Factor V Leiden, Factor II, Protein C, Protein S, and Antithrombin and Ischemic Strokes in Young Adults: A Meta-Analysis
Abstract
:1. Introduction
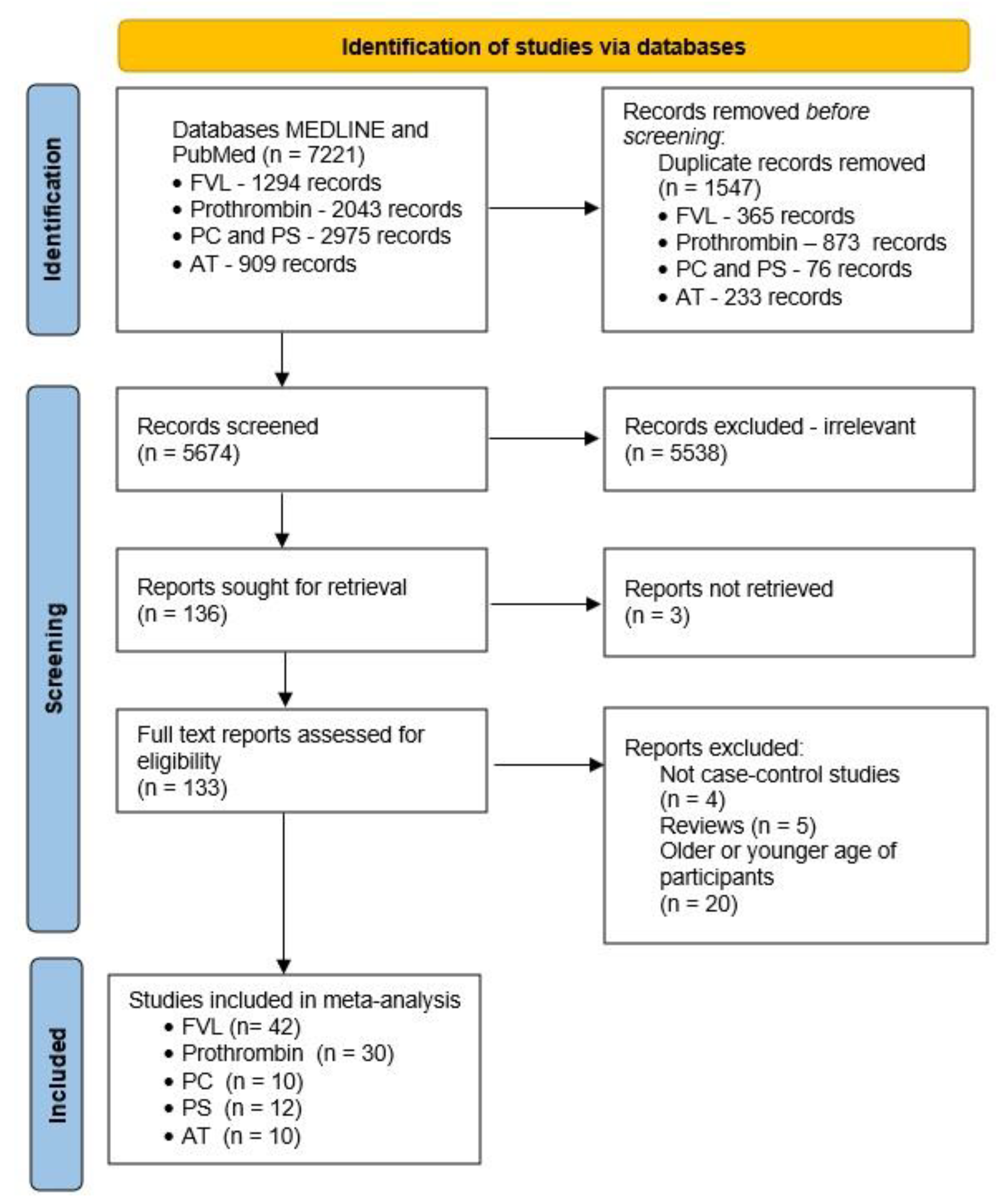
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekker, M.S.; Boot, E.M.; Singhal, A.B.; Tan, K.S.; Debette, S.; Tuladhar, A.M.; de Leeuw, F.-E. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018, 17, 790–801. [Google Scholar] [CrossRef]
- Analyses, National Center of Public Health and Analyses. Mortality Rate of Ischemic Stroke for 2021. 2022. Available online: https://ncpha.government.bg./uploads/statistics/current/2021/insult_21.pdf (accessed on 22 June 2022).
