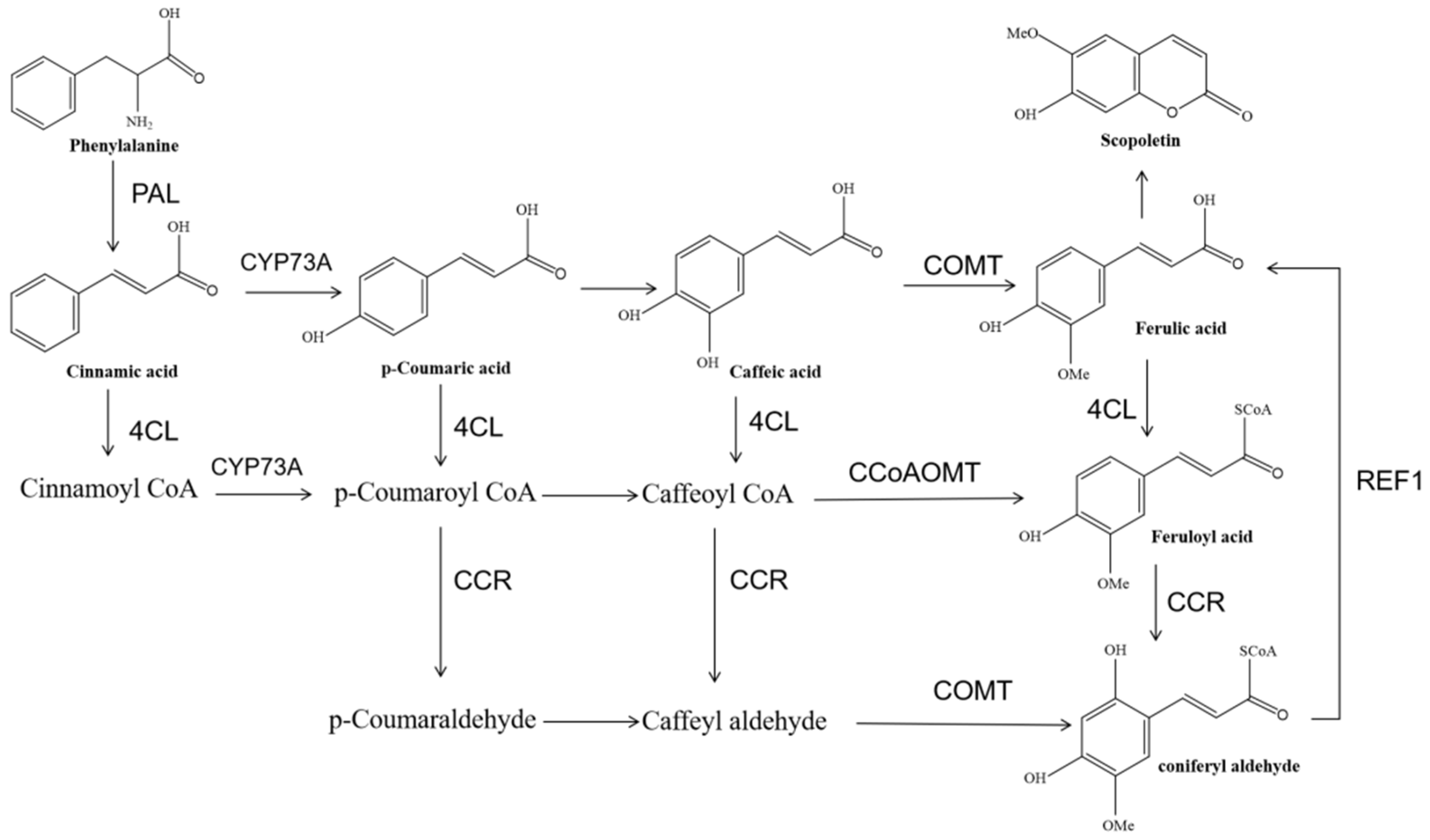
3.3. COMT and CCoAOMT Play a Key Role in the Accumulation of Scopoletin
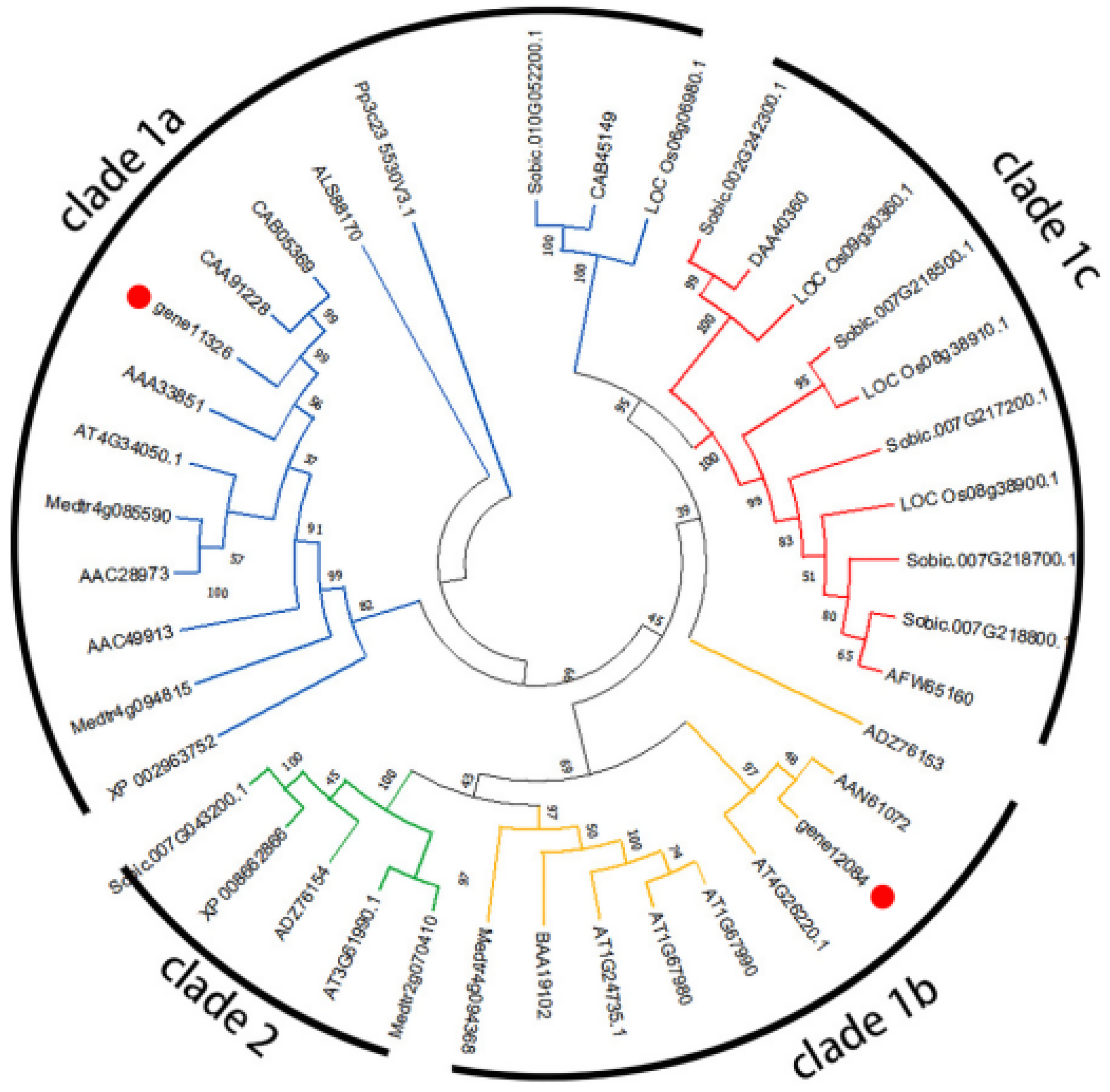
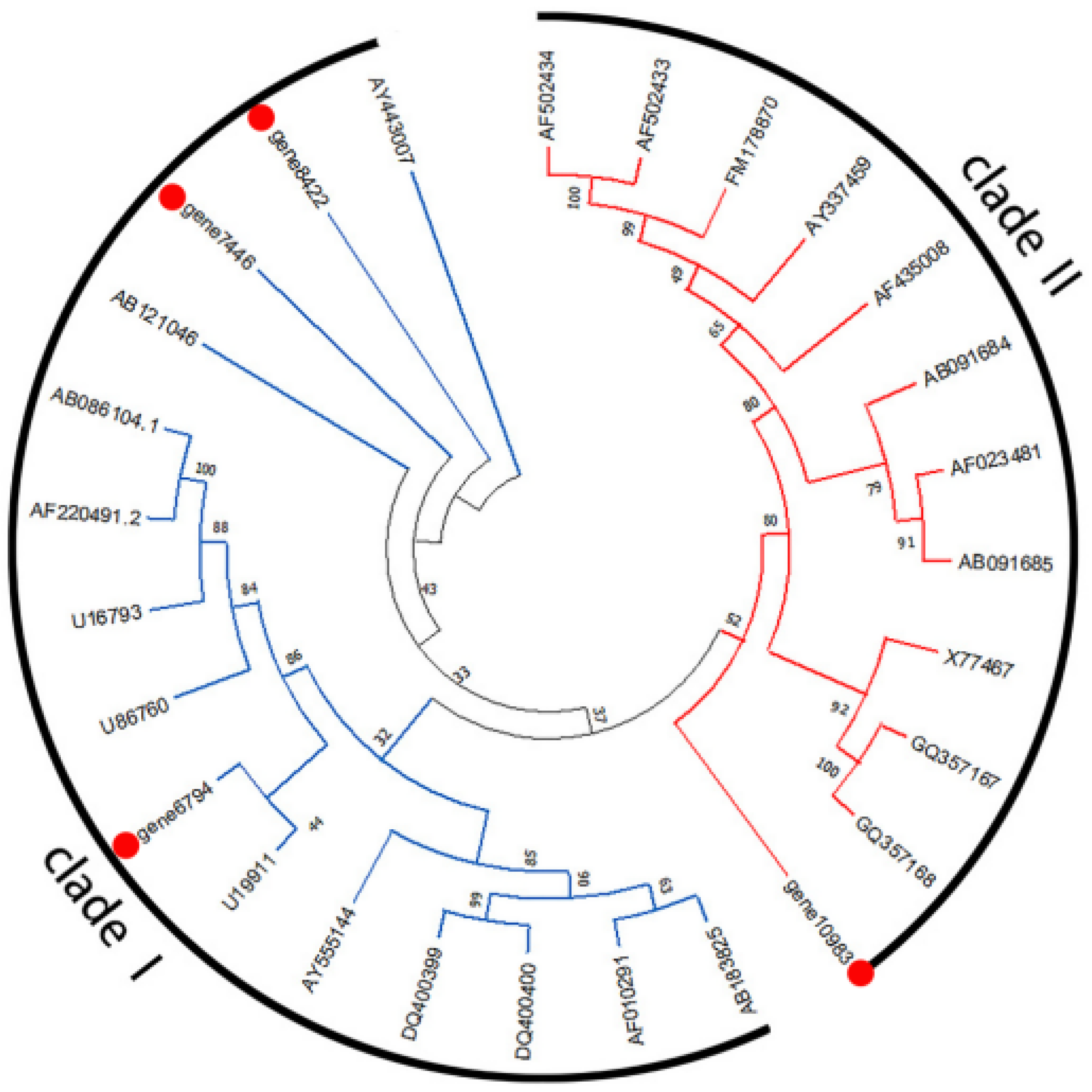
3.4. Phylogenetic Analysis of McCCoAOMTs and McCOMTs
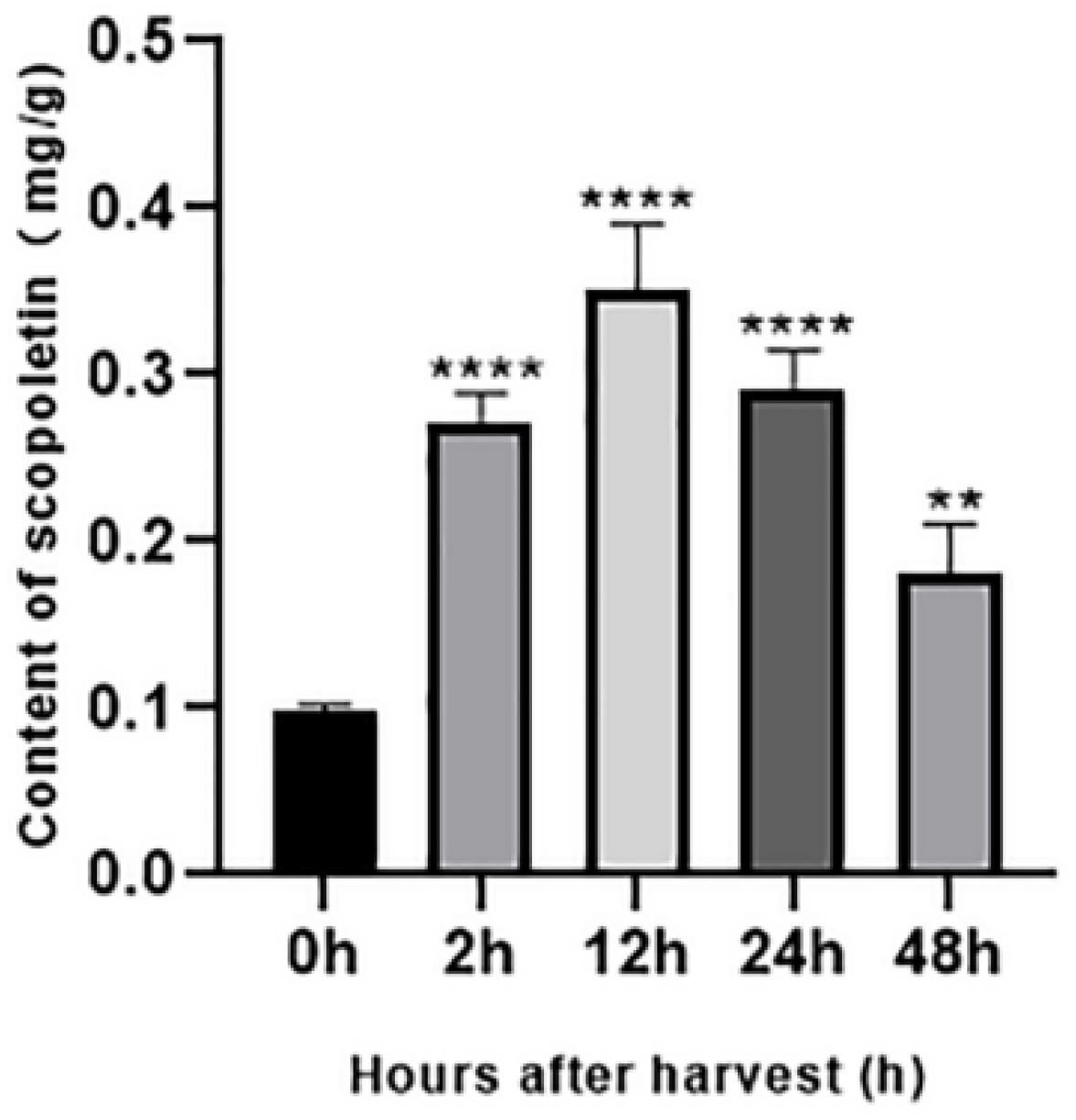
3.5. Accumulation of Scopoletin in Noni Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jahurul, M.; Patricia, M.; Shihabul, A.; Norazlina, M.; George, M.R.; Noorakmar, A.; Lee, J.; Jumardi, R.; Jinap, S.; Zaidul, I. A review on functional and nutritional properties of noni fruit seed (Morinda citrifolia L.) and its oil. Food Biosci. 2021, 41, 101000. [Google Scholar] [CrossRef]
- Marisa, M.W.; Samuel, M.; Matthew, S.S. Volatile changes in Hawaiian noni fruit, Morinda citrifolia L., during ripening and fermentation. J. Sci. Food Agric. 2018, 98, 3391–3399. [Google Scholar] [CrossRef]
- Li, J.; Niu, D.B.; Zhang, Y.; Zeng, X.A. Physicochemical properties, antioxidant and anti proliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chin, Y.W.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Anthraquinones with quinone reductase-inducing activity and benzophenones from Morinda citrifolia (noni) roots. J. Nat. Prod. 2007, 70, 2049–2052. [Google Scholar] [CrossRef]
- Assi, R.A.; Darwis, Y.; Abdulbaqi, I.M.; Khan, A.A.; Lim, V.; Laghari, M. Morinda citrifolia (noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab J Chem. 2015, 10, 691–707. [Google Scholar] [CrossRef]
- Wang, J.F.; Qin, X.C.; Chen, Z.Y.; Ju, Z.R.; He, W.J.; Tan, Y.H.; Zhou, X.J.; Tu, Z.C.; Lu, F.G.; Liu, Y.H. Two new anthraquinones with antiviral activities from the barks of Morinda citrifolia (Noni). Phytochem. Lett. 2016, 15, 13–15. [Google Scholar] [CrossRef]
