Genetic Characterization of Some Saudi Arabia’s Accessions from Commiphora gileadensis Using Physio-Biochemical Parameters, Molecular Markers, DNA Barcoding Analysis and Relative Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical and Enzyme Antioxidant Activities
2.2.1. Phytochemical Parameters
2.2.2. Enzyme Antioxidant Activity
2.3. SDS-PAGE Protein
2.4. Molecular Analysis
2.5. DNA Barcoding
2.6. Gene Expression
2.6.1. RNA Extraction and cDNA Synthesis
2.6.2. Real-Time Quantitative PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. DNA Barcoding
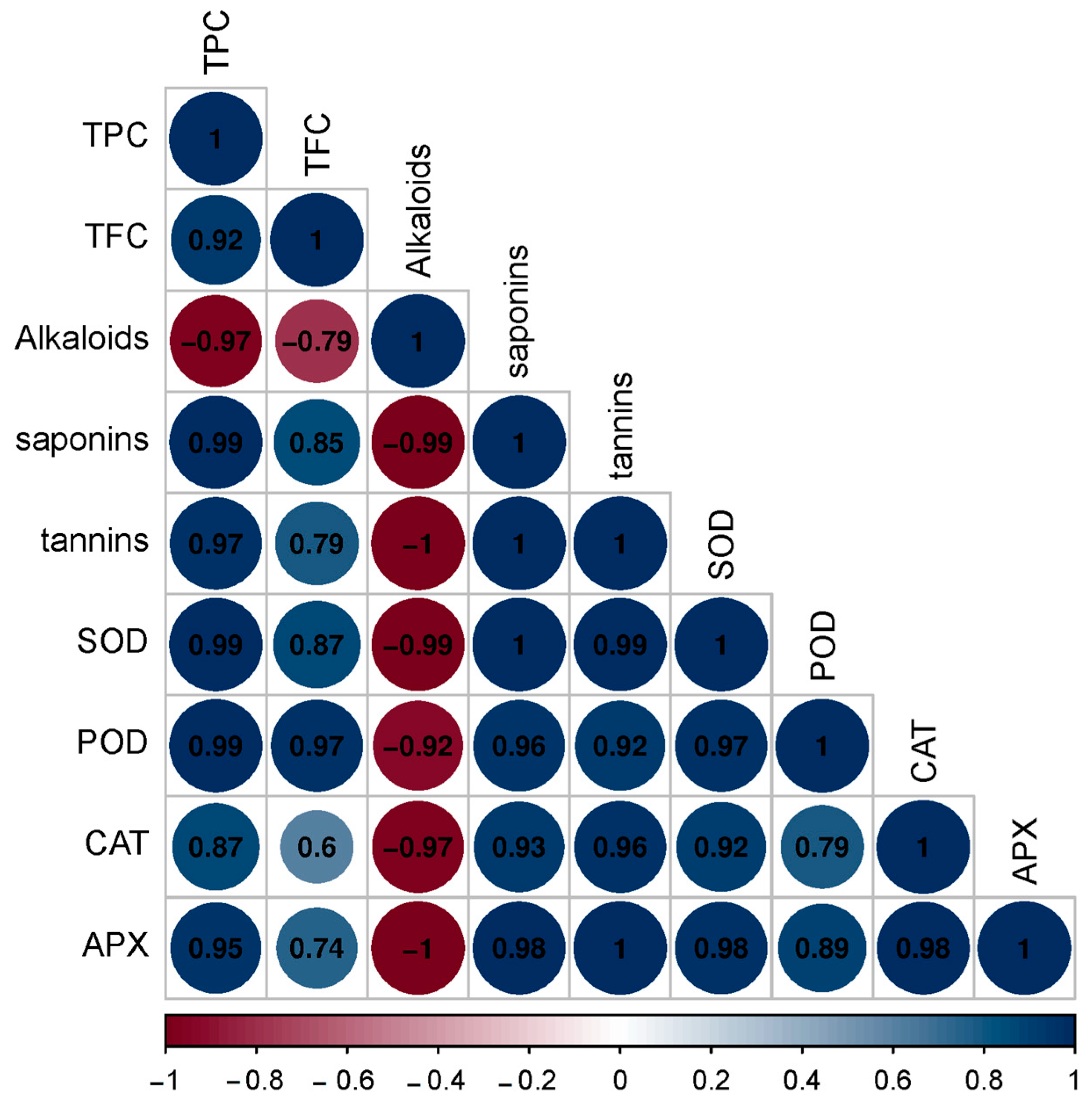
3.2. Phyto-Biochemical and Enzyme Activity Analysis
3.3. Molecular Analysis
3.4. SDS-PAGE Protein Profile
3.5. Relative Gene Expressions
3.6. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byng, J.W. The Flowering Plants Handbook: A Practical Guide to Families and Genera of the World; Plant Gateway Ltd.: Hertford, UK, 2014. [Google Scholar]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology and Ethnobo-Tany; Timber Press: Portland, OR, USA; Cambridge, UK, 2003. [Google Scholar]
- Abdul-Ghani, A.S.; Amin, R. Effect of aqueous extract of Commiphora opobalsamum on blood pressure and heart rate in rats. J. Ethnopharmacol. 1997, 57, 219–222. [Google Scholar] [CrossRef]
- Shen, T.; Wen-Zhu, W.; Xiao-Ning, W.; Ling-Mei, S.; Hui-Qing, Y.; Xiao-Ling, W.; Mei, J.; Hong-Xiang, L. Sesquiterpenoids from the Resinous Exudates of Commiphora opobalsamum (Burseraceae). Helvetica Chimica Acta 2008, 91, 881–887. [Google Scholar] [CrossRef]
- Iluz, D.; Hoffman, M.; Gilboa-Garber, N.; Amar, Z. Medicinal properties of Commiphora gileadensis. Afr. J. Pharm. Pharmacol. 2010, 4, 516–520. [Google Scholar]
- Mugahid, A.M. Flora of Saudi Arabia; King Saud University Press: Riyadh, Saudi Arabia, 1981. [Google Scholar]
- Soromessa, T. Ecological phytogeography: A case study of Commiphora species. Sci. Technol. Arts Res. J. 2013, 2, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water, Kingdom of Saudi Arabia: Riyadh, Saudi Arabia, 2001. [Google Scholar]
- Alfarhan, A.H.; Al-Turki, T.A.; Basahy, A.Y. Flora of Jizan Region; King Abdulaziz City for Science and Technology (KACST): Riyadh, Saudi Arabia, 2005. [Google Scholar]
- Weeks, A.; Simpson, B.B. Molecular phylogenetic analysis of Commiphora (Burseraceae) yields insight on the evolution and historical biogeography of an ‘‘impossible” genus. Mol. Phylogen. Evol. 2007, 42, 62–79. [Google Scholar] [CrossRef]
- Awadh, N.A.; Al Sokari, S.S.; Gushash, A.; Anwar, S.; Al-Karani, K. Ethnopharmacological survey of medicinal plants in Albaha region, Saudi Arabia. Pharmacogn. Mag. 2017, 9, 401–407. [Google Scholar] [CrossRef]