- Rochmah, T.N.; Rahmawati, I.T.; Dahlui, M.; Budiarto, W.; Bilqis, N. Economic burden of stroke disease: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 7552. [Google Scholar] [CrossRef] [PubMed]
- van Alebeek, M.E.; Arntz, R.M.; Ekker, M.S.; Synhaeve, N.E.; Maaijwee, N.A.; Schoonderwaldt, H.; van der Vlugt, M.J.; van Dijk, E.J.; Rutten-Jacobs, L.C.; de Leeuw, F.-E. Risk factors and mechanisms of stroke in young adults: The FUTURE study. J. Cereb. Blood Flow Metab. 2018, 38, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Gomez, F.E.; Amuluru, K.; Elkun, Y.; Al-Mufti, F. Cryptogenic Stroke and Stroke of “Unknown Cause”. In Cerebrovascular Disorders; Springer: Berlin/Heidelberg, Germany, 2021; pp. 293–322. [Google Scholar]
- Favaloro, E.J. (Ed.) Genetic Testing for Thrombophilia-Related Genes: Observations of Testing Patterns for Factor V Leiden (G1691A) and Prothrombin Gene “Mutation” (G20210A). Semin. Thromb. Hemost. 2019, 45, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Majid, Z.; Tahir, F.; Ahmed, J.; Arif, T.B.; Haq, A. Protein C deficiency as a risk factor for stroke in young adults: A review. Cureus 2020, 12, e7472. [Google Scholar] [CrossRef] [Green Version]
- George, M.G. Risk factors for ischemic stroke in younger adults: A focused update. Stroke 2020, 51, 729–735. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Moll, S. Inherited antithrombin deficiency: A review. Haemophilia 2008, 14, 1229–1239. [Google Scholar] [CrossRef]
- Bravo-Pérez, C.; Vicente, V.; Corral, J. Management of antithrombin deficiency: An update for clinicians. Expert Rev. Hematol. 2019, 12, 397–405. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Volume 2, pp. 1–12. [Google Scholar]
- Anadure, R.; Christopher, R.; Nagaraja, D.; Narayanan, C. A genetic study of Factor V Leiden (G1691A) mutation in young ischemic strokes with large vessel disease in a South Indian population. J. Clin. Neurosci. 2017, 44, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Ashjazadeh, N.; Poursadeghfard, M.; Farjadian, S. Factor V G1691A and prothrombin G20210A gene polymorphisms among Iranian patients with cerebral venous thrombosis. Neurol Asia 2012, 17, 199–203. [Google Scholar]
- Aznar, J.; Mira, Y.; Vayá, A.; Corella, D.; Ferrando, F.; Villa, P.; Estellés, A. Factor V Leiden and prothrombin G20210A mutations in young adults with cryptogenic ischemic stroke. Thromb. Haemost. 2004, 91, 1031–1034. [Google Scholar] [PubMed]
- Bondarenko, E.; Shetova, I.; Shamalov, N.; Mocan, E.; Barbacar, N.; Kurochkin, G.; Protopop, S.; Lysyi, L.; Slominsky, P.; Limborska, S. Analysis of acute ischemic stroke DNA markers in Russian and Moldavian populations. Russ. J. Genet. 2011, 47, 1240–1247. [Google Scholar] [CrossRef]
- Botto, N.; Spadoni, I.; Giusti, S.; Ait-Ali, L.; Sicari, R.; Andreassi, M.G. Prothrombotic mutations as risk factors for cryptogenic ischemic cerebrovascular events in young subjects with patent foramen ovale. Stroke 2007, 38, 2070–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, A.; Ranjan, R.; Meena, A.; Akhter, S.; Sharma, V.; Yadav, B.K.; Behari, M.; Saxena, R. Prothrombotic factors and the risk of acute onset non-cardioembolic stroke in young Asian Indians. Thromb. Res. 2009, 124, 397–402. [Google Scholar] [CrossRef]