- West, B.J.; Deng, S.X.; Isami, F.; Uwaya, A.; Jensen, C.J. The Potential Health Benefits of Noni Juice: A Review of Human Intervention Studies. Foods 2018, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Lohania, M.; Majrashib, M.; Govindarajulu, M.; Patel, M.; Ramesh, S.; Bhattacharya, D.; Joshi, S.; Fadan, M.; Nadar, R.; Darien, B.; et al. Immunomodulatory actions of a Polynesian herb noni (Morinda citrifolia L.) and its clinical applications. Complement. Ther. Med. 2019, 47, 102206. [Google Scholar] [CrossRef]
- Thomaz, D.V.; Couto, R.O.; Oliveira Roberth, A.; Oliveira, L.A.R.; Siqueira Leite, K.C.; Freitas Bara, M.T.; Ghedini, P.C.; Bozinis, M.C.V.; Lobón, G.S.; Souza Gil, E.; et al. Assessment of noni (Morinda citrifolia L.) product authenticity by solid state voltammetry. Int. J. Electrochem. Sci. 2018, 13, 8983–8994. [Google Scholar] [CrossRef]
- Jin, M.Y.; Wang, Y.X.; Yang, X.B.; Yin, H.; Nie, S.P.; Wu, X.Y. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Farias, V.A.; Lima, A.D.R.; Costa, A.S.; Freitas, C.D.T.; Araújo, I.M.S.; Garruti, D.D.S.; Figueiredo, E.A.T.; Oliveira, H.D. Noni (Morinda citrifolia L.) fruit as a new source of milk-clotting cysteine proteases. Food Res. Int. 2020, 127, 108689. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, R.; Kim, D.S.; Salleh, L.M.; Lim, S.B. Kinetic Study of Subcritical Water Extraction of Scopoletin, Alizarin, and Rutin from Morinda citrifolia. Foods 2021, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Shimizu, B.; Mizutani, M.; Watanabe, K.; Sakata, K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006, 67, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.H.; Hamad, M.N.; Jaafar, N.S. Phytochemical investigation of Chenopodium murale (Family: Chenopodiaceae) cultivated in Iraq, isolation and identification of scopoletin and gallic acid. Asian J. Pharm. Clin. Res. 2017, 10, 70. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Trivedi, A.; Mishra, S.H. Rapid validated high performance thin layer chromatography method for simultaneous estimation of mangiferin and scopoletin in Canscora decussata (South Indian Shankhpushpi) extract. Rev. Bras. 2015, 25, 193–198. [Google Scholar] [CrossRef]