- Barthet, M. Expression and function of the Chloroplast-Encoded Gene Matk, United States; The Faculty of the Virginia Polytechnic Institute and State, Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2006. [Google Scholar]
- Ali, M.A.; Gábor, G.; Norbert, H.; Balázs, K.; Al-Hemaid, F.M.A.; Pandey, A.K.; Lee, J. The changing epitome of species identification- DNA barcoding. Saudi J. Biol. Sci. 2014, 21, 204–231. [Google Scholar]
- Yao, H.; Song, J.Y.; Liu, C.; Luo, K.; Han, J.P.; Li, Y. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [Green Version]
- Li, D.Z.; Gao, L.M.; Li, H.T.; Wang, H.; Ge, X.J.; Liu, J.Q. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc. Natl. Acad. Sci. USA 2011, 108, 19641–19646. [Google Scholar]
- Hollingsworth, P.M.; Li, D.Z.; van der Bank, M.; Twyford, A.D. Telling plant species apart with DNA: From barcodes to genomes. Phil. Trans. R Soc. B 2016, 371, 20150338. [Google Scholar] [CrossRef] [Green Version]
- Perrino, E.V.; Perrino, P. Crop wild relatives: Know how past and present to improve future research, conservation and utilization strategies, especially in Italy: A review. Resour. Crop Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Agarwal, D.; Aggarwal, K.; Burke-Spolaor, S.; Lorimer, D.R.; Garver-Daniels, N. FETCH: A deep-learning based classifier for fast transient classification. Mon. Not. Royal Astron. Soc. 2020, 497, 1661–1674. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci. Rep. 2018, 8, 10714. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and biological properties of Satureja cuneifolia and Thymus spinulosus Ten.: Two wild officinal species of conservation concern in Apulia (Italy). A preliminary survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Munda, S.; Saikia, R.J.; Begum, T.; Bhandari, S.; Gogoi, A.; Sarma, N.; Tamang, R.; Lal, M. Evaluation of Genetic Diversity Based on Microsatellites and Phytochemical Markers of Core Collection of Cymbopogon winterianus Jowitt Germplasm. Plants 2022, 11, 528. [Google Scholar] [CrossRef]
- Mesfer ALshamrani, S.; Safhi, F.A.; Alshaya, D.S.; Ibrahim, A.A.; Mansour, H.; Abd El Moneim, D. Genetic diversity using biochemical, physiological, karyological and molecular markers of Sesamum indicum L. Front. Genet. 2022, 13, 1035977. [Google Scholar] [CrossRef]
- Gowayed, S.M.H.; Abd El-Moneim, D. Detection of genetic divergence among some wheat (Triticum aestivum L.) genotypes using molecular and biochemical indicators under salinity stress. PLoS ONE 2021, 16, e0248890. [Google Scholar] [CrossRef]
- Horejsi, T.; Stub, J.E. Genetic Variation in Cucumber (Cucumis sativus L.) as Assed by Random Amplified Polymorphic DNA. Genet. Resour. Crop Evol. 1999, 46, 337–350. [Google Scholar] [CrossRef]