- D’Amico, D.; Moschiano, F.; Leone, M.a.; Ariano, C.; Ciusani, E.; Erba, N.; Grazzi, L.; Ferraris, A.; Schieroni, F.; Bussone, G. Genetic abnormalities of the protein C system: Shared risk factors in young adults with migraine with aura and with ischemic stroke? Cephalalgia 1998, 18, 618–621. [Google Scholar] [CrossRef]
- Chatterjee, T.; Gupta, N.; Choudhry, V.P.; Behari, M.; Saxena, R.; Ashraf, M.Z. Prediction of ischemic stroke in young Indians: Is thrombophilia profiling a way out? Blood Coagul. Fibrinolysis 2013, 24, 449–453. [Google Scholar] [CrossRef]
- de Paula Sabino, A.; Ribeiro, D.D.; das Graças Carvalho, M.; Cardoso, J.; Dusse, L.M.S.A.; Fernandes, A.P. Factor V Leiden and increased risk for arterial thrombotic disease in young Brazilian patients. Blood Coagul. Fibrinolysis 2006, 17, 271–275. [Google Scholar] [CrossRef]
- Djordjevic, V.; Stankovic, M.; Brankovic-Sreckovic, V.; Rakicevic, L.; Damnjanovic, T.; Antonijevic, N.; Radojkovic, D. Prothrombotic genetic risk factors in stroke: A possible different role in pediatric and adult patients. Clin. Appl. Thromb. /Hemost. 2012, 18, 658–661. [Google Scholar] [CrossRef]
- Eterović, D.; Titlić, M.; Čulić, V.; Zadro, R.; Primorac, D. Lower contribution of factor V Leiden or G202104 mutations to ischemic stroke in patients with clinical risk factors: Pair-matched case-control study. Clin. Appl. Thromb./Hemost. 2007, 13, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, K.; Herm, J.; Hoppe, B.; Kasabov, R.; Malzahn, U.; Endres, M.; Koscielny, J.; Jungehulsing, G. Thrombophilia screening in young patients with cryptogenic stroke. Hämostaseologie 2012, 32, 147–152. [Google Scholar] [PubMed]
- Hamedani, A.G.; Cole, J.W.; Cheng, Y.; Sparks, M.J.; O’Connell, J.R.; Stine, O.C.; Wozniak, M.A.; Stern, B.J.; Mitchell, B.D.; Kittner, S.J. Factor V leiden and ischemic stroke risk: The Genetics of Early Onset Stroke (GEOS) study. J. Stroke Cerebrovasc. Dis. 2013, 22, 419–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.M.; Ramroodi, N.; Amiri Fard, H.; Talebian, S.; Haghighi Rohani, M.; Rezaei, M.; Noora, M.; Salimi, S. Common variations in prothrombotic genes and susceptibility to ischemic stroke in young patients: A case-control study in Southeast Iran. Medicina 2019, 55, 47. [Google Scholar] [CrossRef] [Green Version]
- Karttunen, V.; Hiltunen, L.; Rasi, V.; Vahtera, E.; Hillbom, M. Factor V Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul. Fibrinolysis 2003, 14, 261–268. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, S.; Sagar, R.; Kumar, P.; Yadav, A.K.; Talwar, P.; Raj, R.; Prasad, K. Relationship between factor V leiden gene variant and risk of ischemic stroke: A case–control study. Ann. Indian Acad. Neurol. 2017, 20, 284. [Google Scholar]
- Kheradmand, E.; Pourhossein, M.; Amini, G.; Saadatnia, M. Factor V Leiden does not have a role in cryptogenic ischemic stroke among Iranian young adults. Adv. Biomed. Res. 2014, 3, 80. [Google Scholar]