- Jamuna, S.; Karthika, K.; Paulsamy, S.; Thenmozhi, K.; Kathiravan, S.; Venkatesh, R. Confertin and scopoletin from leaf and root extracts of Hypochaeris radicata have anti-inflammatory and antioxidant activities. Ind. Crops Prod. 2015, 70, 221–230. [Google Scholar] [CrossRef]
- Ferdinal, N.; Alfajri, R.; Arifin, B. Isolation and characterization of scopoletin from the bark of Fagraea ceilanica thumb and antioxidants tests. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The Coumarins: Secondary Metabolites Playing a Primary Role in Plant Nutrition and Health. Trends Plant Sci. 2020, 26, 248–259. [Google Scholar] [CrossRef]
- He, B.T.; Liu, Z.H.; Li, B.Z.; Yuan, J.Y. Advances in biosynthesis of scopoletin. Microb. Cell Fact. 2022, 21, 152. [Google Scholar] [CrossRef]
- Do, C.T.; Pollet, B.; Thévenin, J.; Silbout, R.; Denoue, D.; Barriere, Y.; Lapierre, C.; Jouanin, L. Both caffeoyl coenzyme A 3-O-methyltransferase and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids, and sinapoyl malate biosynthesis in A. thaliana. Planta 2007, 226, 1117–1129. [Google Scholar] [CrossRef]
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Skata, K.; Shimizu, B. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxolutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 2008, 55, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Kuhlmann, M.; Rutten, T.; Mette, M.F.; Scharfenberg, S.; Petridis, A.; Berreth, D.C.; Mock, H.P. Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant J. 2017, 93, 431–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, N.; Sui, Z.; Huang, C.; Zeng, Z.; Kong, L. The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. Int. J. Mol. Sci. 2019, 20, 1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellenberg, C.; Ohlen, M.; Handrick, V.; Vogt, T. The role of CCoAOMT1 