- Javid, A.; Ghafoor, A.; Anwar, R. Seed Storage Protein Electrophoresis in Groundnut for Evaluating Genetic Diversity. Pak. J. Bot. 2004, 36, 87–96. [Google Scholar]
- Abd El-Moneim, D.; ELsarag, E.I.S.; Aloufi, S.; El-Azraq, A.M.; ALshamrani, S.M.; Safhi, F.A.A.; Ibrahim, A.A. Quinoa (Chenopodium quinoa Willd.): Genetic Diversity According to ISSR and SCoT Markers, Relative Gene Expression, and Morpho-Physiological Variation under Salinity Stress. Plants 2021, 10, 2802. [Google Scholar] [CrossRef]
- Atia, M.A.M.; El-Moneim, D.A.; Abdelmoneim, T.K.; Reda, E.H.; Shakour, Z.T.A.; El-Halawany, A.M.; El-Kashoury, E.-S.A.; Shams, K.A.; Abdel-Azim, N.S.; Hegazy, M.-E.F. Evaluation of genetic variability and relatedness among eight Centaurea species through CAAT-box derived polymorphism (CBDP) and start codon targeted polymorphism (SCoT) markers. Biotechnol. Biotechnol. Equip. 2021, 35, 1230–1237. [Google Scholar] [CrossRef]
- EL-Mansy, A.B.; Abd El-Moneim, D.; ALshamrani, S.M.; Alsafhi, F.A.; Abdein, M.A.; Ibrahim, A.A. Genetic Diversity Analysis of Tomato (Solanum lycopersicum L.) with Morphological, Cytological, and Molecular Markers under Heat Stress. Horticulturae 2021, 7, 65. [Google Scholar] [CrossRef]
- Gerstein, M.; Rozowsky, J.; Yan, K.K. Comparative analysis of the transcriptome across distant species. Nature 2014, 512, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Antje, R.; Kris, M.; John, R.; Geert, G.; Vanessa, H.; Riet, D.R.; Sergej, K.; Jan, V.D.; Jean-Paul, J.; Marnik, V. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Chavan, J.J.; Gaikwad, N.B.; Kshirsagar, P. Total phenolics, flavonoids and antioxidant properties of three Ceropegia species from Western Ghats of India. S. Afr. J. Bot. 2013, 88, 273–277. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Luck, H. Catalase in Methods of Enzymatic Analysis; Bergmeyer, J., Grabi, M., Eds.; Academic Press: New York, NY, USA, 1974; Volume II. [Google Scholar]
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive m method for the quantification of micrograms of protein utilizing the principal dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Cota-Sanchez, H.; Remarchuk, K.; Ubayasenaj, K. Ready-to-use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Mol. Biol. Rep. 2006, 24, 161–167. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.Y.; Maackill, D.J. Start Codon Targted (SCoT) polymorphism: A simple novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Bio. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Anderson, J.A.; Churchill, G.A.; Autrique, J.E.; Tanksley, S.D.; Sorrells, M.E. Optimizing parental selection for genetic linkage maps. Genome 1993, 36, 181–186. [Google Scholar] [CrossRef]