- Longstreth, W., Jr.; Rosendaal, F.; Siscovick, D.; Vos, H.; Schwartz, S.; Psaty, B.; Raghunathan, T.; Koepsell, T.; Reitsma, P. Risk of stroke in young women and two prothrombotic mutations: Factor V Leiden and prothrombin gene variant (G20210A). Stroke 1998, 29, 577–580. [Google Scholar] [CrossRef]
- Lalouschek, W.; Schillinger, M.; Hsieh, K.; Endler, G.; Tentschert, S.; Lang, W.; Cheng, S.; Mannhalter, C. Matched case-control study on factor V Leiden and the prothrombin G20210A mutation in patients with ischemic stroke/transient ischemic attack up to the age of 60 years. Stroke 2005, 36, 1405–1409. [Google Scholar] [CrossRef] [Green Version]
- Lichy, C.; Dong-Si, T.; Reuner, K.; Genius, J.; Rickmann, H.; Hampe, T.; Dolan, T.; Stoll, F.; Grau, A. Risk of cerebral venous thrombosis and novel gene polymorphisms of the coagulation and fibrinolytic systems. J. Neurol. 2006, 253, 316–320. [Google Scholar] [CrossRef]
- Madonna, P.; de Stefano, V.; Coppola, A.; Cirillo, F.; Cerbone, A.M.; Orefice, G.; Di Minno, G. Hyperhomocysteinemia and other inherited prothrombotic conditions in young adults with a history of ischemic stroke. Stroke 2002, 33, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, I.; Battaglioli, T.; Burgo, I.; Di Domenico, S.; Mannucci, P.M. Oral contraceptive use, thrombophilia and their interaction in young women with ischemic stroke. Haematologica 2006, 91, 844–847. [Google Scholar] [PubMed]
- M’barek, L.; Sakka, S.; Meghdiche, F.; Turki, D.; Maalla, K.; Dammak, M.; Kallel, C.; Mhiri, C. MTHFR (C677T, A1298C), FV Leiden polymorphisms, and the prothrombin G20210A mutation in arterial ischemic stroke among young tunisian adults. Metab. Brain Dis. 2021, 36, 421–428. [Google Scholar] [CrossRef]
- Narayan, S.; Chandrasekaran, A.; Basu, D.; Hanumanthappa, N.; Aghoram, R.; Dutta, T.K.; Rejul, V. Prothrombotic Factors Have Significant Association with Arterial and Venous Strokes in Indian Tamilians. J. Appl. Lab. Med. 2021, 6, 101–112. [Google Scholar] [CrossRef]
- Offelli, P.; Zanchetta, M.; Pedon, L.; Marzot, F.; Cucchini, U.; Pegoraro, C.; Iliceto, S.; Pengo, V. Thrombophilia in young patients with cryptogenic stroke and patent foramen ovale (PFO). Thromb. Haemost. 2007, 98, 906–907. [Google Scholar] [CrossRef]
- Pahus, S.H.; Hansen, A.T.; Hvas, A.-M. Thrombophilia testing in young patients with ischemic stroke. Thromb. Res. 2016, 137, 108–112. [Google Scholar] [CrossRef]
- Pestana, C.I.; Torres, A.; Blanco, S.; Rojas, M.J.; Mendez, C.; Lopez, J.L.; de Bosch, N.B.; Porco, A. Factor V Leiden and the Risk of Venous Thrombosis, Myocardial Infarction, and Stroke: A Case–Control Study in Venezuela. Genet. Test. Mol. Biomark. 2009, 13, 537–542. [Google Scholar] [CrossRef]