and COMT1 in Arabidopsis anthers. Planta 2012, 236, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, R.X.; Zhang, X.S.; Zhu, T.T.; Lou, H.X.; Cheng, A.X. The identification and functional characterization of three liverwort class I O-methyltransferases. Phytochemistry 2019, 159, 190–198. [Google Scholar] [CrossRef]
- Rafaliya, R.V.; Sakure, A.A.; Parekh, M.J.; Sushil, K.; Amarjeet, S.S.T.; Desai, P.J.; Patil, G.B.; Mistri, J.G.; Subhash, N. Study of dynamics of genes involved in biosynthesis and accumulation of scopoletin at different growth stages of Convolvulus prostratus Forssk. Phytochemistry 2021, 182, 112594. [Google Scholar] [CrossRef]
- Li, H.M.; Rotter, D.; Hartman, T.G.; Pak, F.E.; Havkin-Frenkel, D.; Belanger, F.C. Evolution of novel O-methyltransferases from the Vanilla planifolia Caffeic Acid O-methyltransferase. Plant Mol. Biol. 2006, 61, 537–552. [Google Scholar] [CrossRef]
- Yu, X.M.; Muhammad, M.A.; Li, B.Q.; Fang, T.; Chen, F.X. Transcriptome data-based identification of candidate genes involved in metabolism and accumulation of soluble sugars during fruit development in ‘Huangguan’ plum. J. Food Biochem. 2021, 45, 13878. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, Y.; Qi, D.; Huo, H.L.; Dong, X.G.; Tian, L.M.; Liu, C.; Cao, Y.F. Metabolomic and transcriptomic analyses highlight the influence of lipid changes on the post-harvest softening of Pyrus ussurian Max. ‘Zaoshu Shanli’. Genomics 2021, 113, 919–926. [Google Scholar] [CrossRef]
- Jia, D.D.; Lan, Z.Q.; Wu, T. Ethylene is the key signal in the accumulation process of scopoletin in noni (Morinda citrifolia). Sci. Hortic. 2020, 261, 108980. [Google Scholar] [CrossRef]