- Nagaraju, J.; Reddy, K.D.; Nagaraja, G.M.; Sethuraman, B.N. Comparison of multilocus RFLPs and PCR-based marker systems for genetic analysis of the silkworm, Bombyx mori. Heredity 2001, 86, 588–597. [Google Scholar] [CrossRef]
- Powell, W.; Morgante, M.; Andre, C.; Hanafey, M.; Vogel, J.; Tingey, S.; Rafalski, A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol. Breed. 1996, 2, 225–238. [Google Scholar] [CrossRef]
- Prevost, A.; Wilkinson, M.J. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [Green Version]
- Kress, W.J.; Garcia-Robledo, C.; Uriarte, M.; Erickson, D.L. DNA barcodes for ecology, evolution, and conservation. Trends Ecol Evol. 2015, 30, 25–35. [Google Scholar] [CrossRef] [PubMed]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, P.S.; Bellampalli, R.; Dobriyal, R.M.; Agarwal, A.; Satyamoorthy, K.; Narayana, D.A. DNA barcoding of authentic and substitute samples of herb of the family Asparagaceae and Asclepiadaceae based on the ITS2 region. J. Ayurveda Integr. Med. 2012, 3, 136–140. [Google Scholar] [PubMed] [Green Version]
- Liu, C.; Liang, D.; Gao, T.; Pang, X.; Song, J.; Yao, H.; Han, J.; Liu, Z.; Guan, X.; Jiang, K.; et al. PTIGS-IdIt, a system for species identification by DNA sequences of the psbA-trnH intergenic spacer region. BMC Bioinform. 2011, 12, S4. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A. Species-specific molecular signature of Commiphora species of Saudi Arabia inferred from internal transcribed spacer sequences of nuclear ribosomal DNA. Saudi J. Biol Sci. 2018, 25, 1298–1301. [Google Scholar] [CrossRef]
- Carneiro de Melo Moura, C.; Brambach, F.; Jair Hernandez Bado, K.; Krutovsky, K.V.; Kreft, H.; Tjitrosoedirdjo, S.S.; Siregar, I.Z.; Gailing, O. Integrating DNA Barcoding and Traditional Taxonomy for the Identification of Dipterocarps in Remnant Lowland Forests of Sumatra. Plants 2019, 8, 461. [Google Scholar] [CrossRef]
- Rajeswari, A. Evaluation of phytochemical constituents, quantitative analysis and antimicrobial efficacy of potential herbs against selected microbes. Asian J. Pharm. Clin. Res. 2015, 8, 232–237. [Google Scholar]
- Al-mahbashi, H.; Shibany, A.; Massarani, S.; Salama, A.; Abudunia, A. Preliminary Phytochemical Composition and Biological Activities of Methanolic Extract of Commiphora Gileadensis L. J. Sci. Technol. 2019, 23, 23–28. [Google Scholar]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signal triggering antioxidant gene defenses. Brazilian J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Demiral, T.; Türkan, I. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J. Plant Physiol. 2004, 161, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.J.M.; Britto, S.J.; Prabhu, S.; Senthilkumar, S.R. Phylogenetic Relationships of Crotalaria Species Based on Seed Protein Polymorphism Revealed by SDS-PAGE. Plant Sci. 2011, 2, 119–128. [Google Scholar]