- Pezzini, A.; Del Zotto, E.; Magoni, M.; Costa, A.; Archetti, S.; Grassi, M.; Akkawi, N.M.; Albertini, A.; Assanelli, D.; Vignolo, L.A. Inherited thrombophilic disorders in young adults with ischemic stroke and patent foramen ovale. Stroke 2003, 34, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Pezzini, A.; Grassi, M.; Zotto, E.D.; Archetti, S.; Spezi, R.; Vergani, V.; Assanelli, D.; Caimi, L.; Padovani, A. Cumulative effect of predisposing genotypes and their interaction with modifiable factors on the risk of ischemic stroke in young adults. Stroke 2005, 36, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Pirhoushiaran, M.; Ghasemi, M.R.; Hami, J.; Zargari, P.; Nezhad, P.S.; Azarpazhooh, M.R.; Nabavi, A.S. The association of coagulation factor V (Leiden) and factor II (prothrombin) mutations with stroke. Iran. Red Crescent Med. J. 2014, 16, e11548. [Google Scholar] [CrossRef] [Green Version]
- Pongracz, E.; Andrikovics, H.; Bernat, I.S.; Nagy, Z. Connection between genetically determined blood coagulation factors and haemorheology. Clin. Hemorheol. Microcirc. 2008, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ranellou, K.; Paraskeva, A.; Kyriazopoulos, P.; Batistatou, A.; Evangelou, A.; El-Aly, M.; Zis, P.; Tavernarakis, A.; Charalabopoulos, K. Polymorphisms in prothrombotic genes in young stroke patients in Greece: A case-controlled study. Blood Coagul. Fibrinolysis 2015, 26, 430–435. [Google Scholar] [CrossRef]
- Rodrigues, C.; Rocha, L.K.A.; Morelli, V.M.; Franco, R.; Lourenço, D. Prothrombin G20210A mutation, and not factor V Leiden mutation, is a risk factor for cerebral venous thrombosis in Brazilian patients. J. Thromb. Haemost. 2004, 2, 1211–1212. [Google Scholar] [CrossRef]
- Sastry, S.; Riding, G.; Morris, J.; Taberner, D.; Cherry, N.; Heagerty, A.; McCollum, C. Young Adult Myocardial Infarction and Ischemic Stroke: The role of paradoxical embolism and thrombophilia (The YAMIS Study). J. Am. Coll. Cardiol. 2006, 48, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Kang, X.; Wang, Y.; Zhou, Y. The coagulation factor V Leiden, MTHFRC677T variant and eNOS 4ab polymorphism in young Chinese population with ischemic stroke. Clin. Chim. Acta 2008, 396, 7–9. [Google Scholar] [CrossRef]
- Slooter, A.; Rosendaal, F.; Tanis, B.; Kemmeren, J.; Van der Graaf, Y.; Algra, A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J. Thromb. Haemost. 2005, 3, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Supanc, V.; Sonicki, Z.; Vukasovic, I.; Solter, V.V.; Zavoreo, I.; Kes, V.B. The role of classic risk factors and prothrombotic factor gene mutations in ischemic stroke risk development in young and middle-aged individuals. J. Stroke Cerebrovasc. Dis. 2014, 23, e171–e176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasdemir, S.; Erdem, H.B.; Sahin, I.; Ozel, L.; Ozdemir, G.; Eroz, R.; Tatar, A. Correlation with platelet parameters and genetic markers of Thrombophilia Panel (Factor II g. 20210G> A, factor V leiden, MTHFR (C677T, A1298C), PAI-1, β-Fibrinogen, factor XIIIA (V34L), Glycoprotein IIIa (L33P)) in ischemic strokes. Neuromolecular Med. 2016, 18, 170–176. [Google Scholar] [CrossRef]
- They-They, T.P.; Battas, O.; Slassi, I.; Rafai, M.A.; Katumbay, D.T.; Nadifi, S. Prothrombin G20210A and factor V Leiden polymorphisms in stroke. J. Mol. Neurosci. 2012, 46, 210–216. [Google Scholar] [CrossRef]