- Rhoads, A.; Au, K.F. PacBio Sequencing and Its Applications. Genom. Pro Teom. Bioinf. 2015, 13, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.P.; Dai, C.; Hu, C.G.; Liu, Z.C.; Kang, C.Y. Global identification of alternative splicing via comparative analysis of SMRT- and Illumina-based RNA-seq in strawberry. Plant J. 2017, 90, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beiki, H.; Liu, H.; Huang, J.; Manchanda, N.; Nonneman, D.; Smith, T.P.L.; Reecy, J.M.; Tuggle, C.K. Improved annotation of the domestic pig genome through integration of Iso-Seq and RNA-seq data. BMC Genom. 2019, 20, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, Q.; Gao, Z.F.; Zhang, D.; Zhao, B.G.; Dong, F.Q.; Fu, C.X.; Liu, L.J.; Wang, B.C. The developmental dynamics of the Populus stem transcriptome. Plant Biotechnol. J. 2018, 17, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Bart, V.R.; Lars, R.K.; Vincent, R. Differential Cryptanalysis of the ICE Encryption Algorithm. Lect. Notes Comput. Sci. 1998, 1372, 270–283. [Google Scholar] [CrossRef] [Green Version]
- Salmela, L.; Rivals, E. LoRDEC: Accurate and efficient long read error correction. Bioinformatics 2014, 30, 3506–3514. [Google Scholar] [CrossRef] [Green Version]
- Limin, F.; Niu, B.; Zhu, Z.W.; Wu, S.T.; Li, W.Z. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Mao, X.Z.; Huang, J.J.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [Green Version]
- Huma, N.; Veda, P.P.; Swati, S.; Upendra, N.D. Structure-function analyses and molecular modeling of caffeic acid-O-methyltransferase and caffeoyl-CoA-O-methyltransferase: Revisiting the basis of alternate methylation pathways during monolignol biosynthesis. Biotechnol. Appl. Bioc. 2013, 60, 170–189. [Google Scholar] [CrossRef]
- Ye, Z.H.; Zhong, R.Q.; Herbert Morrison, W., III; Himmelsbach, D.S. Caffeoyl coenzyme A O-methyltransferase and lignin biosynthesis. Phytochemistry 2001, 57, 1177–1185. [Google Scholar] [CrossRef]