- Sinha, K.N. Electrophoretic Study of Seed Storage Protein in Five Species of Bauhinia. IOSR J. Pharm. Biol. Sci. 2012, 4, 8–11. [Google Scholar] [CrossRef]
- Abd El-Moneim, D. Characterization of ISSR and SCoT markers and TaWRKY gene expression in some Egyptian wheat genotypes under drought stress. J. Plant Prod. Sci. 2020, 8, 31–46. [Google Scholar]
- Abdein, M.A.; El-Mansy, A.B.; Awad, N.S.; Abd El-Moneim, D. Assessment of genetic diversity in summer squash genotypes using some yield traits and DNA markers analysis under sinai conditions. J. Plant Prod. Sci. 2021, 10, 13–29. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Kavane, A.; Sharma, A.; Geetha, K.A.; Samantaray, S.; Maiti, S. Molecular marker analysis of genetic diversity in relation to reproductive behaviour of Commiphora wightii populations distributed in Gujarat and Rajasthan states of India. S. Afr. J. Bot. 2018, 117, 141–148. [Google Scholar] [CrossRef]
- Geetha, K.A.; Kawane, A.; Bishoyi, A.K.; Phurailatpam, A.; Ankita, C.; Malik, S.K.; Srinivasan, R.; Bhat, S.R. Characterization of mode of reproduction of Commiphorawightii [(Arnott) Bhandari] reveals novel pollen-pistil interaction and occurrence of obligate sexual female plants. Trees 2013, 27, 567–581. [Google Scholar] [CrossRef]
- Kawane, A.; Geetha, K.A.; Reddy, M.N.; Maiti, S. Degree of polyembryony among the accessions of Commiphora wightii collected from different natural habitats of India. Curr. Sci. 2014, 107, 361–364. [Google Scholar]
- Abdein, M.A.; El-Moneim, D.A.; Taha, S.S. Molecular characterization and genetic relationships among some tomato genotypes as revealed by ISSR and SCoT markers. Egypt. J. Genet. Cytol. 2018, 47, 139–159. [Google Scholar]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. iMEC: Online Marker Efficiency Calculator. Appl. Plant Sci. 2018, 6, e1159. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Afolayan, A.J. Evaluation of genetic relationship among varieties of Capsicum annuum L. and Capsicum frutescens L. in West Africa using ISSR markers. Heliyon 2019, 5, e01700. [Google Scholar] [CrossRef] [PubMed]
- Rayan, W.A.; Osman, S.A.H. Phylogenetic relationships of some Egyptian soybean cultivars (Glycine max L.) using SCoT marker and protein pattern. Bull. Natl. Res. Cent. 2019, 43, 161. [Google Scholar] [CrossRef] [Green Version]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Yadav, C.; Malik, C.P. Molecular Characterization of Fennel (Foeniculum vulgare Mill.) accessions using Start Codon Targeted (SCoT) markers. J. Plant Sci. 2016, 32, 37–44. [Google Scholar]