- Tosetto, A.; Ruggeri, M.; Castaman, G.; Rodeghiero, F. Inherited abnormalities of blood coagulation in juvenile stroke. A case-control study. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 1997, 8, 397–402. [Google Scholar] [CrossRef]
- Urbanus, R.T.; Siegerink, B.; Roest, M.; Rosendaal, F.R.; de Groot, P.G.; Algra, A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: A case-control study. Lancet Neurol. 2009, 8, 998–1005. [Google Scholar] [CrossRef]
- Weih, M.; Mehraein, S.; Valdueza, J.; Einhäupl, K.; Vetter, B.; Kulozik, A.; Ziemer, S.; Koscielny, J. Increased rate of factor V Leiden mutation in patients with cerebral venous thrombosis. J. Neurol. 1998, 245, 149–152. [Google Scholar] [CrossRef]
- Garcia, E.G.; van Goor, M.; Leebeek, F.; Brouwers, G.J.; Koudstaal, P.; Dippel, D. Elevated prothrombin is a risk factor for cerebral arterial ischemia in young adults. Clin. Neurol. Neurosurg. 2002, 104, 285–288. [Google Scholar] [CrossRef]
- Jiang, B.; Ryan, K.A.; Hamedani, A.; Cheng, Y.; Sparks, M.J.; Koontz, D.; Bean, C.J.; Gallagher, M.; Hooper, W.C.; McArdle, P.F. Prothrombin G20210A mutation is associated with young-onset stroke: The genetics of early-onset stroke study and meta-analysis. Stroke 2014, 45, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Saadatnia, M.; Salehi, M.; Amini, G.; Miri, N.S.A. The impact of prothrombin (G20210A) gene mutation on stroke in youths. ARYA Atheroscler. 2012, 8, 9. [Google Scholar]
- Jerrard-Dunne, P.; Evans, A.; McGovern, R.; Hajat, C.; Kalra, L.; Rudd, A.G.; Wolfe, C.D.; Markus, H.S. Ethnic differences in markers of thrombophilia: Implications for the investigation of ischemic stroke in multiethnic populations: The South London Ethnicity and Stroke Study. Stroke 2003, 34, 1821–1826. [Google Scholar] [CrossRef] [Green Version]
- Zeidman, A.; Levine, Y.; Fradin, Z.; Kanter, P.; Mittelman, M. Clinical and ethnic characteristics of stroke in an Israeli population: A study in a community hospital population. Eur. J. Intern. Med. 2004, 15, 168–171. [Google Scholar] [CrossRef]
- Mojiminiyi, O.A.; Marouf, R.; Al Shayeb, A.R.; Qurtom, M.; Abdella, N.A.; Al Wazzan, H.; Al Humood, S.; Samad, M.A.; El-Muzaini, H. Determinants and associations of homocysteine and prothrombotic risk factors in Kuwaiti patients with cerebrovascular accident. Med. Princ. Pract. 2008, 17, 136–142. [Google Scholar] [CrossRef]
- Margaglione, M.; D’Andrea, G.; Giuliani, N.; Brancaccio, V.; De Lucia, D.; Grandone, E.; De Stefano, V.; Tonali, P.A.; Di Minno, G. Inherited prothrombotic conditions and premature ischemic stroke: Sex difference in the association with factor V Leiden. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1751–1756. [Google Scholar] [CrossRef] [Green Version]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Stroke Foundation. Clinical Guidelines for Stroke Management; Stroke Foundation: Melbourne, Australia, 2022. [Google Scholar]
- Milanov, I.; Stamenova, P. Bulgarian consensus guideline for prophylaxis, diagnosis, and treatment of cerebrovascular diseases. Bulg. Neurol. 2020, 21, 1–31. [Google Scholar]