- Gatadi, S.; Gour, J.; Nanduri, S. Natural product derived promising anti-MRSA drug leads: A review. Bioorg. Med. Chem. 2019, 27, 3760–3774. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; Doss, G.P.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Julie, C.; Rachel, B.; Corinne, S.; Roland, B.; Bernard, F.; Patrick, S. Down regulation of a Pathogen-Responsive Tobacco UDP-Glc:Phenylpropanoid Glucosyltransferase Reduces Scopoletin Glucoside Accumulation, Enhances Oxidative Stress, and Weakens Virus Resistance. Plant Cell 2002, 14, 1093–1107. [Google Scholar] [CrossRef] [Green Version]
- Vivek, Y.; Wang, Z.Y.; Wei, C.H.; Aduragbemi, A.; Bilal, A.; Yang, X.Z.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zuo, D.; Xiao, S.; Wang, Q.; Cheng, H.; Lv, L.; Zhang, Y.; Li, P.; Song, G. Genome-Wide Identification and Characterization of GhCOMT Gene Family during Fiber Development and Verticillium Wilt Resistance in Cotton. Plants 2021, 10, 2756. [Google Scholar] [CrossRef]
- Seong, E.S.; Yoo, J.H.; Lee, J.G.; Kim, H.Y.; Hwang, I.S.; Heo, K.; Kim, J.K.; Lim, J.D.; Sacks, E.J.; Yu, C.Y. Antisense-overexpression of the MsCOMT gene induces changes in lignin and total phenol contents in transgenic tobacco plants. Mol. Biol. Rep. 2013, 40, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Covarrubias, O.A.; Douches, D.S.; Hammerschmidt, R.; daRocha, A.; Kirk, W.W. Effect of photoperiod and temperature on resistance against Phytophthora infestans in susceptible and resistant potato cultivars: Effect on deposition of structural phenolics on the cell wall and resistance to penetration. Am. J. Potato Res. 2006, 83, 325–334. [Google Scholar] [CrossRef]
- Abdelali, B.; Alex, C.; Norzawani, B.M.Y.; Joseph, S.P.; Sun, Z.C.; John, E.C. Comparative genomics and evolutionary analyses of the O-methyltransferase gene family in Populus. Gene 2011, 479, 37–46. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, Y.Z.; Pei, J.B.; Li, Y.D.; Sun, H.Y. Genome-wide identification and characterization of COMT gene family during the development of blueberry fruit. BMC Plant Biol. 2021, 21, 5. [Google Scholar] [CrossRef]