- Pour-Aboughadareh, A.; Mohmoudi, A.M.; Ahmadi, J.; Moghaddam, M.; Mehrabi, A.A. Agromorphological and molecular variability in Triticum boeoticum accessions from Zagros Mountains. Iran Genet. Resour. Crop. Evol. 2017, 64, 545–556. [Google Scholar] [CrossRef]
- Jin, Q.; Yao, Y.; Cai, Y.; Lin, Y. Molecular cloning and sequence analysis of a phenylalanine ammonia-lyase gene from Dendrobium. PLoS ONE 2013, 8, e62352. [Google Scholar] [CrossRef] [Green Version]
- Van der Weerden, N.L.; Anderson, M.A. Plant defensins: Common fold, multiple functions. Fungal Biol. Rev. 2013, 26, 121–131. [Google Scholar]
- Upadhyay, P.; Rai, A.; Kumar, R.; Singh, M.; Sinha, B. Differential Expression of Pathogenesis Related Protein Genes in Tomato during Inoculation with A. Solani. J. Plant Pathol. Microbiol. 2014, 5, 217. [Google Scholar]
- Juškytė, A.D.; Mažeikienė, I.; Stanys, V. Putative Genes of Pathogenesis-Related Proteins and Coronatine-Insensitive Protein 1 in Ribes spp. Plants 2022, 11, 355. [Google Scholar] [CrossRef]







| Nuclear Region | Accessions | GC% | GenBank Accession No. | Pairwise Identity (PI) | Nucleotide (bp) |
|---|---|---|---|---|---|
| ITS | C1 | 56% | OL411968 | 100% | 1517 |
| C2 | 62.9% | ON129562 | 100% | 420 | |
| C3 | 64.3% | ON128707 | 95.45% | 686 | |
| 18 S | C1 | 50.1% | ON171463 | 100% | 827 |
| C2 | 62.7% | ON176685 | 91.13% | 859 | |
| C3 | 50.9% | ON148092 | 100% | 753 |
| Marker | Primer Name | Sequence | Fragment Size (bp) | MB | PB | TB | PIC | EMR | MI | RP | P% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UB | NB | |||||||||||
| ISSR | ISSR1 | (AG)8T | 192–905 | 4 | 4 | 1 | 9 | 0.46 | 2.78 | 1.28 | 12.00 | 55.56 |
| ISSR2 | (GA)8T | 210–895 | 5 | 3 | 1 | 9 | 0.36 | 1.78 | 0.64 | 13.33 | 44.44 | |
| ISSR3 | (CT)8T | 196–720 | 6 | 0 | 1 | 7 | 0.08 | 0.14 | 0.01 | 13.33 | 14.29 | |
| ISSR4 | (CT)8A | 326–515 | 3 | 0 | 1 | 4 | 0.14 | 0.25 | 0.04 | 7.33 | 25.00 | |
| ISSR5 | (CA)8T | 245–995 | 4 | 4 | 3 | 11 | 0.47 | 4.45 | 2.09 | 14.67 | 63.64 | |
| ISSR6 | (GT)8A | 200–420 | 2 | 0 | 3 | 5 | 0.33 | 1.80 | 0.59 | 8.00 | 60.00 | |
| ISSR7 | (AG)8C | 365–475 | 1 | 2 | 0 | 3 | 0.59 | 1.33 | 0.79 | 3.33 | 66.67 | |
| ISSR8 | (AG)8G | 242–645 | 3 | 2 | 2 | 7 | 0.41 | 2.29 | 0.94 | 10.00 | 57.14 | |
| ISSR9 | (GA)8C | 364–764 | 2 | 3 | 1 | 6 | 0.54 | 2.67 | 1.44 | 7.33 | 66.67 | |
| ISSR10 | (CT)8G | 294–1190 | 1 | 6 | 2 | 9 | 0.72 | 7.11 | 5.12 | 8.67 | 88.89 | |
| Total | 31 | 24 | 15 | 70 | 4.10 | 24.60 | 12.94 | 97.99 | 55.71 | |||
| Average | 3.10 | 2.40 | 1.50 | 7 | 0.41 | 2.46 | 1.29 | 9.80 | 55.71 | |||