- Bowen, A.; Young, G. National Clinical Guideline for Stroke Prepared by the Intercollegiate Stroke Working Party; Royal College of Physicians: London, UK, 2016. [Google Scholar]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Chiasakul, T.; De Jesus, E.; Tong, J.; Chen, Y.; Crowther, M.; Garcia, D.; Chai-Adisaksopha, C.; Messé, S.R.; Cuker, A. Inherited thrombophilia and the risk of arterial ischemic stroke: A systematic review and meta-analysis. J. Am. Heart Assoc. 2019, 8, e012877. [Google Scholar] [CrossRef]
- Kim, R.J.; Becker, R.C. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: A meta-analysis of published studies. Am. Heart J. 2003, 146, 948–957. [Google Scholar] [CrossRef]
- Hamedani, A.G.; Cole, J.W.; Mitchell, B.D.; Kittner, S.J. Meta-analysis of factor V Leiden and ischemic stroke in young adults: The importance of case ascertainment. Stroke 2010, 41, 1599–1603. [Google Scholar] [CrossRef]
- Simone, B.; De Stefano, V.; Leoncini, E.; Zacho, J.; Martinelli, I.; Emmerich, J.; Rossi, E.; Folsom, A.R.; Almawi, W.Y.; Scarabin, P.Y. Risk of Venous Thromboembolism Associated with Single and Combined Effects of Factor V Leiden, Prothrombin 20210A and Methylenetethraydrofolate Reductase C677T: A Meta-Analysis Involving over 11,000 Cases and 21,000 Controls; Springer: Berlin/Heidelberg, Germany, 2013; pp. 621–647. [Google Scholar]
- Gemmati, D.; Longo, G.; Franchini, E.; Araujo Silva, J.; Gallo, I.; Lunghi, B.; Moratelli, S.; Maestri, I.; Serino, M.L.; Tisato, V. Cis-Segregation of c. 1171C> T Stop Codon (p. R391*) in SERPINC1 Gene and c. 1691G> A Transition (p. R506Q) in F5 Gene and Selected GWAS Multilocus Approach in Inherited Thrombophilia. Genes 2021, 12, 934. [Google Scholar] [CrossRef]
- Salehi Omran, S.; Hartman, A.; Zakai, N.A.; Navi, B.B. Thrombophilia testing after ischemic stroke: Why, when, and what? Stroke 2021, 52, 1874–1884. [Google Scholar] [CrossRef]
- Omran, S.S.; Lerario, M.P.; Gialdini, G.; Merkler, A.E.; Moya, A.; Chen, M.L.; Kamel, H.; DeSancho, M.; Navi, B.B. Clinical impact of thrombophilia screening in young adults with ischemic stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Bauduer, F.; Lacombe, D. Factor V Leiden, prothrombin 20210A, methylenetetrahydrofolate reductase 677T, and population genetics. Mol. Genet. Metab. 2005, 86, 91–99. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsalta-Mladenov, M.; Levkova, M.; Andonova, S. Factor V Leiden, Factor II, Protein C, Protein S, and Antithrombin and Ischemic Strokes in Young Adults: A Meta-Analysis. Genes 2022, 13, 2081. https://doi.org/10.3390/genes13112081
Tsalta-Mladenov M, Levkova M, Andonova S. Factor V Leiden, Factor II, Protein C, Protein S, and Antithrombin and Ischemic Strokes in Young Adults: A Meta-Analysis. Genes. 2022; 13(11):2081. https://doi.org/10.3390/genes13112081
Chicago/Turabian StyleTsalta-Mladenov, Mihael, Mariya Levkova, and Silva Andonova. 2022. "Factor V Leiden, Factor II, Protein C, Protein S, and Antithrombin and Ischemic Strokes in Young Adults: A Meta-Analysis" Genes 13, no. 11: 2081. https://doi.org/10.3390/genes13112081