- Paul, D.; Christopher, M.; Marta, M.; Helena, O.; Catherine, L.; Robbie, W.; Jennifer, S.; David, M.; Abdellah, B.; Yukiko, T.; et al. RNAi-suppression of barley caffeic acid O-methyltransferase modifies lignin despite redundancy in the gene family. Plant Biotechnol. J. 2019, 17, 594–607. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, S.; Boudet, A.; Grima-Pettenati, J. Characterisation of caffeic acid O-methyltransferase and cinnamyl alcohol dehydrogenase gene expression patterns by in situ hybridisation in Eucalyptus gunnii Hook. Plantlets. Plant Sci. 2003, 164, 165–173. [Google Scholar] [CrossRef]
- Hamberger, B.; Ellis, M.; Friedmann, M.; Souza, C.A.; Barbazuk, B.; Douglas, C.J. Genome-wide analyses of phenylpropanoid-related genes in Populus trichocarpa, Arabidopsis thaliana, and Oryza sativa: The Populus lignin toolbox and conservation and diversification of angiosperm gene families. Can. J. Bot. 2007, 85, 1182–1201. [Google Scholar] [CrossRef]
- Xu, R.X.; Zhao, Y.; Gao, S.; Zhang, Y.Y.; Li, D.D.; Lou, H.X.; Cheng, A.X. Functional characterization of a plastidal cation-dependent O-methyltransferase from the liver-wort Plagiochasma appendiculatum. Phytochemistry 2015, 118, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Widiez, T.; Hartman, T.G.; Dudai, N.; Yan, Q.; Lawton, M.; Havkin-Frenkel, D.; Belanger, F.C. Functional characterization of two new members of the caffeoyl CoA O-methyltransferase-like gene family from Vanilla planifolia reveals a new class of plastid-localized O-methyltransferases. Plant Mol. Biol. 2011, 76, 475–488. [Google Scholar] [CrossRef]
- Li, L.L.; Tao, S.T.; Zhang, H.W.; Huang, W.J.; Dunwell, J.M.; Li, M. Identification and Characterization of the CCoAOMT Gene Family in Apple, Chinese White Pear, and Peach. J. Am. Soc. Hortic. Sci. 2021, 146, 184–195. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zou, D.M.; Wu, B.S.; Lin, D.H.; Zhang, Z.H.; Wu, J.C. Cloning and expression analysis of a CCoAOMT homolog in loquat fruit in response to low-temperature storage. Postharvest Biol. Technol. 2015, 105, 45–50. [Google Scholar] [CrossRef]