| SCoT | SCoT1 | CAACAATGGCTACCACC | 180–740 | 6 | 0 | 3 | 9 | 0.19 | 1.00 | 0.19 | 0.19 | 33.33 |
| SCoT2 | CAACAATGGCTACCACG | 200–785 | 2 | 11 | 0 | 13 | 0.75 | 9.31 | 6.98 | 0.75 | 84.62 | |
| SCoT3 | AAGCAATGGCTACCACC | 203–385 | 3 | 2 | 2 | 7 | 0.41 | 2.29 | 0.94 | 0.41 | 57.14 | |
| SCoT4 | ACGACATGGCGACCAAC | 195–428 | 3 | 1 | 1 | 5 | 0.29 | 0.80 | 0.23 | 0.29 | 40.00 | |
| SCoT5 | ACCATGGCTACCACCGA | 63–925 | 5 | 7 | 8 | 20 | 0.53 | 11.25 | 5.96 | 0.53 | 75.00 | |
| SCoT6 | CACCATGGCTACCACCA | 186–1430 | 1 | 8 | 5 | 14 | 0.71 | 12.07 | 8.57 | 0.71 | 92.86 | |
| SCoT7 | ACCATGGCTACCACCGC | 189–1950 | 0 | 12 | 7 | 19 | 0.77 | 19.00 | 14.63 | 0.77 | 100.00 | |
| SCoT8 | ACGACATGGCGACCCAC | 80–1040 | 3 | 10 | 4 | 17 | 0.66 | 11.53 | 7.61 | 0.66 | 82.35 | |
| SCoT9 | CCATGGCTACCACCGCA | 185–1011 | 4 | 3 | 5 | 12 | 0.45 | 5.33 | 2.40 | 0.45 | 66.67 | |
| SCoT10 | ACGACATGGCGACCGCG | 192–1018 | 5 | 2 | 5 | 12 | 0.41 | 4.08 | 1.67 | 0.41 | 58.33 | |
| SCoT11 | CAACAATGGCTACCACCC | 260–1050 | 2 | 3 | 5 | 10 | 0.54 | 6.40 | 3.46 | 0.54 | 80.00 | |
| Total | 34 | 59 | 45 | 138 | 5.71 | 83.06 | 52.64 | 166.66 | 75.36 | |||
| Average | 5.36 | 4.09 | 12.55 | 5.36 | 0.52 | 7.55 | 4.79 | 15.15 | 75.36 | |||
| Features | Molecular Markers | SDS-PAGE Protein | |
|---|---|---|---|
| ISSR | SCoT | ||
| Band size range | 192–1190 bp | 63–1950 bp | 56–137 KDa |
| Total bands | 70 | 138 | 7 |
| Polymorphic bands | 39 | 104 | 2 |
| Unique bands | 8 (C1), 4(C2), 12 (C3) | 23 (C1), 7(C2), 29 (C3) | 2 (C1), 0 (C2 & C3) |
| % Polymorphism | 55.71% | 75.36% | 28.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safhi, F.A.; ALshamrani, S.M.; Jalal, A.S.; El-Moneim, D.A.; Alyamani, A.A.; Ibrahim, A.A. Genetic Characterization of Some Saudi Arabia’s Accessions from Commiphora gileadensis Using Physio-Biochemical Parameters, Molecular Markers, DNA Barcoding Analysis and Relative Gene Expression. Genes 2022, 13, 2099. https://doi.org/10.3390/genes13112099
Safhi FA, ALshamrani SM, Jalal AS, El-Moneim DA, Alyamani AA, Ibrahim AA. Genetic Characterization of Some Saudi Arabia’s Accessions from Commiphora gileadensis Using Physio-Biochemical Parameters, Molecular Markers, DNA Barcoding Analysis and Relative Gene Expression. Genes. 2022; 13(11):2099. https://doi.org/10.3390/genes13112099
Chicago/Turabian StyleSafhi, Fatmah Ahmed, Salha Mesfer ALshamrani, Areej Saud Jalal, Diaa Abd El-Moneim, Amal A. Alyamani, and Amira A. Ibrahim. 2022. "Genetic Characterization of Some Saudi Arabia’s Accessions from Commiphora gileadensis Using Physio-Biochemical Parameters, Molecular Markers, DNA Barcoding Analysis and Relative Gene Expression" Genes 13, no. 11: 2099. https://doi.org/10.3390/genes13112099