- Zhang, X.S.; Ni, R.; Wang, P.Y.; Zhu, T.T.; Sun, C.J.; Lou, H.X.; Cheng, A.X. Isolation and functional characterization of two Caffeoyl Coenzyme A 3-O- methyltransferases from the fern species Polypodiodes amoena. Plant Physiol. Bioch. 2019, 136, 169–177. [Google Scholar] [CrossRef]
- Yao, R.L.; Zhao, Y.C.; Liu, T.T.; Huang, C.L.; Xu, S.; Sui, Z.W.; Luo, J.; Kong, L.Y. Identification and functional characterization of a p-coumaroyl CoA 2’-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn. Plant Mol. Biol. 2017, 95, 199–213. [Google Scholar] [CrossRef]
- Jia, D.D.; Lan, Z.Q.; Wu, T. Studies on genetic transformation of seedlessness gene to noni (Morinda citrifolia) with its stem segments as the expalnts. J. Southwest. 2018, 40, 7–12. [Google Scholar] [CrossRef]
- Liu, J.; Lan, Z.Q.; Wu, T.; Yang, Z.Y. Transformation of non-seeded gene into noni root segment by Agrobacterium. Biotechnology 2018, 28, 473–477+466. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, D.; Jin, C.; Gong, S.; Wang, X.; Wu, T. RNA-Seq and Iso-Seq Reveal the Important Role of COMT and CCoAOMT Genes in Accumulation of Scopoletin in Noni (Morinda citrifolia). Genes 2022, 13, 1993. https://doi.org/10.3390/genes13111993
Jia D, Jin C, Gong S, Wang X, Wu T. RNA-Seq and Iso-Seq Reveal the Important Role of COMT and CCoAOMT Genes in Accumulation of Scopoletin in Noni (Morinda citrifolia). Genes. 2022; 13(11):1993. https://doi.org/10.3390/genes13111993
Chicago/Turabian StyleJia, Dandan, Can Jin, Shusen Gong, Xuan Wang, and Tian Wu. 2022. "RNA-Seq and Iso-Seq Reveal the Important Role of COMT and CCoAOMT Genes in Accumulation of Scopoletin in Noni (Morinda citrifolia)" Genes 13, no. 11: 1993. https://doi.org/10.3390/genes13111993




